Abstract
Aims
This study examined the contribution of transmissible risk, in conjunction with family and peer contextual factors during childhood and adolescence, on development of cannabis use disorder in adulthood.
Design
The family high risk design was used to recruit proband fathers with and without substance use disorder and longitudinally track their sons from late childhood to adulthood.
Setting
The families were recruited under aegis of the Center for Education and Drug Abuse Research in Pittsburgh, Pennsylvania.
Participants
The oldest son in the family was studied at ages 10–12, 16, 19, and 22.
Measurements
The transmissible liability index (TLI) (Vanyukov et al., 2009) along with measures of quality of parent child relationship, cooperative behavior at home, social attitudes, and peer milieu were administered to model the developmental pathway to cannabis use disorder.
Findings
Affiliation with socially deviant peers and harboring non-normative attitudes (age 16) mediate the association between transmissible risk for SUD (age 10–12) and use of illegal drugs (age 19) leading to cannabis use disorder (age 22).
Conclusions
Deviant socialization resulting from transmissible risk and poor parent-child relationship is integral to development of cannabis use disorder in young adulthood.
Keywords: deviant socialization, cannabis use, transmissible liability index (TLI)
Introduction
Externalizing behavior during childhood frequently progresses to social deviancy and delinquency during adolescence[1] and antisocial personality disorder in young adulthood [2]. Externalizing behavior is also the most ubiquitous predisposing characteristic and correlate of substance abuse and substance use disorder (SUD) [3–8]. Commensurate with findings showing that attention deficit hyperactivity disorder (ADHD), conduct disorder, and antisocial personality disorder in adulthood share significant genetic variance with risk for SUD [9–10], it has been demonstrated that the variety of SUDs can be ordered on a latent trait measuring externalizing disorder severity [11]. The SUDs located toward the left pole (less severe disorders) are consequent to consumption of legal drugs (e.g. alcohol, tobacco) whereas SUDs consequent to using illegal drugs are located toward the right pole (more severe disorders) on the externalizing trait, These findings indicate that use of illegal drugs and associated SUDs are integrally related to low adherence to societal norms; however, numerous other psychological characteristics, particularly disturbances in emotion regulation and cognition also amplify risk for SUD [12–13].
Up to 100% of genetic variance and up to 80% of phenotypic variance are congenerous to risk for all categories of SUD [14,15]. Accordingly, the psychological characteristics predisposing to SUD are largely common across all diagnostic categories. Thus, contrary to the gateway hypothesis, which asserts that unique factors are associated with use of each particular compound [16], the evidence points instead to primarily shared psychological characteristics.
Based on genetic and biobehavioral evidence documenting common liability [see 17 for review], Vanyukov and colleagues proposed an innovative strategy involving the use of item response theory methodology [18] to quantify on a continuous interval scale the transmissible component of risk for all SUDs. Notably, the transmissible liability index (TLI) derived by Vanyukov and colleagues [19] has been shown to have excellent psychometric properties, including discriminative and predictive validity [19,20]. Significantly, a modified version of the TLI using the variables contained in the National Epidemiological Survey of Alcohol and Related Conditions (NESARC) predicts all SUD categories [21]. Moreover, studies conducted on twins have revealed that between 75% [22] and 85%[19] of variance on the TLI is accounted by heritability. Among youths who develop cannabis use disorder, the score on the TLI linearly increases in severity from the time of first cannabis use to diagnosis [23].
Based on many studies showing genetic, phenotypic and developmental overlap between SUD and antisociality, it was hypothesized that non-normative socialization mediates the association between transmissible risk in childhood and cannabis use disorder in adulthood. Deviant socialization, manifested during adolescence as affiliation with norm-violating peers and harboring non-traditional attitudes, was theorized to result from three correlated influences during childhood: i) individual predisposition (transmissible risk) for SUD; ii) quality of parent child relationship; and, iii) childhood propensity for prosocial behavior indicated by cooperatively performing household tasks. Numerous studies have shown that a poor relationship with parents is associated with consuming illegal drugs, delinquency, and affiliation with socially deviant peers during adolescence [24–31]. Moreover, harboring unconventional attitudes has also been reported frequently in substance abusing youths [24,29]. However, it has not yet been determined whether these latter indicators of deviant socialization mediate the relation between transmissible risk and SUD outcome. Demonstrating that cannabis use disorder is the outcome of non-normative socialization determined by psychological disposition having largely genetic basis in conjunction with quality of parent-child relationship has important ramifications for SUD prevention. Specifically, rather than emphasizing desistence from drugs as the primary focus of prevention, it would appear that potentiating normative socialization by inculcating attitudes and behaviors that conform to societal mores and laws would reduce the propensity to initiate consumption of substances having abuse potential.
