Abstract
Purpose
Increased adrenergic activity in response to chronic stress is known to promote tumor growth by stimulating the tumor microenvironment. The focus of the current study was to determine whether dopamine, an inhibitory catecholamine, could block the effects of chronic stress on tumor growth.
Experimental Design
Expression of dopamine receptors (DR1-DR5) was analyzed by real time reverse transcription-PCR and by Western blotting. In vitro effects of dopamine on cell viability, apoptosis and migration were examined. For in vivo therapy, murine and human DR2-siRNA’s were incorporated into chitosan nanoparticles (CH).
Results
In this model of chronic stress, tumoral norepinephrine levels remained elevated while dopamine levels were significantly decreased compared to non-stressed animals. Daily restraint stress resulted in significantly increased tumor growth in both immunodeficient (SKOV3ip1 and HeyA8) and immunocompetent (ID8) ovarian cancer models. This increase was completely blocked with daily dopamine treatment. Dopamine treatment also blocked the stress induced increase in angiogenesis. Endothelial and ovarian cancer cells expressed all dopamine receptors except for the lack of DR3 expression in ovarian cancer cells. DR2 was responsible for the inhibitory effects of dopamine on tumor growth and microvessel density (MVD) as well as the stimulatory effect on apoptosis, since the DR2-antagonist eticlopride, reversed these effects. Dopamine significantly inhibited cell viability and stimulated apoptosis in vitro. Moreover, dopamine reduced cAMP levels and inhibited norepinephrine and VPF/VEGF induced Src kinase activation.
Conclusions
Dopamine depletion under chronic stress conditions creates a permissive microenvironment for tumor growth that can be reversed by dopamine replacement.
Keywords: Ovarian cancer, stress, catecholamines
Introduction
The stress response is a complex process arising from interactions between environmental contexts and the organism’s evaluation of potential threat and its capacity to respond. These factors initiate a cascade of information processing in both central and peripheral nervous systems as well as hormonal cascades.(1) This results in activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis (2, 3). Norepinephrine and epinephrine are known to be elevated in the plasma and tumor microenvironment of individuals with acute and chronic stress (4, 5). We have recently demonstrated that both norepinephrine and epinephrine levels are elevated in a sustained fashion in ovarian and other peritoneal tissues in preclinical models of chronic stress (6). These hormonal increases were related to greater tumor burden, which was mediated by increased angiogenesis. Recent evidence suggests that the third catecholamine, dopamine, has the opposite effect with regard to effects on tumor angiogenesis, growth and development of ascites (7, 8). In vivo and in vitro studies have shown that dopamine, via its specific DR2 receptors, inhibits tumor growth by suppressing the actions of vascular permeability factor/vascular endothelial growth factor-A (VPF/VEGF) on both tumor endothelial cells and bone-marrow-derived endothelial progenitor cells (9). Dopamine inhibits VEGF-induced angiogenesis by suppressing VEGFR-2 phosphorylation (10–12), and inhibits MAP kinase and focal adhesion kinase activation (12). Dopamine can also inhibit mobilization of endothelial progenitor cells (EPCs) from the bone marrow (13).
It is known that dopamine levels are increased in the brain during acute stress (14). In contrast, under chronic stress conditions, dopamine levels are lower as a consequence of decreased release of dopamine (15). However, it is not known whether dopamine levels are depleted in the tumor microenvironment in response to chronic stress. Moreover, it is not known whether dopamine can counteract the stimulatory effects of norepinephrine on tumor growth. These unanswered questions along with underlying mechanisms are addressed in the current manuscript.
Materials and Methods
Reagents
Dopamine (DA), bromocriptine (dopamine receptor 2 (DR2)-agonist), eticlopride (DR2-antagonist), SKF 3839 (DR1 agonist), butaclamol (DR1 antagonist) and norepinephrine (NE) were obtained from Sigma Aldrich (Detroit, MI); recombinant human VEGF from R&D Systems (Minneapolis, MN). Annexin V- and Tunel staining kits were purchased from BD Pharmingen (San Diego, CA) and Promega (Madison, WI) respectively.
Cell lines and culture conditions
The ovarian non-transformed (HIO-180) and cancer cells (SKOV3ip1, HeyA8, A2780. RMG2 and IGROV) were maintained in RPMI1640, 15% FBS and 0.1% gentamicin sulfate (Gemini Bioproducts, Calabasas, CA) (16, 17). Endothelial cells isolated from the mesentery or ovary of the immortomouse (MOEC) were a kind gift from Dr. Robert Langley (18) and were maintained in DMEM, 10% FBS. All in vitro experiments were conducted at 60% to 80% confluence, unless otherwise specified. For in vivo injections, cancer cells were trypsinized and centrifuged at 1000 rpm for 7 min at 4°C, washed twice, and reconstituted in Hank’s balanced salt solution (Gibco, Carlsbad, CA).
Determination of dopamine concentration in tumor and normal tissue
Dopamine levels were determined by HPLC-EC in the College of Pharmacy at the University of Iowa. The method uses electrochemical detection to quantitate dopamine levels; HPLC is used to separate one catecholamine from another. Values were calculated by comparing the peak height of the unknown (sample) to that of a pure standard of known concentration, these were expressed in pg dopamine/mg wet tissue.
RT-PCR analysis of dopamine receptors
Total RNA was isolated by using Qiagen RNeasy kit. cDNA was synthesized by using the SuperScript First-Strand kit (Invitrogen) as per the manufacturer’s instructions. cDNA was subjected to PCR using specific primers sequences for human dopamine receptors (DR1-DR5) previously reported (19). Specific primers sequences for murine dopamine receptors (DR1-DR5) were designed based on the reported NCBI-nucleotide sequences using the Oligo Perfect software (Invitrogen). β-actin was used as a housekeeping gene.
