Abstract
A mood stabilizing and antidepressant response to lithium is only found in a subgroup of bipolar disorder and depression patients. Identifying strains of mice that are responsive and non-responsive to lithium may elucidate genomic and other biological factors that play a role in lithium responsiveness. Mouse strains were tested in the forced swim, tail suspension, and open field tests after acute and chronic systemic, and intracerebroventricular and chronic lithium treatments. Serum and brain lithium levels were measured. Three (129S6/SvEvTac, C3H/HeNHsd, C57BL/6J) of the eight inbred strains tested, and one (CD-1) of the three outbred strains, showed an antidepressant-like response in the forced swim test following acute systemic administration of lithium. The three responsive inbred strains, as well as the DBA/2J strain, were also responsive in the forced swim test after chronic administration of lithium. However, in the tail suspension test, acute lithium resulted in an antidepressant-like effect only in C3H/HeNHsd mice. Only C57BL/6J and DBA/2J were responsive in the tail suspension test after chronic administration of lithium. Intracerebroventricular lithium administration resulted in a similar response profile in BALB/cJ (non-responsive) and C57BL/6J (responsive) strains. Serum and brain lithium concentrations demonstrated that behavioral results were not due to differential pharmacokinetics of lithium in individual strains, suggesting that genetic factors likely regulate responsiveness to lithium. Our results indicate that responsiveness to lithium in tests of antidepressant efficacy varies among genetically diverse mouse strains. These results will assist in identifying genomic factors associated with lithium responsiveness and the mechanisms of lithium action.
Keywords: mouse strains, lithium, mood disorder, mood stabilizer, antidepressant, pharmacogenetics
INTRODUCTION
Mood disorders, including major depression and bipolar disorder, are serious human health problems and affect a significant portion of the population (15% in the case of major depression and 1–3% in the case of bipolar disorder) (Fava & Kendler, 2000, Kessler et al., 2003, Kessler et al., 2005, Sullivan et al., 2000). Lithium has efficacy both as a mood stabilizer and an antidepressant (Geddes et al., 2004). Extensive clinical evidence indicates that lithium has robust effects when used as an adjunct antidepressant (Bauer et al., 2010), and in addition clinical studies show that lithium monotherapy has efficacy in treatment of depression (Smoller & Finn, 2003, Watanabe et al., 1975). Evidence suggests that lithium-responsiveness might be a marker by which distinct subtypes of bipolar disorder can be stratified (Alda et al., 2005, Smeraldi et al., 1984, Smoller & Finn, 2003). For example, lithium-responsive bipolar disorder patients are more likely than non-responders to have relatives with bipolar disorder (Mendlewicz et al., 1973), and those relatives in turn are more likely to be responsive to the therapeutic effects of lithium (Grof et al., 2002). However, the neurobiological and genetic mechanisms that underlie lithium-responsiveness are not entirely known.
Elucidating the genetic underpinnings of lithium responsiveness in validated animal tests can assist in identifying the mechanisms of action of lithium and genetics of the lithium response in humans. One way of achieving this goal is to identify particular genetic patterns of responsiveness in closely related but genetically diverse animal lines, such as different strains of mice (Jacobson & Cryan, 2007). Identifying differences between standard mouse strains has been a widely used approach to investigate responses to various antidepressants (Bai et al., 2001, Cervo et al., 2005, Crowley et al., 2005, David et al., 2003, Dubocovich et al., 1990, Dulawa et al., 2004, Liu & Gershenfeld, 2001, Lucki et al., 2001, Nomura et al., 1991, Porsolt et al., 1978, Ripoll et al., 2003, Van Der Heyden et al., 1987), as well as the antimanic-like effects of lithium (Gould et al., 2007b, Gould et al., 2001, Hamburger-Bar et al., 1986) and effects of lithium on pre-pulse inhibition (O’neill et al., 2003, Ong et al., 2005). However, no study to date has compared the antidepressant-like effects of lithium in different strains of mice systematically and comprehensively. We examined responsiveness to lithium’s antidepressant-like effects in various mouse strains using multiple treatment paradigms.
MATERIALS and METHODS
Subjects
Male mice, 7–8 weeks old, were obtained from commercial breeding colonies. A/J, BALB/cJ, C57BL/6J, CBA/J, DBA/2J, FVB/NJ, (The Jackson Laboratories, Bar Harbor, Maine), 129S6/SvEvTac, and Black Swiss (Taconic, Germantown, NY), C3H/HeNHsd and CD-1 (Charles River, Wilmington, MA), and NIH Swiss (Harlan, Indianapolis, Indiana) were transported to our laboratory, and allowed to acclimatize for at least one week. Mice were housed as four individuals per cage in an animal room with a constant temperature (22±1°C) and a 12-h light/dark cycle (lights on/off at 07.00/19.00), with free access to food and water. Experiments were performed in the light phase of the cycle. All experimental procedures were approved by the University of Maryland, Baltimore Animal Care and Use Committee, and were conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Lithium Administration
For the systemic acute lithium administration experiment, lithium chloride (Sigma, Saint Louis, MO) solutions were made by dissolving LiCl in 0.9% saline. Mice were given i.p. injections of either saline, 200 mg/kg, 300 mg/kg, or 400 mg/kg LiCl in an injection volume of 4 ml/kg, five hours prior to the commencement of behavioral tests. For the systemic chronic lithium administration experiments, two to five days after the arrival of the mice, regular food was removed from all cages and lithium chow containing 4 g/kg LiCl (Bioserv, Frenchtown, NJ) was provided in half of the cages, while the other half were given control chow. In both groups mice received a water bottle, and a 0.9% saline bottle to reduce ion imbalances that are caused by chronic lithium.
At least one week prior to the intracerebroventricular (ICV) lithium administration experiments, cannulas were stereotaxically implanted in the right lateral ventricle. Stereotaxic coordinates were calculated from the bregma and they were −0.60 mm AP, and +1.00 mm ML. Three μl of artificial cerebrospinal fluid (aCSF) containing 600 mmol/L LiCl (0.076 mg of LiCl per subject) or vehicle was administered through a cannula at a rate of 1 μl/min with a Hamilton syringe under isoflurane anesthesia. Thirty minutes after the infusion, subjects were assessed in behavioral tests. All subjects were later infused ICV with 3 μl tryphan blue dye to confirm the site of cannulation. Those subjects with no dye diffusion into either left lateral or third ventricles were excluded from this study.
Lithium Concentration Assays
Subjects were anesthetized under isoflurane. Blood was collected through a cardiac puncture, and the brain was removed and frozen at −80 C. Entire brains were homogenized with a polytron homogenizer (Kinematica AG, Model PT-MR 2100, Littau, Switzerland) in 3 volumes of 0.5 N trichloroacetic acid, followed by centrifugation (Gould et al., 2007a, Gould et al., 2008, Hamburger-Bar et al., 1986). Lithium levels of both serum (mmol/L) and brain (mmol/kg, wet weight) samples were measured with a flame photometer (Cole-Palmer Model 2655-00, Chicago, IL).