Methods
Subjects
Probands were adult men who had a 10–12 year old biological son and either qualified for a lifetime diagnosis of SUD concomitant to use of an illegal drug or had no adult onset psychiatric disorder. Eighty percent of the SUD+ fathers were recruited via public service announcements, advertisement and a market research firm that conducted random digit dialing. The remaining 20% of the SUD+ men were enrolled following discharge from treatment facilities. Recruitment of SUD+ men with and without treatment history maximizes the likelihood that the sample encompasses the full spectrum of SUD severity so that transmissible risk measured in their children likewise reflects the full range of severity. The most frequent SUD diagnoses (abuse or dependence) in the fathers pertained to use of cannabis (34.2%), cocaine (23.8%), opiates (11.2%), and amphetamines (8.4%). An alcohol use disorder without a co-occurring SUD concomitant to consuming an illegal drug was a disqualifying criterion. Comorbid alcohol use disorder was, however, diagnosed in 42.6% of the SUD+ men. The most frequent non-SUD psychiatric diagnoses in the men were depression (15.8%), antisocial personality disorder (12.2%) and anxiety spectrum disorder (8.8%). The same methods were used to recruit the SUD- men except that none were accrued from treatment facilities.
The oldest 10–12 year old son in each family (N=500) participated in this longitudinal study. The sample at baseline consisted of 250 boys having SUD+ fathers and 250 boys having SUD- fathers. At the time of recruitment, the biological sons of the SUD+/− men underwent a physical examination, urine drug screen, and intelligence evaluation using the WISC-III-R. To qualify for enrollment in the study, the boys were required to be in good health, drug free, and have at least low normal intelligence. Follow-up evaluations were conducted when the boys attained 16, 19 and 22 years of age. A total of 254 boys participated in the age 22 follow-up. The remainder from the baseline sample of 500 either declined participation (N = 154) or had not yet attained 22 years of age (N = 92) at the time this report was prepared. Comparisons between subjects retained and attrited at the age 22 follow-up when they were 10–12 years of age (baseline evaluation) did not reveal systematic differences. Socioeconomic status using Hollingshead criteria was similar in retained and attrited participants (41.0 vs. 38.8). Grade in school was identical in the two groups (4.5). Ethnic distribution was also not related to attrition. The baseline sample that was retained for follow-up was 76% European American and 24% African American. The distribution of the attrited sample was 73% European American and 27% African American. Attrition was about 10% higher (44.5% vs. 55.5%) in sons of SUD+ fathers. Full scale WISC-III-R IQ score was somewhat lower (110 vs. 104, F=14.73; p<.001) in the baseline participants who did not complete the age 22 evaluation.
Instrumentation
Diagnostic Ascertainment
Diagnostic assessment was conducted using an expanded version of the Structured Clinical Interview for DSM-III-R (SCID) [32]. The results of the SCID in conjunction with medical, legal, psychiatric, and social history information obtained from official records and other questionnaires were reviewed by a clinical committee consisting of a psychiatrist certified in addiction psychiatry (chair), another psychiatrist or clinical psychologist, and the clinical associates who conducted the interviews. Following a review of all information, the committee arrived at a consensus regarding axis I and II lifetime diagnoses using the method recommended by Leckman et al. [33]. The same procedure was employed to formulate axis I and II diagnoses in the biological fathers, biological mothers, and their sons when they attained 22 years of age. The outcome variable, current diagnosis of cannabis use disorder (abuse or dependence), was present in 27% of the boys.
Transmissible Liability Index (TLI) (age 10–12)
Transmissible liability denotes the portion of phenotypic variance associated with risk for SUD that has intergenerational continuity. The rationale and method of deriving the transmissible liability index (TLI) have been previously documented [17,18]. In addition, construct, predictive and discriminative validity have been described [19,20].