Short interfering RNA (siRNA) preparation
Specific siRNA sequences targeted against murine dopamine receptor 2 (DR2) (duplex of: 5′GAUUCACUGUGACAUCUUU and 5AAAGAUGUCACAGUGAAUC) and human DR2 (duplex of: 5′CACACAUCCUGAACAUCA and 5′UGUAUGUUCAGGAUGUGUG) were obtained from Sigma Aldrich. These sequences were incorporated into chitosan nanoparticles (CH) using a gelation method of anionic tripolyphosphate (TPP) (20). Briefly, predetermined TPP (0.25% w/v) and siRNA (1 μg/μL) were added in CH solution, resulting in siRNA-CH-NP generated under constant stirring at room temperature. After incubating at 4°C for 40 min, the siRNA-CH was collected by centrifugation (Thermo Biofuge, Germany) at 12,000 rpm for 40 min at 4°C. The CH-NPs were purified 3 times and stored at 4°C until used.
Chronic stress model and treatment schema
Female athymic nude and immunocompetent (C57BL6) mice (10 to 12-week-old) were obtained from the US National Cancer Institute. All experiments were approved by the Institutional Animal Care and Use Committee of the M.D. Anderson Cancer Center. The animals were experimentally stressed using a well-characterized restraint system, in which periodic immobilization induces high levels of HPA and SNS activity characteristic of chronic stress (6). Ovarian cancer cells were injected intraperitoneally into mice 7 days after starting daily stress applied for 2 hours. Then, mice were further divided into treatment groups (10 animals/group) as follows: 1) control PBS, 2) dopamine (75 mg/kg daily i.p), 3) dopamine + eticlopride (10 mg/kg daily i.p); 4) control siRNA-CH; 5) dopamine + murine dopamine receptor 2-siRNA (mDR2-siRNA-CH (5 μg/injection) or 6) dopamine + human dopamine receptor 2-siRNA (hDRD2-siRNA) (5 μg/injection). SiRNA treatments (150 μg/kg) were given through the intravenous route twice per week. Following 3 weeks of treatment, mice were euthanized, and, tumor weight and nodules were recorded. Tumor and relevant tissue samples were collected.
Cell viability assay
To examine the effect of dopamine on cell viability, MTT assay was performed as previously described (21).
Cyclic AMP determination
The effect of dopamine on cAMP accumulation was examined exposing cells to 0, 10 and 50 μM dopamine for 30 min at 37°C. cAMP levels were measured in total cell lysates using an enzyme immunoassay-kit (Biomol, PA).
Cell Invasion assay
Invasion through human-defined matrix was assessed using the Membrane Invasion Culture System (MICS), as previously described (22). 1.5 × 105 SKOV3ip1-cells were loaded into the upper chamber in media only or in media containing the stimulant of interest. Agonist (DR1: SKF 3839; DR2: bromocriptine) and antagonist (DR1: butaclamol; DR2: eticlopride) were used at 50 μM. NE (10 μM) and VEGF (10 ng/ml). Cells were allowed to invade in a humidified incubator for 24 hours. Cells that had invaded through the basement membrane were collected, stained and counted by light microscopy in 5 random fields (original magnification × 200) per sample.
Western Blot and Immunoprecipitation analyses
Western blot analysis was performed as previously described (21). Briefly, lysates from cultured cells were prepared using modified RIPA buffer, protein concentrations were determined using a BCA Protein Assay Reagent kit (Pierce Biotechnology, Rockford, IL). Protein lysates were subjected to 10 % SDS-PAGE separation and transferred to a nitrocellulose membrane. Blots were probed with primary antibodies: DR2 (Santa Cruz, Biotechnology); Src and pSrcY416 kinase (Cell Signaling, Biotechnology) and HRP-conjugated secondary antibody (Amersham) and developed with enhanced chemiluminescence detection kit (ECL, Pierce). Equal protein loading was confirmed reprobing membranes with an anti-β actin antibody (Sigma Aldrich). To examine the association between Gαi1 protein and DR2, MOEC cells were exposed to 10 μM dopamine for 0, 10 and 30 min at 37° C. Cell lysates were than prepared and immunoprecipitated with DR2 antibody at 4°C for 2 hours. Immunocomplexes were captured with 2% protein A–agarose beads (Upstate). Protein was eluted in reducing sample Laemmli buffer, subjected to 10% SDS-PAGE separation and transferred to a nitrocellulose membrane. Anti-Gαi (Abcam) was used as primary antibody. Immunodetection of Gαi1 protein was performed as described above.
Immunohistochemistry
Analysis of microvessel density (MVD) and assessment of tumor and endothelial cell apoptosis was performed following procedures previously described (18, 21, 23, 24). Double Immunofluorescence for DR2/CD31 and for DR2/Tunel was performed in frozen tissue-sections as follows: after acetone-fixation and blocking with gelatin (4%), tissues were incubated with rabbit DR2 antibody (Santa Cruz-Biotech, (1:100) at 4 °C overnight. Samples were washed in PBS and incubated with blocking solution for 10 minutes and then with a goat anti-rabbit Alexa 488 antibody (1:1000) for 1 hour. Afterwards, tissues were subjected to CD31 and Tunel stainings, as previously described.(18, 21, 23, 24).
Immunofluorescence and confocal microscopy
Immunofluorescence microscopy was performed using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 100-W Hg lamp and narrow bandpass excitation filters. Representative images were obtained using a cooled charge-coupled device Hamamatsu C5810 camera (Hamamatsu Photonics, Bridgewater, NJ) and Optimas software (Media Cybernetics, Silver Spring, MD). Confocal fluorescence images were collected using 20x objectives on a Zeiss LSM 510 laser scanning microscopy system (Carl Zeiss Inc., Thornwood, NY) equipped with a motorized Axioplan microscope, argon laser (458/477/488/514 nm, 30 mW), HeNe laser (413 nm, 1 mW, and 633 nm, 5 mW), LSM 510 control and image acquisition software, and appropriate filters (ChromaTechnology Corp., Brattleboro, VT). Composite images were constructed with Photoshop software (Adobe Systems, Inc., Mountain View, CA).