Behavioral Tests
Forced Swim Test (FST)
Subjects were placed in Plexiglas cylinders (30 cm height x 20 cm diameters) filled with 15 cm of tap water at a temperature of 24 ± 1°C for six minutes and videotaped. The last four minutes of each session were scored by a trained observer who was blind to the group assignments. Mobility was defined as any movements other than those necessary to maintain the head above the water.
Tail Suspension Test (TST)
Each mouse was suspended for six minutes by a 15 cm length of tape attached to the end of its tail. The entire six minutes of each session was videotaped and then scored for mobility time by a trained observer who was blind to the group assignments. Mobility was defined as movements of the hind legs, and/or other escape oriented behaviors. A modification was made for the C57BL/6J strain, which is known for its behavior of climbing tails during the TST (Cryan et al., 2005, Mayorga & Lucki, 2001). Plexiglas cylinders (1.5 cm diameter, 4 cm length, 3.5 g weight) were placed around the tails, where they rest on the tail base during the procedure. This modification completely eliminates tail climbing in the C57BL/6J strain (Dao et al., 2010).
Open-Field Test (OFT)
Locomotion of animals was assessed in 50 cm × 50 cm × 38 cm arenas under 30–35 lux illumination. The test was 30 minutes in duration. Each session was videotaped. The distance moved was calculated with Top Scan software from CleverSys Inc (Reston, VA).
Statistics
All statistical analyses were performed with GraphPad v.5.02 or SPSS 17 software. One-way ANOVAs was followed by Dunnett’s post hoc test, in which the saline group was compared pairwise with all other groups. In cases in which there were only two groups, unpaired t-tests were used. Pearson correlation coefficients were calculated between mean brain lithium levels and mean percent change in immobility in the FST of the Experiment 2. Since each strain was tested on a different day, no interstrain statistical comparisons were made. Outlier analyses of lithium concentrations were performed with the Grubbs’ test, and any outliers detected were excluded from the experiment. Linear regression analyses (SPSS 17) were conducted to assess if lithium levels in serum and brain change differentially in response to systemic acute lithium treatment between strains that underwent the FST. Comparisons of the linear regression slopes were assessed by Bonferroni post hoc tests. Data are reported as mean±SEM. All analyses were two-tailed, and statistical significance level was set as p<0.05.
RESULTS
Experiment 1: Time Course of Serum and Brain Lithium Concentrations
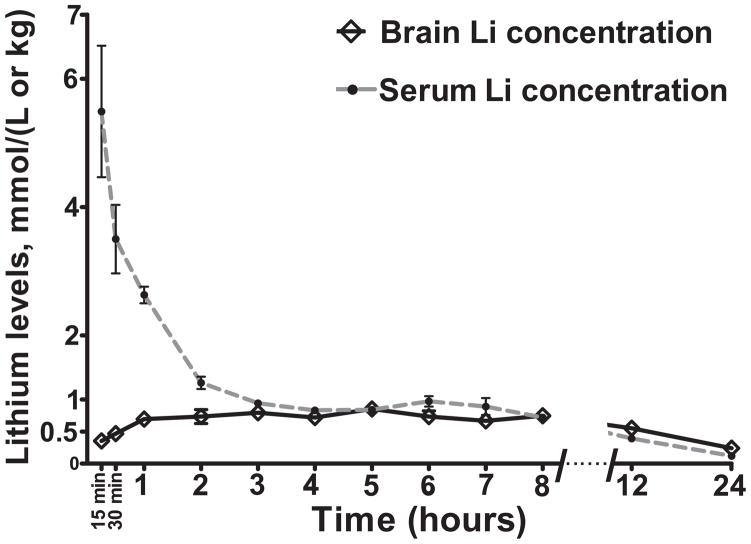
We first conducted a time course experiment to determine lithium concentrations in serum and brain at a different time periods following i.p. injection of 200 mg/kg LiCl in the C57BL/6J strain. The aim was to find an optimal time point when the brain levels of lithium were high and within the human therapeutic range (0.6–1.3 mM) but serum levels were relatively low, thus limiting peripheral effects of lithium. We found that in the C57BL/6J strain, brain lithium concentrations were at their highest level (0.85± 0.04 mmol/kg) and within the human therapeutic range five hours after injection, whereas serum levels were relatively low at ~15% of their initial levels, thus decreasing the likelihood that behavior outcomes would be due to peripheral side effects of high lithium concentrations (Figure 1).
Figure 1. Time course of changes in lithium concentrations in serum and brain after a single i.p. injection of 200mg/kg lithium in C57BL/6J mice.
Data are expressed as mean ± SEM. n:6 animals per time point.
Experiment 2: Systemic Acute Administration of Lithium
Forced Swim Test
Eleven strains were tested for responsiveness to lithium in the FST. The means and SE of serum and brain lithium concentration levels are listed in Table 1. One subject from C57BL/6J 300 mg/kg group and one from DBA/2J 400 mg/kg group were excluded from the experiment, since their brain lithium levels were outliers (z=2.36, p<0.05; z=2.29, p<0.05, respectively). Lithium levels were generally either in or moderately above the human therapeutic range (0.6 to 1.3 mM) in 200 mg/kg and 300 mg/kg treatment groups. Consistent with a previous report, we found that lithium clearance rates were rapid in the C57BL/6J strain compared to other inbred strains (El-Kassem & Singh, 1983). The relationship between the dose of LiCl administered and resulting serum and brain lithium levels was significant in all strains (p<0.001). For serum lithium levels we found statistically significant (p<0.0001) R2 values for all strains (C57BL/6J R2=0.477, NIH Swiss R2=0.447, all the other strains R2=0.820–0.903). For brain lithium levels, we found statistically significant (p<0.001) R2values for all strains (C57BL/6J R2=0.355, NIH Swiss R2=0.305, all the other strains R2=0.821–0.974). One-way ANOVA indicated that the slopes corresponding to LiCl dose and serum [F(10,248)=6.88, p<0.0001] and brain [F(10,248)=6.11, p<0.0001] lithium levels were significantly different between strains. Bonferroni post-hoc tests indicated that the change in serum levels in relation to the dose of LiCl was significantly different between C57BL/6J and 129S6/SvEvTac, BALB/cJ, Black Swiss, C3H/HeNHsd; FVB/NJ and 129S6/SvEvTac, BALB/cJ, Black Swiss; NIH Swiss and 129S6/SvEvTac, BALB/cJ; 129S6/SvEvTac and A/J, CD-1 strains. The change in brain levels in relation to dose of LiCl was significantly different between C57BL/6J and A/J, BALB/cJ, Black Swiss, C3H/HeNHsd, CBA/J, DBA/2J; NIH Swiss and A/J, BALB/cJ, Black Swiss, C3H/HeNHsd, CBA/J, DBA/2J; Black Swiss and FVB/NJ.