The TLI items, shown at www.pitt.edu/~CEDAR/TLIdocument.html, encompass processes reflecting psychological self-regulation related to modulation of affect, behavior control and attention. Accordingly, the items measure characteristics such as irritability, arousability to aggression, control of impulses, and susceptibility to distraction. In addition, the TLI items capture certain interpersonal manifestations of poor self-regulation (e.g. propensity to annoy others, defiance of authority), risk taking, suicide thoughts, and psychological as well as physical (nailbiting) correlates of anxiety.
Parent-Child Relationship (age 10–12)
Items were selected from four questionnaires based on their face validity. The questionnaires were: i) Children’s Report on Parental Behavior Inventory [34], ii) Areas of Change Questionnaire [35], iii) Supervision/Involvement Scale [36], and iv) Family Assessment-Dyadic Relationship Scale [37]. Principal components analysis of the items revealed a factor accounting for 31% of variance. Factor loadings of the items ranged from .30 to .90. The large gap between eigenvalue of the first (11.2) and second (3.71) factor, along with their ratio (3.02), points to unidimensionality. Items having factor loading less than .40 were pruned from the construct. Confirmatory factor analysis conducted on the remaining items revealed acceptable model data fit (χ2=137.19; df=120; p =.14, RMSEA=.02). Item response theory methods performed on the factor revealed that the item discrimination parameter was moderate (M=.46, sd=.15) and the threshold parameter was low (M=−1.77, sd=1.03). The alpha coefficient was .89. Skewness and kurtosis were 0.04 and 0.11. Before conducting the analyses, the factor scores were transformed to a z-scale.
Cooperative Behavior (age 10–12)
Performing household tasks that benefit the welfare of all family members is a harbinger of normative socialization. Accordingly, the mother was queried about her son’s prosocial behavior at home. She responded to three questions administered in an interview: 1) “If you ask him to do something around the house, can you be sure it will get done without your having to watch or check on him?” 2) “Does he help clean up and put things away when he is finished…...?” and, 3) “When you are working or doing some kind of job around the house, does he want to help or join in?” A 4-point Likert scale recorded frequency of cooperative behavior ranging from “always” to “never”. Alpha coefficient was .71. The sample obtained a mean score of 7.09 (SD =1.58). The total score, ranging from 4–12, had skewness of 0.10 and kurtosis of 0.13.
Normative Social Attitudes (age 16)
The 25-item Traditionalism Scale of the self-report Multidimensional Personality Questionnaire (MPQ)[38] measures conformity to societal norms. The alpha coefficient in this sample is .81. The mean score and standard deviation in the sample are respectively 16.52 and 5.2. Skewness and kurtosis are −0.35 and −0.27.
Peer Environment (age 16)
The Peer Milieu Index (PMI), previously developed by Feske and colleagues [39], measures adherence to social mores and the law by the friendship network. This 38-item self-report administered at age 10–12 is a significant predictor of cannabis use disorder at age 22 [39]. Alpha coefficient is .80. The z-transformed score in this sample has skewness of 0.10 and kurtosis of 0.13.
Consumption of Illegal Drugs (age 19)
Section 1A of the revised Drug Use Screening Inventory (DUSI-R)[40] recorded frequency of consumption of illegal drugs during the month prior to study participation. The score used in the analyses was computed by adding the number of occasions cannabis, amphetamines, cocaine/crack, opiates, and prescription drugs without medical supervision were consumed. The log transformed mean score in the sample was 1.38 (SD = 2.52). Skewness and kurtosis were 0.98 and −0.18.
Procedure
Written assent and written informed consent were obtained respectively from the boys and their parents prior to administering the research protocols. Informed consent was also obtained from the boys when they attained 19 and 22 years of age. The research protocols have been approved annually by the University of Pittsburgh Institutional Review Board since 1990. A urine drug screen was performed before the test session to ensure that the results were not confounded or biased by the acute effects of or withdrawal from alcohol or drugs. A positive result required rescheduling the participant. The assessments were individually conducted by experienced master-level research associates in a sound attenuated room. Prior to discharge from the laboratory, the participants were debriefed and compensated for their time.