In vitro assessment of apoptosis
MOEC and SKOV3ip1 cells were exposed to different dopamine concentrations (0–50 μM) for 48 hours. The relative percentage of apoptotic cells was assessed using the Annexin V-FITC apoptosis detection Kit-1 (BD Pharmingen, San Diego, CA) according to the manufacturer’s protocol.
Statistical analysis
Continuous variables were compared with the Student’s t-test (between two groups) or analysis of variance (for all groups) if normally distributed and the Mann-Whitney rank sum test if distributions were nonparametric. For in vivo therapy experiments, 10 mice in each group were used, and a p<0.05 on 2-tailed testing was considered significant. To control for the effects of multiple comparisons, a Bonferroni adjustment was made; for the analysis, a p value ≤ 0.017 was considered statistically significant.
Results
Dopamine levels during chronic stress
We determined dopamine and norepinephrine concentrations in tumors and peritoneal tissues including ovary, liver, and omentum from mice subjected to daily stress for 1, 3, 7 or 14 days. NE levels increased after 1 day of stress and remained elevated until day 14 in all tissues samples. In contrast, DA concentrations increased significantly after 1 day of stress, but declined after 3 to 14 days of stress in tumor, ovarian and omental tissues (Supplementary Figure 1).
Dopamine- dose-response in vivo
We have previously demonstrated that chronic stress establishes favorable conditions for promoting angiogenesis in the tumor microenvironment by SNS activation (6). Since the levels of the anti-angiogenic catecholamine, dopamine, are decreased in the tumor microenvironment under chronic stress, we asked whether dopamine could block the stimulatory effects of SNS on ovarian cancer growth. Prior to performing such blocking experiments, we first tested several doses of dopamine to identify the lowest dose required for inhibiting cancer growth in non-stressed or stressed mice. SKOV3ip1-tumor bearing nude mice (n=10 per group) were treated with either: 1) control PBS, 2) dopamine 50 mg/kg, 3) dopamine 75 mg/kg, or 4) dopamine 100 mg/kg. Treatment was started 1 week after injection of SKOV3ip1-ovarian cancer cells. In stressed mice, doses of 75 and 100 mg/kg significantly reduced tumor growth (p<0.05), with the greatest decrease noted at the 75 mg/kg dose. All three doses significantly decreased the number of tumor nodules (p<0.05) compared to controls (Supplementary Figure 2A). In contrast, in non-stressed mice no significant changes in tumor weight or tumor nodules were observed at any of the dopamine doses tested. Given the known effects of dopamine on angiogenesis (7), we also assessed MVD. There was a significant reduction in MVD at all dopamine doses tested (p<0.05; Supplementary Figure 2B). Dopamine 75 mg/kg was selected for all subsequent in vivo experiments due to its inhibitory effect on cancer growth.
To assess the longitudinal effects of dopamine, we performed an in vivo experiment in SKOV3ip1-tumor bearing mice. Tumor growth curves in non-stressed and stressed mice are presented in Supplementary Figure 2D. While there was no significant effect of dopamine in the non-stress setting, dopamine completely blocked the growth stimulatory effects of daily restraint stress (Supplementary Figure 2D).
Dopamine blocks stress-induced ovarian cancer growth via DR2
As expected, daily stress increased tumor growth by 2.4-fold (Figure 1). In stressed mice, daily dopamine-treatments resulted in 68% reduction in tumor growth compared to control mice injected with PBS (p<0.05). We also tested the effects of dopamine in an immune-competent syngeneic mouse model of ovarian cancer (ID8-C57BL6 model). Consistent with the other models, daily restraint stress increased tumor growth by 50% (p<0.01; Supplementary Figure 3) in the ID8 model. Moreover, dopamine treatment significantly reduced tumor growth (47%; p<0.02) in the non-stressed group and blocked the stress mediated increase in tumor growth (78%, p<0.001; Supplementary Figure 3).
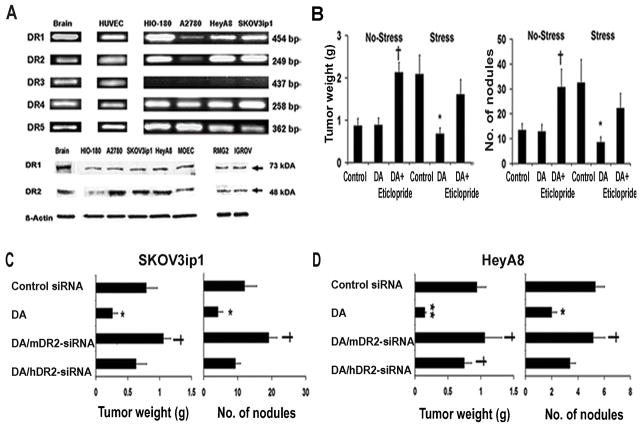
Figure 1.
Dopamine blocks stress-mediated tumor growth by acting through DR2. (A) Tumor weight and tumor nodules in non-stressed and stressed mice bearing SKOV3ip1-tumors treated with: PBS (Control), DA, and DA/eticlopride. DA blocked significantly tumor growth and reduced number of tumor nodules in stressed mice. This effect was abrogated by the combined treatment of DA/eticlopride. In non-stressed mice DA/Eticlopride treatment significantly increased tumor growth compared to controls. Data are the mean ± SE; n =10 mice per group. *p <0.05 compared to stress controls, †p< 0.005 compared to non-stress controls. (B) RT-PCR analysis of DR1-DR5 and protein expression of DR1-DR2 in endothelial and ovarian cancer cell lines. HUVEC and MOEC express all 5 receptors, whereas DR3 was not detected in ovarian cancer cells. (C and D) Tumor weight and tumor nodules in stressed mice bearing SKOV3ip1 and HeyA8 tumors treated with: Control siRNA, DA, DA/mDR2-siRNA-CH and DA/hDR2-SiRNA-CH. DA reduced significantly tumor growth and tumor nodules in the SKOV3 ip1 (p<0.05) and HeyA8 models (p<0.01). The combined treatment DA/mDR2-siRNA significantly abrogated the dopamine-inhibitory effect on tumor growth in the SKOV3ip1 model. In the HeyA8 model, both combined treatments (DA/mDR2-siRNA-CH and DA/hDR2) reversed the dopamine-blocking effect on tumor growth. Data are the mean ± SE; n =10 mice per group. *p<0.05, **p<0.01 compared to controls, † p< 0.05, compared to DA-treated mice.