Table 1. Serum and brain lithium levels of subjects immediately after the forced swim test in Experiment 2.
Strains are indicated in the left hand column. Other column headers refer to lithium concentration (200, 300, or 400 mg/kg) of the injection five hours prior to the test. n:6–8 animals per group in each strain.
| Strain | Serum Lithium Levels mmol/L ±S.E.M. | Brain Lithium Levels mmol/kg wet weight ±S.E.M. | ||||
|---|---|---|---|---|---|---|
| 200 mg/kg | 300 mg/kg | 400 mg/kg | 200 mg/kg | 300 mg/kg | 400 mg/kg | |
| 129S6/SvEvTac | 0.88±0.05 | 1.57±0.07 | 2.52±0.10 | 0.82±0.05 | 1.36±0.05 | 2.11±0.07 |
| A/J | 0.96±0.03 | 1.49±0.03 | 1.93±0.09 | 1.04±0.02 | 1.84±0.05 | 2.49±0.07 |
| BALB/cJ | 0.84±0.08 | 1.62±0.11 | 2.39±0.11 | 0.91±0.10 | 1.69±0.09 | 2.37±0.12 |
| Black Swiss | 0.70±0.03 | 1.5±0.09 | 2.16±0.09 | 0.90±0.03 | 1.84±0.09 | 2.70±0.15 |
| C3H/HeNHsd | 0.77±0.03 | 1.39±0.08 | 2.14±0.08 | 1.01±0.02 | 1.88±0.10 | 2.56±0.11 |
| C57BL/6J | 0.64±0.04 | 1.02±0.05 | 1.32±0.18 | 0.84±0.03 | 1.27±0.04 | 1.55±0.24 |
| CBA/J | 0.62±0.02 | 1.19±0.07 | 2.08±0.12 | 0.69±0.03 | 1.38±0.05 | 2.14±0.03 |
| CD-1 | 0.53±0.03 | 0.97±0.11 | 1.46±0.07 | 0.62±0.03 | 1.27±0.13 | 1.79±0.08 |
| DBA/2J | 0.65±0.03 | 1.23±0.04 | 1.92±0.18 | 0.86±0.02 | 1.55±0.06 | 2.30±0.14 |
| FVB/NJ | 0.43±0.02 | 0.94±0.05 | 1.17±0.08 | 0.58±0.02 | 1.09±0.03 | 1.47±0.09 |
| NIH Swiss | 0.60±0.08 | 1.01±0.08 | 1.80±0.22 | 0.60±0.11 | 1.29±0.14 | 1.37±0.22 |
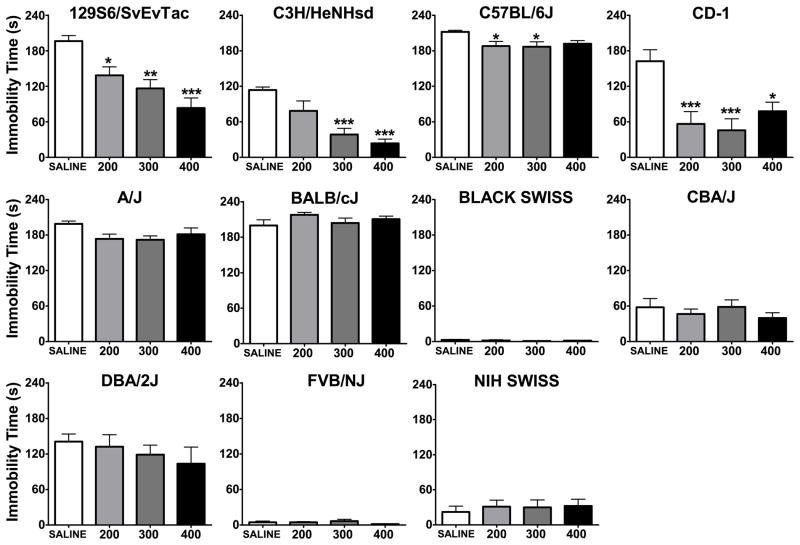
Of the three outbred strains, Black Swiss and NIH Swiss showed very low baseline levels of immobility (2.6 and 21.9 seconds respectively) and no significant differences were found between groups. However, in the case of the CD-1 strain, one-way ANOVA revealed that there was a significant effect of LiCl treatment and Dunnets’ post hoc test confirmed that 200, 300, and 400 mg/kg groups were significantly less immobile than the saline group (Figure 2; Table 2).
Figure 2. Immobility time in the forced swim test five hours after a single injection of saline, 200, 300, or 400 mg/kg lithium.
*:p<0.05, **:p<0.01, ***:p<0.001 denote a significant difference compared to saline group, Dunnett’s post hoc test. Data are expressed as mean ± SEM. n:6–8 animals per group for each strain.
Table 2.
Statistical test results of the Experiment 2 forced swim test (Figure 2).
| Forced Swim Test | Dunnett’s post hoc test comparison to the saline group | ||||
|---|---|---|---|---|---|
| Strain | df | One-way ANOVA | 200 mg/kg | 300 mg/kg | 400 mg/kg |
| 129S6/SvEvTac | (3,31) | F=11.28, p<0.001 | p<0.05 | p<0.01 | p<0.001 |
| A/J | (3,31) | F=2.54, p=0.077 | NA | NA | NA |
| BALB/cJ | (3,31) | F=1.17, p=0.34 | NA | NA | NA |
| Black Swiss | (3,31) | F=0.74, p=0.54 | NA | NA | NA |
| C3H/HeNHsd | (3,31) | F=14.55, p<0.001 | NS | p<0.001 | p<0.001 |
| C57BL/6J | (3,30) | F=3.40, p<0.05 | p<0.05 | p<0.05 | NS |
| CBA/J | (3,31) | F=0.68, p=0.57 | NA | NA | NA |
| CD-1 | (3,23) | F=7.90, p<0.01 | p<0.01 | p<0.001 | p<0.05 |
| DBA/2J | (3,30) | F=0.66, p=0.58 | NA | NA | NA |
| FVB/NJ | (3,23) | F=1.20, p=0.33 | NA | NA | NA |
| NIH Swiss | (3,30) | F=0.17, p=0.92 | NA | NA | NA |
NA: not applicable, NS: not significant.
Out of eight inbred strains studied, 129S6/SvEvTac, C3H/HeNHsd, and C57BL/6J were found to be responsive to lithium at least in one dose level in the FST (Figure 2). The A/J strain showed a trend toward a significant effect of treatment (p=0.077). 129S6/SvEvTac mice were significantly less immobile in all lithium treatment groups. C3H/HeNHsd mice showed lower immobility in 300 mg/kg and 400 mg/kg LiCl groups, and C57BL/6J were less immobile in 200 mg/kg and 300 mg/kg LiCl groups compared to the baseline (Table 2). One inbred strain, FVB/NJ, had very low baseline levels of immobility (4.33 seconds on the average).
We observed differential patterns of lithium responsiveness. For example while the 129S6/SvEvTac strain showed a clear increase in response with dose, the C57BL/6J strain did not. In order to determine whether an antidepressant-like response to lithium in the FST is due to differences in pharmacokinetics of lithium in individual strains, correlation analyses between mean brain lithium levels and mean percent change in immobility compared to the baseline levels of each strain were made at each dose level. Black Swiss, FVB/NJ, and NIH Swiss strains were not included in these analyses, since these strains had very low baseline immobility levels in the FST. At the 200 mg/kg dose, there was no statistically significant correlation between average brain lithium concentration levels and percent change in immobility in the FST (r=0.54, p=0.17). The same was true for the 300 mg/kg (r=0.054, p=0.88), and the 400 mg/kg (r=−0.108, p=0.79) doses.
Tail Suspension Test
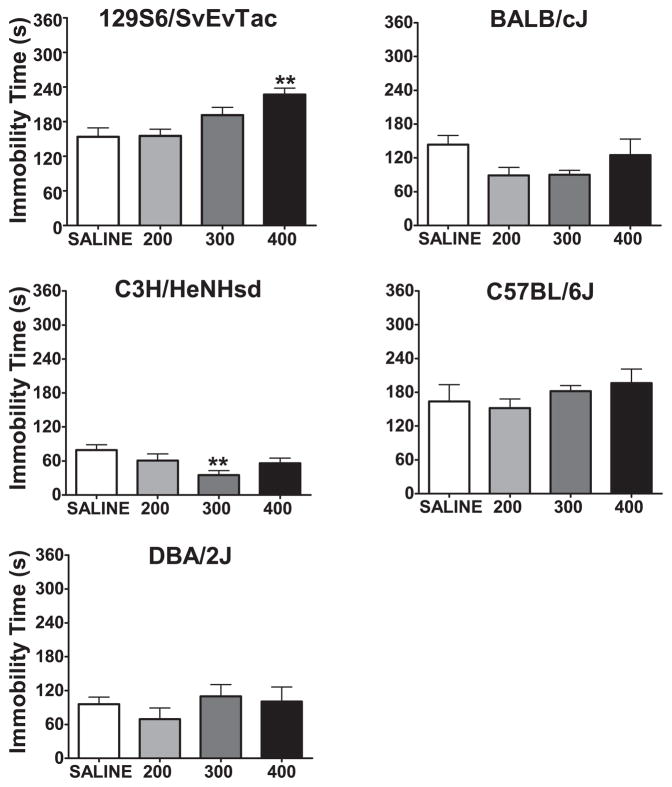
A subgroup of inbred strains was selected on the basis of their responsiveness to lithium in the FST and tested in the TST. Serum and brain samples showed similar levels of lithium concentrations to what we found in the FST (Table 3). Among the five inbred strains tested; 129S6/SvEvTac, BALB/cJ, C3H/HeNHsd, C57BL/6J, and DBA/2J, only two manifested a response to lithium (Figure 3). 129S6/SvEvTac mice were significantly more immobile after treatment with 400 mg/kg than with vehicle, and therefore did not show an antidepressant-like effect. C3H/HeNHsd mice, at the 300 mg/kg LiCl level, had significantly lower levels of immobility. The other strains, BALB/cJ, C57BL/6J, and DBA/2J did not significantly change their immobility behavior in response to lithium treatment in the TST (Table 4).
Table 3. Serum and brain lithium levels of subjects immediately after the tail suspension test in Experiment 2.
Strains are indicated in the left hand column. Other column headers refer to lithium concentration (200, 300, or 400 mg/kg) of the injection that was five hours prior to the test. n:7–9 animals per group in each strain.
| Strain | Serum Lithium Levels mmol/L ±S.E.M. | Brain Lithium Levels mmol/kg wet weight ±S.E.M. | ||||
|---|---|---|---|---|---|---|
| 200 mg/kg | 300 mg/kg | 400 mg/kg | 200 mg/kg | 300 mg/kg | 400 mg/kg | |
| 129S6/SvEvTac | 0.78±0.07 | 1.31±0.07 | 2.37±0.11 | 0.82±0.04 | 1.39±0.07 | 2.20±0.07 |
| BALB/cJ | 0.63±0.04 | 1.35±0.10 | 2.26±0.24 | 0.90±0.07 | 1.69±0.11 | 2.45±0.17 |
| C3H/HeNHsd | 0.86±0.06 | 1.27±0.05 | 2.17±0.04 | 1.07±0.04 | 1.60±0.03 | 2.68±0.09 |
| C57BL/6J | 0.58±0.06 | 1.00±0.13 | 1.97±0.10 | 0.66±0.09 | 1.12±0.14 | 1.88±0.09 |
| DBA/2J | 1.05±0.03 | 2.17±0.14 | 3.29±0.22 | 1.14±0.05 | 2.14±0.07 | 3.07±0.10 |
Figure 3. Immobility time in the tail suspension test five hours after a single injection of saline, 200, 300, or 400 mg/kg lithium.
**:p<0.01 denotes a significant difference compared to saline group, Dunnett’s post hoc test. Data are expressed as mean ± SEM. n:7–9 animals per group for each strain.
Table 4.
Statistical test results of the Experiment 2 tail suspension test (Figure 3).
| Tail Suspension Test | Dunnett’s post hoc test comparison to the saline group | ||||
|---|---|---|---|---|---|
| Strain | df | One-way ANOVA | 200 mg/kg | 300 mg/kg | 400 mg/kg |
| 129S6/SvEvTac | (3,27) | F=7.18, p<0.01 | NS | NS | p<0.01 |
| BALB/cJ | (3,31) | F=2.19, p=0.11 | NA | NA | NA |
| C3H/HeNHsd | (3,31) | F=3.68, p<0.05 | NS | p<0.01 | NS |
| C57BL/6J | (3,21) | F=0.95, p=0.44 | NA | NA | NA |
| DBA/2J | (3,31) | F=0.72, p=0.55 | NA | NA | NA |
NA: not applicable, NS: not significant.