Since dopamine can signal through multiple receptors, we examined the RNA expression of DR1-DR5 in ovarian and endothelial cell lines. Figure 1A shows the RNA expression in ovarian non-transformed epithelial (HIO-180), cancer (A2780, HeyA8, and SKOV3ip1) and in endothelial (HUVEC) cells. While all dopamine receptors are expressed in HUVEC, DR3 was not detected in any of the ovarian cell lines tested. Protein expression of DR1 and DR2 was also confirmed by Western blotting in these and other ovarian cancer cell lines (RMG2 and IGROV), and MOEC (Figure 1A).
Given the suspected role of DR2 in mediating the anti-angiogenic effects of dopamine (7), we next examined whether the suppression of tumor growth by dopamine was specifically mediated by DR2. In stressed mice, addition of a DR2 antagonist (eticlopride) to dopamine treatment reversed the protective effects of dopamine (Figure 1B). These data suggest that dopamine indeed acted through DR2 to block stress mediated ovarian cancer growth.
Dopamine targets murine endothelial and human cancer cells
To further examine whether the inhibitory effects of dopamine were mediated by targeting (host) endothelial and/or (human) tumor cells through their corresponding DR2 receptors, we utilized siRNA incorporated into chitosan nanoparticles. The specificity of siRNA sequences for mouse and human DR2, was confirmed using RT-PCR (Supplementary Figure 4). SKOV3ip1-tumor bearing mice were divided into 4 experimental groups: 1) control siRNA, 2) dopamine alone, 3) dopamine + mDR2-siRNA and 4) dopamine + hDR2-siRNA; each group was used for both non-stress and stress conditions (Figure 1C). In daily stressed mice, dopamine treatment significantly blocked tumor weight (67%) and the number of tumor nodules (65%). This growth inhibitory effect of dopamine was significantly abrogated by dopamine/mDR2-siRNA-CH, but not by dopamine/hDR2-siRNA-CH combined treatment (p=0.02; Figure 1C). These results suggest that in the SKOV3ip1 model, dopamine mediated effects occur primarily through DR2 on endothelial cells. The effects of dopamine were also tested in a second ovarian cancer model, HeyA8 (Figure 1D). Compared to mice injected with control siRNA, dopamine treatment blocked tumor growth by 84% (p=0.007) and resulted in a 63% decrease in the number of nodules (p=0.02). Interestingly, in this model both, both dopamine/mDR2-siRNA-CH and dopamine/hDR2-siRNA-CH treatments significantly blocked the inhibitory effects of dopamine
Effect of dopamine on angiogenesis and cell viability
Given the known effects of catecholamines on angiogenesis, we examined microvessel density in the SKOV3ip1-tumors harvested from dopamine treated animals. In stressed mice, dopamine-treatment resulted in a significant reduction (61%) in MVD compared to controls (Figure 2A; p<0.01). In contrast, dopamine/eticlopride treatment led to a significant increase (Figure 2A; p<0.01) in MVD, compared to dopamine-treated mice. We also examined effects on tumor cell apoptosis since it is known that norepinephrine can reduce sensitivity of cancer cells to apoptosis (19). Tissues from dopamine-treated mice revealed a 2.2-fold increase (p<0.01) in tumor cell apoptosis compared to control tissues (Figure 2A). The combined dopamine/eticlopride treatment abrogated the dopamine-mediated effects on cell apoptosis. In addition, the combined treatment of dopamine/mDR2siRNA and dopamine/hDR2-siRNA-CH reversed the effects of dopamine on MVD (p<0.01) and cell apoptosis (p<0.01; Figure 2B). Colocalization of DR2 in tumor cells and tumor associated endothelial cells was confirmed by double immunofluorescence staining for CD31 antigen/DR2 and Tunel/DR2. Confocal images are included in Figure 2A and 2B.
Figure 2.