Open Field Test
In order to verify that the antidepressant-like results that we found in the earlier antidepressant efficacy tests were not due to non-specific increases in general activity, we tested a subset of lithium responsive and non-responsive inbred strains, 129S6/SvEvTac, BALB/cJ, and C57BL/6J, in the OFT (Table 5). In all strains tested, distance traveled was significantly reduced in the 400 mg/kg LiCl treatment group compared to the saline group (for 129S6/SvEvTac [F(3,31)=3.9, p<0.05, Dunnett’s post hoc test p<0.01], for BALB/cJ [F(3,30)=3.59, p<0.05, Dunnett’s post hoc test p<0.05], and for C57BL/6J [F(3,21)=11.35, p<0.001, Dunnett’s post hoc test p<0.001]. At the other doses (200 and 300 mg/kg) total distance travelled tended to be less, though there were no statistically significant differences.
Table 5. Total average distance moved in the Experiment 2 open field test.
Data are in meters and expressed as mean ± SEM, n:6–8 animals per group in each strain.
| Strain | Total Distance Moved (m) | |||
|---|---|---|---|---|
| Saline | 200 mg/kg | 300 mg/kg | 400 mg/kg | |
| 129S6/SvEvTac | 34.3±4 | 27.2±5.1 | 21.0±5.6 | 12.7±3.6 |
| BALB/cJ | 87.5±9.5 | 80.2±11.9 | 69.3±9 | 41.0±11.4 |
| C57BL/6J | 71.2±5.8 | 83.7±7 | 62.7±14.2 | 16.8±4 |
Experiment 3: Systemic Chronic Lithium Administration
Mice from 129S6/SvEvTac, BALB/cJ, C3H/HeNHsd, C57BL/6J, and DBA/2J strains were tested for the effects of chronic lithium administered in their food. These strains were selected as representative responsive and non-responsive strains from the acute lithium administration studies. After chronic lithium treatment, all strains weighed less than control chow treated mice. This loss was similar across strains and was ~15–20 %. Overall, serum lithium levels were ~0.7–1.0 mmol/L and brain lithium levels were ~0.8–1.0 mmol/kg (Table 6), which are within the human therapeutic range of 0.6 to 1.3 mM.
Table 6. Serum and brain lithium levels of subjects that were that were chronically treated with 0.4% LiCl in food (Experiment 3).
n:10–12 animals per group in each strain.
| Strain | Serum Lithium mmol/L ±S.E.M. | Brain Lithium mmol/kg ±S.E.M. |
|---|---|---|
| 129S6/SvEvTac | 0.69±0.04 | 0.80±0.02 |
| BALB/cJ | 0.81±0.03 | 0.95±0.03 |
| C3H/HeNHsd | 0.83±0.03 | 1.02±0.23 |
| C57BL/6J | 1.00±0.05 | 0.93±0.03 |
| DBA/2J | 0.82±0.06 | 0.94±0.04 |
Open Field Test
Mice were tested after three weeks of treatment (Table 7). Chronic lithium administration had no significant effect on locomotion in any of the strains studied in this experiment: 129S6/SvEvTac t(22)=0.79, p=0.43, for BALB/cJ t(20)=0.61, p=0.54, for C3H/HeNHsd t(22)=0.33, p=0.74, for C57BL/6J t(29)=1.72, p=0.09, and for DBA/2J t(22)=1.76, p=0.09.
Table 7. Total average distance moved in the Experiment 4 open field test.
Data are in meters and expressed as mean ± SEM, n:5–8 animals per group in each strain.
| Strain | Total Distance Moved (m) | |
|---|---|---|
| Control Chow | Lithium Chow | |
| 129S6/SvEvTac | 42.4±2.3 | 38.3±4.5 |
| BALB/cJ | 109.5±7 | 100.5±13.8 |
| C3H/HeNHsdd | 48.3±3.3 | 46.1±5.6 |
| C57BL/6J | 95.9±6.3 | 80.9±6.1 |
| DBA/2J | 78.4±3.8 | 89.6±5.1 |
Tail Suspension Test
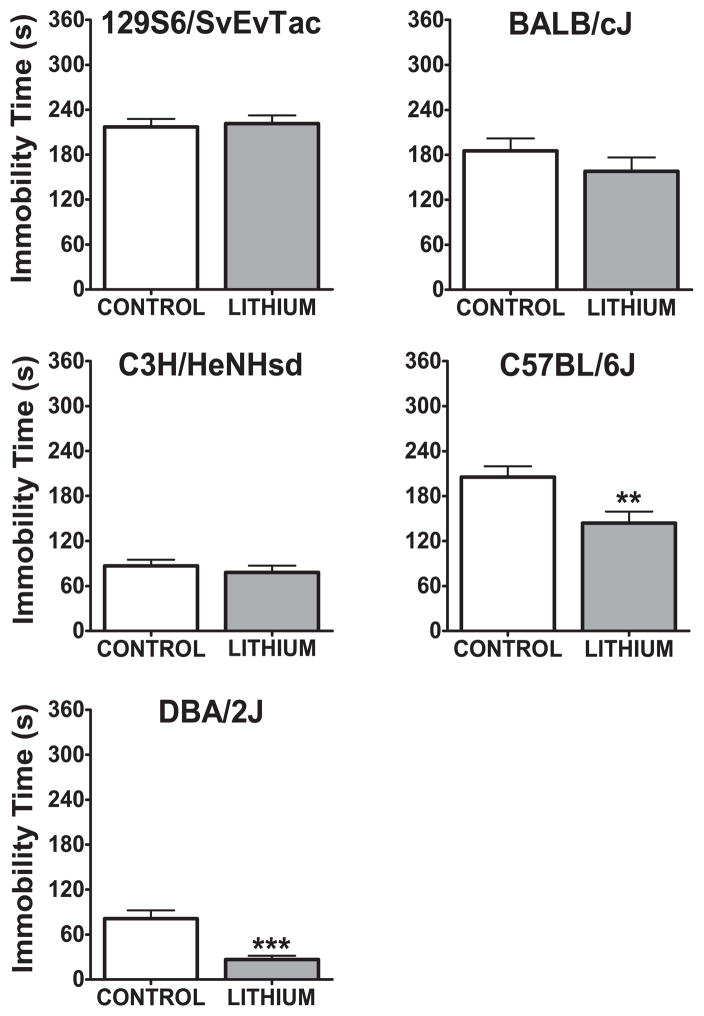
Two days after the OFT, mice were tested in the TST. Only in the C57BL/6J (t(29)=2.88, p<0.01) and DBA/2J (t(22)=4.5, p<0.001) strains was there a significant decrease in immobility (Figure 4). There was no statistically significant change in immobility as a result of chronic lithium treatment in other strains (129S6/SvEvTac t(22)=0.29, p=0.77, for BALB/cJ t(20)=1.1, p=0.28, and C3H/HeNHsd t(22)=0.72, p=0.48).
Figure 4. Immobility time in the tail suspension test after three weeks of chronic lithium administration.
**:p<0.01, ***:p<0.001 denote a significant, unpaired t-test. Data are expressed as mean ± SEM. n:10–12 animals per group for each strain.
Forced Swim Test
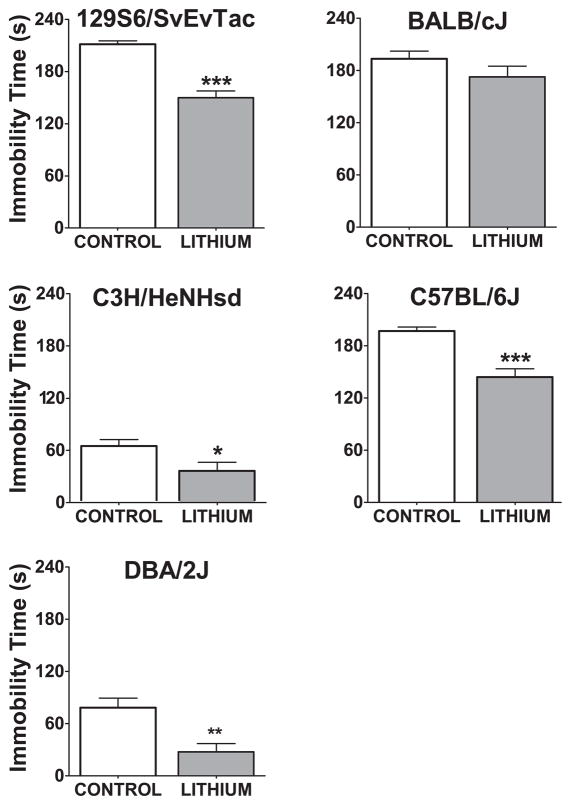
A week after the TST, the same mice were tested in the FST (Figure 5). All strains except BALB/cJ showed an antidepressant-like effect of chronic lithium treatment (129S6/SvEvTac t(22)=7, p<0.001, C3H/HeNHsd t(22)=2.31, p<0.05, C57BL/6J t(29)=4.84, p<0.001, DBA/2J t(22)=3.5, p<0.01, BALB/cJ t(20)=1.4, p=0.17).
Figure 5. Immobility time in the forced swim test after 3 weeks of chronic lithium administration.
*:p<0.05, **:p<0.01, ***:p<0.001 denote a significant difference; unpaired t-test. Data are expressed as mean ± SEM. n:10–12 animals per group for each strain.
Experiment 4: Intracerebroventricular Acute Lithium Administration
C57BL/6J and BALB/cJ strains were tested in OFT and FST after ICV administration of lithium, as representative responsive and non-responsive strains respectively. The aim was to confirm that the antidepressant-like effects of lithium (or lack thereof) were the result of central, rather than peripheral, effects of lithium. To assess brain and serum lithium levels following ICV administration, we infused 3 μl of a 600 mmol/L LiCl aCSF solution (0.076 mg of LiCl per subject) in a subset of mice not used in behavioral testing. Brain and serum lithium levels were 0.98±0.22 mmol/kg and 0.08±0.01 mmol/L in BALB/cJ mice (n=5) and 1.3± 0.16 mmol/kg and 0.09±0.01 mmol/L in C57BL/6J mice (n=6) respectively. After the surgery and at least one week of recovery, both strains were tested in the OFT, and in 6–7 days after that they were tested in the FST.
Open Field Test
In the C57BL/6J strain, vehicle-treated mice traveled a greater distance (52±5.4 m) than the lithium-treated subjects (17.7±9.9 m), and this difference was statistically significant (t(11)=3.17, p<0.01). In the BALB/cJ strain, vehicle-treated mice (23.5±4.7 m) travelled a greater distance than the lithium-treated subjects (10.2±4.7 m). There was a statistical trend for lithium to decrease locomotion in the BALB/cJ strain (t(11)=1.9, p=0.08), which was significant when an outlier (z=2.28, p<0.05) was removed (t(10)=3.81, p<0.01).
Forced Swim Test
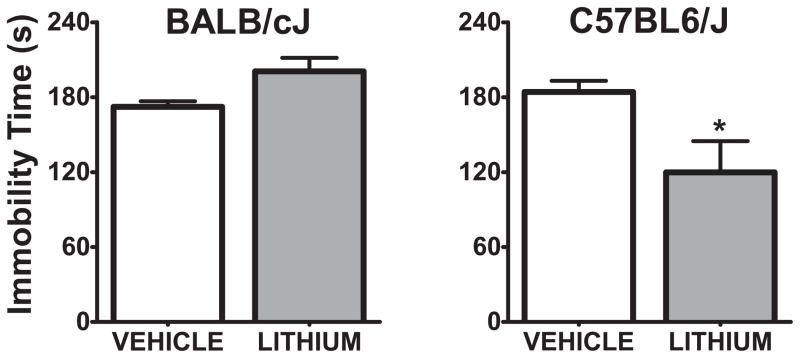
In the FST, lithium-treated subjects were significantly less immobile (t(11)=2.57, p<0.05) than vehicle-treated mice in the C57BL/6J strain. There was a trend for lithium-treated BALB/cJ subjects to show increased immobility when compared to controls (t(11)=2.01, p=0.069) (Figure 6).
Figure 6. Immobility time in forced swim test 30 minutes after ICV administration of 3 μl, 600 mmol/L LiCl into the right lateral ventricle.
*:p<0.05 denotes a significant difference, unpaired t-test. Data are expressed as mean ± SEM. n:5–8 animals per group for each strain.
DISCUSSION
We examined strain-dependent effects of lithium, administered via different routes, in two behavioral tests of antidepressant efficacy: the FST and the TST. In the FST, we examined eight inbred strains of mice: 129S6SvEvTac, A/J, BALB/cJ, C3H/HeNHsd, C57BL/6J, CBA/J, DBA/2J, FVB/NJ; and three outbred strains: Black Swiss, CD-1, and NIH Swiss. Among the outbred strains, only CD-1 was responsive to lithium. Inbred strains 129S6/SvEvTac, C3H/HeNHsd, and C57BL/6J manifested an antidepressant-like response to acute lithium. Black Swiss, NIH Swiss, and FVB/NJ strains manifested low baseline levels of immobility, presenting a floor effect. There was no relationship between baseline immobility levels and response to lithium. For example, while A/J, BALB/cJ, 129S6/SvEvTac, and C57BL/6J have similarly high baseline immobility levels, A/J and BALB/cJ strains did not manifest antidepressant-like responses to lithium in the FST, but 129S6/SvEvTac and C57BL/6J did. This outcome is consistent with the results of earlier mouse strain differences studies, which found no correlation between baseline immobility levels and response to selective norepinephrine and serotonin reuptake inhibitors in the FST (David et al., 2003, Lucki et al., 2001). Additionally, we did not observe a significant correlation between the pattern of lithium responsiveness and brain concentrations of lithium.