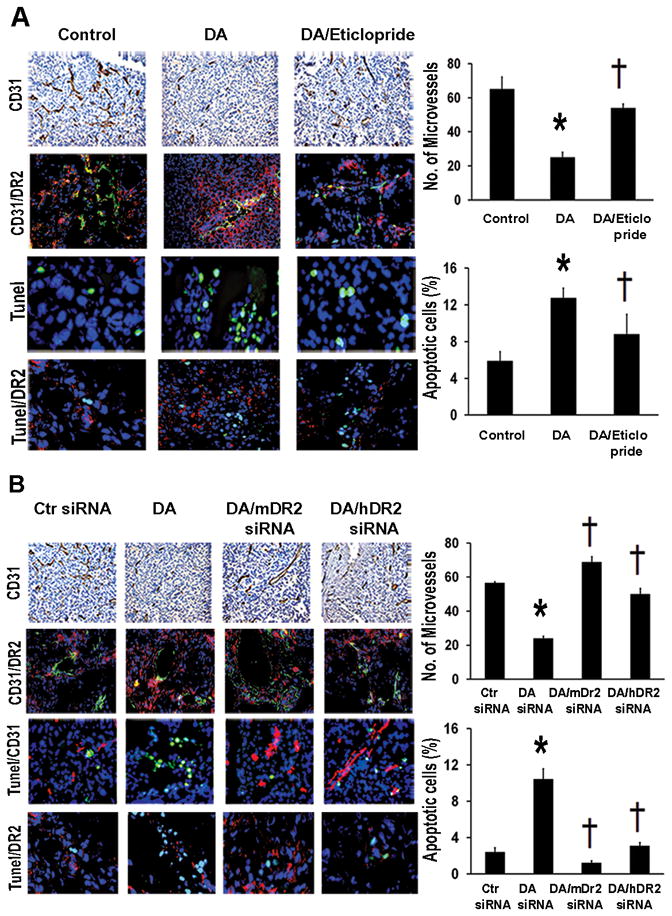
Dopamine decreases stress-stimulated angiogenesis and stimulates tumor cell apoptosis via its DR2. (A) MVD was significantly decreased by DA-treatment in SKOV3ip1-tumor tissues of stressed mice (*p<0.01). DA/eticlopride treatment significantly (†p<0.01) reversed DA-inhibitory effect on MVD. MVD was evaluated by immunohistochemical analysis of CD31 (DAB-staining). Microvessels were counted in five fields at 100X of each tissue sample (n=5). Values are means ± SE. DA treatment caused significantly (*p<0.005) higher apoptotic rates compared to control mice. This effect was also significantly († p< 0.005) reversed by DA/eticlopride combined treatment. Confocal images (200X) showing colocalization of DR2 (red fluorescence) in CD31-positive tumor endothelial cells (green fluorescence). Apoptotic cells (green fluorescence) were detected by Tunel staining (Promega kit). Percentages of apoptotic cells were calculated in five fields at 200X of each tissue sample (n=5). Values are means ± SE. Detection of DR2 (red fluorescence) in tumor apoptotic cells (green fluorescence) (Confocal images 200X). (B) DA/mDR2-siRNA and DA/hDR2-siRNA-CH combined treatments induced significant increase (†p <0.005) in MVD in stressed mice, reverting the DA-inhibitory effect on MVD (*p<0.005). Values are means ± SE. Detection of DR2 (red fluorescence) in tumor CD31-positive endothelial cells (green fluorescence) (Confocal images 200X). DA-stimulatory effect on tumor cell apoptosis was also significantly abrogated by the DA/mDR2-siRNA and DA/hDR2-siRNA treatments (†p <0.005). Values are means ± SE. Confocal images (200X) revealing colocalization of DR2 (red fluorescence) in tumor apoptotic cells (green fluorescence).
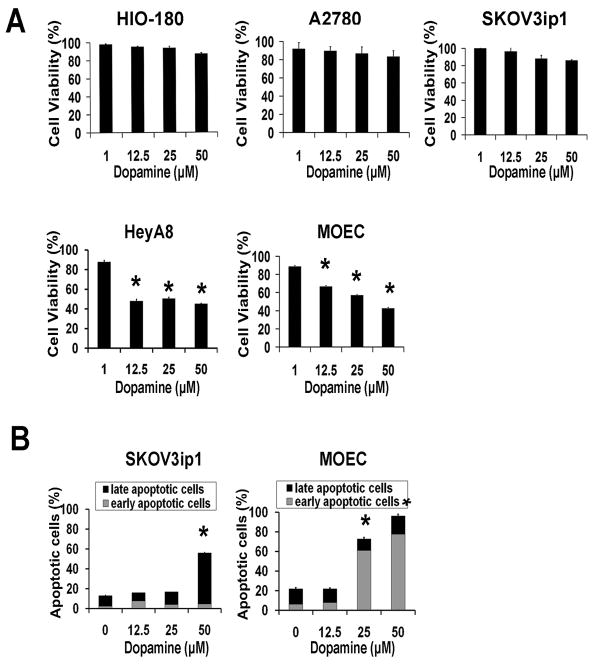
To determine whether the effects of dopamine on tumor growth are direct or indirect, we performed a series of in vitro experiments. First, effects of dopamine on in vitro viability of ovarian cancer or endothelial cells were tested. Dopamine did not significantly affect viability of the non-transformed (HIO-180) or ovarian cancer cells (A2780 and SKOV3ip1; Figure 3). However, cell viability of HeyA8 ovarian cancer cells was significantly decreased at dopamine doses from 12.5 to 50 μM. This is consistent with the in vivo inhibitory effect of dopamine on HeyA8 tumor growth. In MOEC, there was a dose-dependent decrease in cell viability with increasing doses of dopamine (p<0.01; Figure 3).
Figure 3.
Effect of dopamine on cell viability and in vitro cell apoptosis.
(A) Dopamine (12.5– 50 μM) significantly reduced cell viability of HeyA8 ovarian cancer cells (*p<0.005) and caused a dose-dependent decrease in MOEC cell viability (*p<0.01). (B) Apoptotic rates were significantly increased by DA exposure (25 and 50 μM) in MOEC; in SKOV3 ip1 cells at 50 μM DA. Values are means ± SE of three independent experiments.
We also examined the effects of dopamine on apoptosis in MOEC and SKOV3ip1 cells after treatment for 48 hours. The percentage of early apoptosis increased proportionally to dopamine concentrations used in MOEC (Figure 3), whereas late apoptotic rates did not vary. Total apoptotic rates in these cells were significantly elevated at 25 (51%) and 50 μM (75%) compared to untreated cells (p<0.01). SKOV3ip1 cells showed significantly higher percentages of late apoptotic cells at 50 μM dopamine (p<0.01).
Dopamine counteracts the stimulatory effect of VEGF and norepinephrine on tumor cell invasion
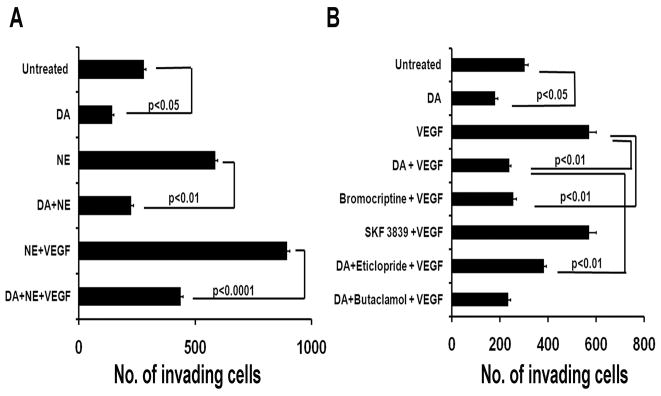
While dopamine did not affect cell viability of all ovarian cancer cells tested, we explored whether it could affect other steps in the metastatic cascade. We examined the effects of dopamine on cell invasion. We analyzed the effect of dopamine and various dopamine agonists and antagonists on the invasive potential of SKVO3ip1 cells. Dopamine 10 μM, inhibited VPF/VEGF induced cell invasion (22) by 59% (p<0.05; Fig. 4), as did the DR2 agonist bromocriptine (56%; p<0.05). However, the DR1 agonist SKF 38393 (50 μM) had no effect. Eticlopride (50 μM), a specific DR2 receptor antagonist, significantly abrogated the dopamine-mediated inhibition of cancer cell invasion (p<0.01). These results further suggest that the inhibitory action of dopamine on cell invasion was mediated specifically through DR2.