After the completion of this initial FST screening, we selected a subgroup of inbred strains (129S6/SvEvTac, BALB/cJ, C3H/HeNHsd, C57BL/6J and DBA/2J) to examine the effects of systemically administered acute lithium in the TST. Two strains showed a statistically significant change in immobility in response to lithium. 300 mg/kg lithium lowered immobility in the C3H/HeNHsd strain, but 400 mg/kg lithium increased immobility in 129S6/SvEvTac. While the results of FST and TST do not completely match, differences between results of FST and TST have been reported in previous studies in response to particular antidepressants (Cervo et al., 2005, Crowley et al., 2005, David et al., 2003, Ripoll et al., 2003).
Since behaviors manifested in the FST and TST could also be affected by a non-specific effect of lithium on general activity, we measured open field locomotion in 129S6/SvEvTac, BALB/cJ, and C57BL/6J strains. Acute lithium administration resulted in a significant effect on locomotion only at the 400 mg/kg dose in these strains. Lithium at this high dose decreased locomotion, an effect that may be related to lithium toxicity. However, this effect is unlikely to play a role in lower immobility times that we observed in the FST. None of the strains that were found to be responsive to lithium in the FST were responsive solely at the 400 mg/kg dose level, and chronic lithium, which did not significantly change locomotion, also resulted in antidepressant-like effects. Furthermore, the direction of change in locomotion (decrease) after 400 mg/kg LiCl administration is the opposite of the increased activity observed in the FST. Finally, these strains show different responses to lithium in the FST despite the similar effect on locomotion.
In clinical practice, lithium is most effective when administrated chronically. We therefore conducted a chronic systemic administration of lithium study with 129S6/SvEvTac, BALB/cJ, C3H/HeNHsd, C57BL/6J, and DBA/2J strains, in which we delivered the lithium through food over three to four weeks. This treatment did not lead to any significant change in locomotor activity. In the TST, chronic lithium administration decreased immobility time in C57BL/6J and DBA/2J strains. All strains, except for BALB/cJ, manifested lower levels of immobility in the FST in response to chronic lithium administration.
Finally, we assessed the effects of acute administration of ICV lithium in the OFT and FST. BALB/cJ and C57BL/6J were selected as representative non-responsive and responsive strains respectively. We observed that lithium reduced locomotor activity in the OFT in both strains. Consistent with our earlier findings, ICV lithium administration resulted in an antidepressant-like effect of lithium only in the C57BL/6J strain, but not in the BALB/cJ strain. The C57BL/6J results complement those of a previous study, in which chronic ICV lithium administration was observed to decrease immobility time in the FST and TST in C57BL/6J mice (Gould et al., 2008).
Examination of mouse strain variation in response to antidepressants has recently played a major role in defining the role of stress and fluoxetine on pre-mRNA editing of the serotonin 2C receptor (Bhansali et al., 2007, Englander et al., 2005) as well as the genetics and biochemistry that underlie activity of tryptophan hydroxylase-2 (TPH2), the enzyme responsible for brain serotonin synthesis and response to citalopram in the mouse FST (Cervo et al., 2005, Zhang et al., 2004). These findings have influenced the progression of clinical studies: for example, polymorphisms in TPH2 gene have been associated with the risk of developing both major depression and bipolar disorder (Cichon et al., 2008, Harvey et al., 2004, Van Den Bogaert et al., 2006, Zhang et al., 2005, Zhou et al., 2005, Zill et al., 2004).
Our study is limited in the sense that it only investigates the antidepressant effects of lithium. Because there is no mouse model that adequately represents the cycling of mood characteristic of bipolar disorder, the antidepressant and antimanic effects of lithium are generally examined in isolation. A strain differences study that focused on the effect of lithium on d-amphetamine-induced hyperlocomotion, a model of the antimanic efficacy, showed that the C57BL/6J strain was responsive to the antimanic effect of lithium (Gould et al., 2007b). This study did not test the BALB/cJ strain, because these mice do not show increased locomotion in response to d-amphetamine (Davis et al., 1974, Moisset & Welch, 1973).
The final goal of these experimental studies will be to use these strain differences as a tool to assist in determining lithium’s mechanisms of action, and to identify genes that modify mouse as well as human response to lithium. A logical follow-up to the present experiments is a quantitative trait loci (QTL) study using C57BL/6J and BALB/cJ strains as parental strains to identify regions in the mouse genome that harbor genes mediating the behavioral responses to lithium, followed by a comparison of these results to similar human studies in human subjects. In the past, Crowley, Lucki, and colleagues mapped QTLs for response to citalopram in the TST to three chromosomal loci (Crowley et al., 2006). Subsequent studies are expected to focus on identifying citalopram response genes that may be located within these regions (Crowley et al., 2006). Using a QTL approach, Tomida and colleagues recently identified Ubiquitin-specific peptidase 46 (Usp46) as a primary mediator of baseline FST and TST differences between C57BL/6J and CS inbred strains (Tomida et al., 2009). These recent successes indicate the capability of QTL approach to identify specific chromosomal regions and genes associated with mouse strain variation in complex behaviors. Such studies could complement ongoing human studies designed to identify genetic variants that modify human therapeutic response to lithium (Mccarthy et al., 2010, Perlis et al., 2009, Schulze et al., 2010).
Acknowledgments
This study has been supported by the grant NIHM R21 MH084043 to TDG.
References
- Alda M, Grof P, Rouleau GA, Turecki G, Young LT. Investigating responders to lithium prophylaxis as a strategy for mapping susceptibility genes for bipolar disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:1038–1045. doi: 10.1016/j.pnpbp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Bauer M, Adli M, Bschor T, Pilhatsch M, Pfennig A, Sasse J, Schmid R, Lewitzka U. Lithium’s Emerging Role in the Treatment of Refractory Major Depressive Episodes: Augmentation of Antidepressants. Neuropsychobiology. 2010:36–42. doi: 10.1159/000314308. [DOI] [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Canetta A, Calcagno E, Burbassi S, Sacchetti G, Caccia S, Fracasso C, Albani D, Forloni G, Invernizzi RW. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Winge I, Mattheisen M, Georgi A, Karpushova A, Freudenberg J, Freudenberg-Hua Y, Babadjanova G, Van Den Bogaert A, Abramova LI, Kapiletti S, Knappskog PM, McKinney J, Maier W, Jamra RA, Schulze TG, Schumacher J, Propping P, Rietschel M, Haavik J, Nothen MM. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum Mol Genet. 2008;17:87–97. doi: 10.1093/hmg/ddm286. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic Evaluation of the Antidepressant Citalopram in the Mouse Tail Suspension Test. Neuropsychopharmacology. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dao D, Mahon P, Cai X, Kovacsics C, Blackwell R, Arad M, Shi J, Zandi P, O’Donnel lP, Knowles J, Weissman M, Coryell W, Scheftner W, Lawson W, Levinson D, Thompson S, Potash J, Gould T Bipolar Genome Study (BiGS) Consortium. Mood Disorder Susceptibility Gene CACNA1C Modifies Mood-Related Behaviors in Mice and Interacts with Sex to Influence Behavior in Mice and Diagnosis in Humans. Biological Psychiatry. 2010;68:801–810. doi: 10.1016/j.biopsych.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–382. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- Davis WM, Babbini M, Pong SF, King WT, White CL. Motility of mice after amphetamine - Effects of strain, aggregation and illumination. Pharmacology Biochemistry and Behavior. 1974;2:803–809. doi: 10.1016/0091-3057(74)90113-0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Mogilnicka E, Areso PM. Antidepressant-like activity of the melatonin receptor antagonist, luzindole (N-0774), in the mouse behavioral despair test. Eur J Pharmacol. 1990;182:313–325. doi: 10.1016/0014-2999(90)90290-m. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- El-Kassem M, Singh SM. Strain dependent rate of Li+ elimination associated with toxic effects of lethal doses of lithium chloride in mice. Pharmacology Biochemistry and Behavior. 1983;19:257–261. doi: 10.1016/0091-3057(83)90049-7. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-Term Lithium Therapy for Bipolar Disorder: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Psychiatry. 2004;161:217–222. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007a;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007b;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium’s reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O’Donovan C, Alda M. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63:942–947. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- Hamburger-Bar R, Robert M, Newman M, Belmaker RH. Interstrain correlation between behavioural effects of lithium and effects on cortical cyclic AMP. Pharmacol Biochem Behav. 1986;24:9–13. doi: 10.1016/0091-3057(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Harvey M, Shink E, Tremblay M, Gagne B, Raymond C, Labbe M, Walther DJ, Bader M, Barden N. Support for the involvement of TPH2 gene in affective disorders. Mol Psychiatry. 2004;9:980–981. doi: 10.1038/sj.mp.4001557. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Cryan J. Feeling Strained? Influence of Genetic Background on Depression-Related Behavior in Mice: A Review. Behavior Genetics. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology. 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11:1439–1465. doi: 10.2217/pgs.10.127. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Fieve RR, Stallone F. Relationship Between the Effectiveness of Lithium Therapy and Family History. Am J Psychiatry. 1973;130:1011–1013. doi: 10.1176/ajp.130.9.1011. [DOI] [PubMed] [Google Scholar]
- Moisset B, Welch BL. Effects of d-amphetamine upon open field behaviour in two inbred strains of mice. Cellular and Molecular Life Sciences. 1973;29:625–626. doi: 10.1007/BF01926708. [DOI] [PubMed] [Google Scholar]
- Nomura S, Okada H, Naruse R, Yamaoka K. The tail suspension test for screening antidepressant drugs: comparison of movement in ICR and NMRI mice. Jpn J Psychiatry Neurol. 1991;45:113–114. [PubMed] [Google Scholar]
- O’Neill HC, Schmitt MP, Stevens KE. Lithium alters measures of auditory gating in two strains of mice. Biol Psychiatry. 2003;54:847–853. doi: 10.1016/s0006-3223(03)00184-7. [DOI] [PubMed] [Google Scholar]
- Ong JC, Brody SA, Large CH, Geyer MA. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315:1163–1171. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Smoller JW, Ferreira MAR, McQuillin A, Bass N, Lawrence J, Sachs GS, Nimgaonkar V, Scolnick EM, Gurling H, Sklar P, Purcell S. A Genomewide Association Study of Response to Lithium for Prevention of Recurrence in Bipolar Disorder. Am J Psychiatry. 2009;166:718–725. doi: 10.1176/appi.ajp.2009.08111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Alda M, Adli M, Akula N, Ardau R, Bui ET, Chillotti C, Cichon S, Czerski P, Del Zompo M, Detera-Wadleigh SD, Grof P, Gruber O, Hashimoto R, Hauser J, Hoban R, Iwata N, Kassem L, Kato T, Kittel-Schneider S, Kliwicki S, Kelsoe JR, Kusumi I, Laje G, Leckband SG, Manchia M, MacQueen G, Masui T, Ozaki N, Perlis RH, Pfennig A, Piccardi P, Richardson S, Rouleau G, Reif A, Rybakowski JK, Sasse J, Schumacher J, Severino G, Smoller JW, Squassina A, Turecki G, Young LT, Yoshikawa T, Bauer M, McMahon FJ. The International Consortium on Lithium Genetics (ConLiGen): An Initiative by the NIMH and IGSLI to Study the Genetic Basis of Response to Lithium Treatment. Neuropsychobiology. 2010;62:72–78. doi: 10.1159/000314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeraldi E, Petroccione A, Gasperini M, Macciardi F, Orsini A, Kidd KK. Outcomes on lithium treatment as a tool for genetic studies in affective disorders. Journal of Affective Disorders. 1984;6:139–151. doi: 10.1016/0165-0327(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. American Journal of Medical Genetics. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tomida S, Mamiya T, Sakamaki H, Miura M, Aosaki T, Masuda M, Niwa M, Kameyama T, Kobayashi J, Iwaki Y, Imai S, Ishikawa A, Abe K, Yoshimura T, Nabeshima T, Ebihara S. Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat Genet. 2009;41:688–695. doi: 10.1038/ng.344. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaert A, Sleegers K, De Zutter S, Heyrman L, Norrback KF, Adolfsson R, Van Broeckhoven C, Del-Favero J. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and bipolar disorder in a Northern Swedish, isolated population. Arch Gen Psychiatry. 2006;63:1103–1110. doi: 10.1001/archpsyc.63.10.1103. [DOI] [PubMed] [Google Scholar]
- van der Heyden JA, Molewijk E, Olivier B. Strain differences in response to drugs in the tail suspension test for antidepressant activity. Psychopharmacology (Berl) 1987;92:127–130. doi: 10.1007/BF00215493. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ishino H, Otsuki S. Double-Blind Comparison of Lithium Carbonate and Imipramine in Treatment of Depression. Arch Gen Psychiatry. 1975;32:659–668. doi: 10.1001/archpsyc.1975.01760230125010. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu J-M, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan Hydroxylase-2 Controls Brain Serotonin Synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, Enoch MA, Virkkunen M, Goldman D. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. 2005;62:1109–1118. doi: 10.1001/archpsyc.62.10.1109. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]