Figure 4.
Effect of dopamine and various dopamine agonists and antagonists on VPF/VEGF and NE-stimulated SKOV3ip1-cell invasion. (A) Dopamine and the DR2 agonist (bromocriptine) significantly inhibited the VPF/VEGF induced cell invasion (p<0.05); Eticlopride, a DR2 specific antagonist, abrogated the DA-mediated inhibition of VPF/VEGF induced cell invasion, whereas butaclamol, a DR1 specific antagonist did not affect cell invasion. B) DA blocked the stimulatory effect of NE alone (p<0.05), and NE+VEGF (p<0.001) on cell invasion. Values are means ± SE of three independent experiments.
We also examined whether dopamine can block the stimulatory effect of norepinephrine on SKOV3ip1-cell invasion, previously described by our group (25). Dopamine blocked the NE-mediated effect by 61% (p<0.05) in SKOV3ip1 cells exposed to NE plus dopamine. In addition, the combined treatment of dopamine/NE/VEGF led to a 52% reduction (p<0.001) in cell invasion compared to NE/VEGF-treatment, indicating the ability of DA to counteract the stimulatory effects of NE and VEGF on cell invasion (Figure 4).
Dopamine decreases cAMP levels in MOEC and SKOV3ip1 cells
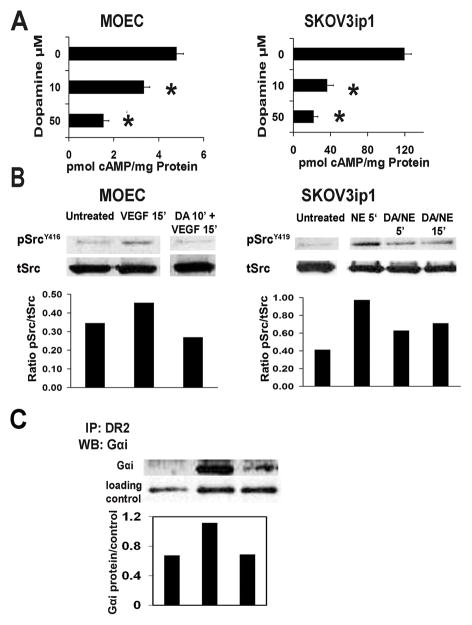
Dopamine, acting through G-protein coupled receptors, exerts stimulatory (DR1, DR5) or inhibitory (DR2-DR4) effects on adenylate cyclase leading to increased and decreased intracellular cAMP levels, respectively. We examined the effects of dopamine on cAMP levels in MOEC and SKOV3ip1 cells. Dopamine 10 μM resulted in a reduction of intracellular cAMP levels by 31% and 70% in MOEC and SKOV3ip1 cells, respectively (Figure 5A). Further decreases in cAMP levels were noted at higher doses (50 μM) of dopamine.
Figure 5.
Dopamine decreases intracellular cAMP levels and inhibits VPF/VEGF- and NE-stimulated Src-activation. (A) DA at 10 and 50 μM caused a significant decrease of intracellular cAMP levels in MOEC and SKOV3ip1 cells. Values are means ± SE of three independent experiments; *p<0.001 for MOEC; *p<0.05 for SKOV3 ip1 cells. (B) In MOEC, pre-incubation with DA 10 μM for 10 min inhibited the VPF/VEGF stimulated phosphorylation of SrcY416. In SKOV3 ip1 cells, DA reverted the stimulatory effect of NE on SrcY419 phosphorylation by combined treatment of NE+DA for 5 and 15 min. Phospho- Src densitometric data were normalized to total Src; these are representative of two independent experiments. (C) Dopamine induces association of DR2 to Gαi-protein. MOEC were exposed to 10 μM DA, for 10 and 30 min. MOEC lysates were then immunoprecipitated with DR2 antibody and analyzed by Western blotting. Gαi-protein was immunodetected using a specific anti-Gαi antibody (Abcam). DA caused a 1.6 fold higher interaction between DR2 and Gαi-protein after 10 min of exposure, compared to untreated cells. The densitometry data were normalized to a loading control (β-actin from original lysate) and are representative of two independent experiments.
Dopamine inhibits VEGF and NE induced cell signaling
The effect of dopamine on phosphorylation of several key effectors of the VEGF-angiogenic pathway was analyzed in MOEC and SKOV3ip1. In MOEC, VEGF (10 ng/ml) for 15 min resulted in increased phosphorylation of SrcY416 (Figure 5B). This effect was blocked by a 10 min- pre-incubation of cells with DA 10 μM. Similarly, SKOV3ip1 cells stimulated with NE (10 μM) for 5 min revealed a significant increase (2.4-fold) of pSrcY419 (Figure 5B), which was blocked by combined treatment of DA + NE for 5 and 15 min.
Our in vitro data confirmed that dopamine decreases intracellular cAMP levels in MOEC as well as in SKOV3ip1 cells. This strongly suggested that dopamine could be acting through the DR2-cAMP-signaling pathway to exert its inhibitory effects in vivo on tumor growth. To address this question, we examined the association of DR2 to Gαi1 protein in MOEC exposed to dopamine (10 μM) for 10 and 30 min. There was a 1.6-fold increase in the interaction between DR2 and Gαi1 protein after 10 min of dopamine-exposure, compared to untreated cells (Figure 5C).
Discussion
The main findings of this study are that dopamine significantly reduces stress-mediated cancer growth in ovarian carcinoma. Our data strongly suggest that dopamine retards tumor growth by inhibiting tumor angiogenesis and stimulating tumor cell apoptosis. In addition, we provide the first evidence that dopamine is able to block the stimulatory effects of chronic stress on cancer growth.
The physiological actions of dopamine are mediated by at least 5 distinct G-protein-coupled receptor subtypes. (26, 27). Two DR1-like receptor subtypes (DR1 and DR5) couple to the G protein Gs, activate adenylate cyclase and increase cAMP levels. The other receptor subtypes belong to the DR2-like subfamily (DR2, DR3 and DR4) and are prototypic of G protein-coupled receptors that inhibit adenylate cyclase and decrease cAMP production. The ovarian non-transformed, cancer and endothelial cells tested in this work showed expression of DR1 and DR2-like dopamine receptors, indicating that dopamine might regulate stimulatory and/or inhibitory processes in these cells.
Dysfunction of dopaminergic system is known to be associated with various disorders, including schizophrenia and Parkinson’s disease (28). The consequences of dopamine dysfunction indicate the importance of maintaining dopamine functionality through homeostatic mechanisms based on the delicate balance between synthesis, storage, release, metabolism and reuptake (28). A decrease in dopamine in the brain has been implicated as the cause of Parkinson’s disease. In contrast, it is argued that a functional excess of DA or oversensitivity of certain DR’s is one of the causal factors in schizophrenia (29). Interestingly, the incidence of cancer in patients with schizophrenia has been reported to be lower than in the general population (30–32). Whether this reduced incidence is related to hyperactive dopaminergic system is not known.
It has been demonstrated that dopamine concentrations are lower in the tumor microenvironment compared to normal tissues (11, 33). These findings prompted us to consider whether the increases in tumor growth and angiogenesis may result from a permissive microenvironment created by a relative shift toward growth-promoting catecholamines. In the present study, we demonstrate that dopamine blocks stress induced tumor growth by activating DR2. The central role of DR2 was confirmed with the DR2-antagonist eticlopride in combination therapy with dopamine, as well as with siRNA targeted against murine or human DR2. The dopamine-suppressing effect on tumor growth in our stress models was significantly blunted by DR2 gene silencing. These findings indicate that dopamine targets both host murine endothelial cells and human cancer cells through DR2 to exert its growth suppressive effects. Our results are supported by the reported growth inhibitory effects of dopamine under non-stress conditions (7, 9, 34).
Our data also indicate that dopamine, via DR2, blocked the VPF/VEGF or norepinephrine mediated invasion of ovarian cancer cells. The inhibitory effects of dopamine on cell invasion would explain, in part, the in vivo blocking effect of dopamine on tumor progression and metastasis under chronic stress. Our in vitro studies demonstrate that dopamine decreases viability not only of endothelial cells, as previously described, (7, 12) but also of ovarian cancer cells. These results were further confirmed using in vivo models of ovarian carcinoma. The dual cell targeting (endothelial and tumor cells) of dopamine in the tumor microenvironment underlines the significance of dopamine as a potential modality to block the growth stimulatory effects of chronic stress.
Dopamine and DR2-agonists have been used for many years for the treatment of Parkinson’s disease and hyperprolactinemia (35, 36) as well as for management of cardiovascular disorders and renal dysfunction.(37, 38). Dopamine agonists such as bromocriptine mesylate (Parlodel, oral) have also been used clinically to treat hyperprolactinemia and are well tolerated (39). Furthermore, effective shrinkage of prolactinomas has been observed after injection of long-acting form of Parlodel (40). Such agents may represent a new strategy for blocking the effects of chronic stress on tumor growth. With such therapies, however, some adverse reactions such as nausea, hallucinations, and orthostatic hypotension have been reported and would require careful monitoring.
To identify the signaling pathways by which dopamine affects endothelial and ovarian cancer cell function, we examined the effects of dopamine on phophorylation and activation of various effectors in the metastatic cascade. In MOEC, dopamine inhibited VEGF-induced phophorylation and activaton of Src kinase. In addition, dopamine reversed NE-stimulated Src-phosphorylation. Src is a key mediator for multiple signaling pathways that regulate critical cellular functions (41). In ovarian cancer, Src has been shown to play a functional role in cancer cell invasion and angiogenesis (42). Src is also a regulator of VEGF-mediated vascular permeability (43). Recent studies in HUVEC have shown that dopamine reduces VEGF-mediated permeability by inhibiting VEGF-induced Src activation (44). These studies coupled with our data implicate blockade of Src activation as a key mediator of the inhibitory effects of dopamine on ovarian cancer growth.
Overall, our data suggest that dopamine inhibits stress hormone stimulated Src activation in endothelial and ovarian cancer cells. Moreover, we conclude that dopamine-replacement effectively counteracts the stimulatory effects of norepinephrine on ovarian cancer growth during chronic stress. Considering the stimulatory effects of chronic stress on cancer growth (6), our findings implicate dopamine as a potential therapeutic strategy for blocking the deleterious effects of chronic stress.
Supplementary Material
Acknowledgments
Portions of this work were supported by the NIH (CA110793, CA109298, P50 CA 083639, CA128797, RC2GM092599; U54 CA151668), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the DOD (OC093146), the Zarrow Foundation, the Marcus Foundation, and the Betty Anne Asche Murray Distinguished Professorship. RLS is supported by NCI-DHHS-NUH T32 Training Grant (T32 CA101642). MMKS is supported by the NIH/NICHD WRHR Grant (HD050128) and the GCF-Molly Cade Ovarian Cancer Research Grant.
References
- 1.Weiner H. Perturbing the Organism: The biology of stressful experience. Chicago: University of Chicago, Press; 1992. [Google Scholar]
- 2.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 3.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt C, Kraft K. Beta-endorphin and catecholamine concentrations during chronic and acute stress in intensive care patients. Eur J Med Res. 1996;1:528–32. [PubMed] [Google Scholar]
- 5.Lutgendorf SK. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain, behavior and Immunity. 2009;23:176–83. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Nagy JA, Pal S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–74. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 8.Teunis MA, Kavelaars A, Voest E, et al. Reduced tumor growth, experimental metastasis formation, and angiogenesis in rats with a hyperreactive dopaminergic system. Faseb J. 2002;16:1465–7. doi: 10.1096/fj.02-0145fje. [DOI] [PubMed] [Google Scholar]
- 9.Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69:3727–30. doi: 10.1158/0008-5472.CAN-08-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu S, Sarkar C, Chakroborty D, et al. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Research. 2004;64:5551–5. doi: 10.1158/0008-5472.CAN-04-1600. [DOI] [PubMed] [Google Scholar]
- 11.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–56. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–10. doi: 10.1158/1078-0432.CCR-07-1778. [DOI] [PubMed] [Google Scholar]
- 13.Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–9. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res. 1991;554:217–22. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- 15.Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194–9. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- 16.Buick RN, Pullano R, Trent JM. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985;45:3668–76. [PubMed] [Google Scholar]
- 17.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 18.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Research. 2003;63:2971–6. [PubMed] [Google Scholar]
- 19.Zarei S, Frieden M, Rubi B, et al. Dopamine modulates von Willebrand factor secretion in endothelial cells via D2–D4 receptors. Journal of Thromobosis and Haemostasis. 2006;4:1588–95. doi: 10.1111/j.1538-7836.2006.01998.x. [DOI] [PubMed] [Google Scholar]
- 20.Han HD, Mangala LS, Lee JW, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res. 16:3910–22. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landen CN, Jr, Lu C, Han LY, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–70. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 22.Spannuth WA, Nick AM, Jennings NB, et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer. 2009;124:1045–53. doi: 10.1002/ijc.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landen CN, Merritt WM, Mangala LS, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5:1708–13. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 24.Thaker PH, Lloyd M, Jennings N, et al. Chronic Stress Promotes Angiogenesis in Ovarian Carcinoma. The 36th Annual Meeting of the Society of Gynecologic Oncologists; 2005; Miami, FL. 2005. [Google Scholar]
- 25.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 27.Civelli O. Molecular Biology of the Dopamine Receptor Subtypes. Lippincott Williams & Wilkins; 1995. [Google Scholar]
- 28.Best J, Nijhout HN, Reed MC. Homoestatic mechanisms in dopamine synthesis and release: a mathematical model. Theoretical Biology and Medical Modelling. 2009;6:21. doi: 10.1186/1742-4682-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birtwistle J, Baldwin D. Role of dopamine in schizophrenia and Parkinson’s disease. Br J Nurs. 1998;7:832–4. doi: 10.12968/bjon.1998.7.14.5636. [DOI] [PubMed] [Google Scholar]
- 30.Mortensen PB. The incidence of cancer in schizophrenic patients. J Epidemiol Community Health. 1989;43:43–7. doi: 10.1136/jech.43.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldacre M, Kurina L, Wotton C, Yeates D, Seagroatt V. Schizophrenia and cancer: an epidemiological study. British Journal of Psychiatry. 2005;187:334–8. doi: 10.1192/bjp.187.4.334. [DOI] [PubMed] [Google Scholar]
- 32.Asada M, Ebihara S, Numachi Y, et al. Reduced tumor growth in a mouse model of schizophrenia lacking the dopamine transporter. Int J Cancer. 2008;123:511–8. doi: 10.1002/ijc.23562. [DOI] [PubMed] [Google Scholar]
- 33.Basu S, Dasgupta PS. Role of dopamine in malignant tumor growth. Endocrine. 2000;12:237–41. doi: 10.1385/ENDO:12:3:237. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1554–60. doi: 10.1152/ajpheart.00272.2004. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Lloret S, Bondon-Guitton E, Rascol O, Montastruc JL. Adverse drug reactions to dopamine agonists: a comparative study in the French Pharmacovigilance Database. Mov Disord. 2010;25:1876–80. doi: 10.1002/mds.23204. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Lloret S, Rascol O. Dopamine receptor agonists for the treatment of early or advanced Parkinson’s disease. CNS Drugs. 2010;24:941–68. doi: 10.2165/11537810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Kerr JL, Timpe EM, Petkewicz KA. Bromocriptine mesylate for glycemic management in type 2 diabetes mellitus. Ann Pharmacother. 2010;44:1777–85. doi: 10.1345/aph.1P271. [DOI] [PubMed] [Google Scholar]
- 38.McGrath BP. Dopamine: clinical applications iii. cardiovascular Australian Prescriber. 1994;17:44–5. [Google Scholar]
- 39.Bankowski BJ, Zacur HA. Dopamine agonist therapy for hyperprolactinemia. Clin Obstet Gynecol. 2003;46:349–62. doi: 10.1097/00003081-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Schettini G, Lombardi G, Merola B, et al. Rapid and long-lasting suppression of prolactin secretion and shrinkage of prolactinomas after injection of long-acting repeatable form of bromocriptine (Parlodel LAR) Clin Endocrinol (Oxf) 1990;33:161–9. doi: 10.1111/j.1365-2265.1990.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 41.Han LY, Landen CN, Trevino JG, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66:8633–9. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyd DD, Wang H, Avila H, et al. Combination of an SRC kinase inhibitor with a novel pharmacological antagonist of the urokinase receptor diminishes in vitro colon cancer invasiveness. Clin Cancer Res. 2004;10:1545–55. doi: 10.1158/1078-0432.ccr-1565-02. [DOI] [PubMed] [Google Scholar]
- 43.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharya R, Sinha S, Yang SP, et al. The neurotransmitter dopamine modulates vascular permeability in the endothelium. Journal of Molecular Signaling. 2008;3:2187. doi: 10.1186/1750-2187-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.