Statistical Analysis
Path analysis with dichotomous outcome was conducted to model the trajectory to cannabis use disorder taking into account transmissible liability, parent-child relationship and cooperative behavior at baseline (age 10–12), normative attitudes and peer environment during mid-adolescence (age 16), use of illegal drugs in early adulthood (age 19), and cannabis use disorder (age 22). The method, described by West and Aiken [41], was employed to assess mediation. The analyses were conducted using MPlus software.
In addition, the temporal sequence of the variables was tested as a causal model using Directed Acyclic Graph (DAG) analysis [42] DAG analysis has certain advantages over the path model for analyzing the causal effects of model factors. i) it does not assume the presence of a linear relation among the variables, ii) it examines the relation among the variables based on their joint distributions and conditional independence, and, iii) it removes bias which could arise when a third variable is introduced between mediators and outcome. Essentially, DAG analysis involves testing the equality of two competing models: 1) a model with direct causal effects only; and, 2) a model consisting of direct and indirect effects. The likelihood ratio test is used to determine the best model data fit.
Results
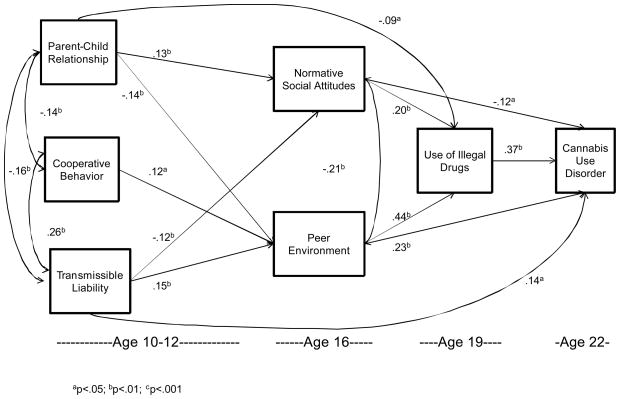
As can be seen in Figure 1, TLI, cooperative behavior and parent-child relationship are intercorrelated (TLI and parent-child relationship: b=−.16, z=−3.80, p<.001; TLI and cooperative behavior: b=.26, z=8.74, p<.001; parent-child relationship and cooperative behavior: b=−.14, z=−3.76, p<.001). Moreover, each variable predicts peer environment 4–6 years later (parent-child relationship: b=−.14, z=−3.12, p=.002; TLI: b=.15, z=3.39, p=.001; and cooperative behavior: b=.12, z=2.54, p=.011). Peer environment, in turn, predicts use of illegal drugs at age 19 (b=.44, z=10.42, p<.001) and that in turn predicts cannabis use disorder at age 22 (b=.37, z=6.26, p<.001). TLI (b=−.12, z=−2.63, p=.008) and parent-child relationship (b=.13, z=2.80, p=.005), but not cooperative behavior at age 10–12, predicts normative social attitudes which, in turn, predicts use of illegal drugs (b=.20, z=4.29, p<.001) three years later leading to cannabis use disorder (b=−.12, z=−1.96, z=.05) six years later. Peer environment and normative social attitudes are correlated (b=−.21, z=−4.80, p<.001) at age 16. In addition, TLI at age 10–12 (b=.14, z=2.10, p=.035) and peer environment at age 16 (b=.23, z=3.95, p<.001) directly predict cannabis use disorder at age 22. The overall model data fit is excellent (χ2 = 2.86, df = 4, p = .58, CFI = .99, TLI = .99, RMSEA < .001).
Figure 1.
Path model depicting development of cannabis use disorder
Use of illegal drugs (age 19) mediates the association between peer environment (age 16) and cannabis use disorder at (age 22) (b = .19, t = 5.35, p<.001). Use of illegal drugs (age 19) also mediates the relation between normative attitudes (age 16) and cannabis use disorder (age 22) (b=.01, t=3.25, p=.001). Furthermore, normative attitudes (age 16) mediate the association between parent child relationship (age 10–12) and use of illegal drugs (age 19) (b=.02, t=2.27, p=023). Moreover, use of illegal drugs mediates the association between parent-child relationship (age 10–12) and cannabis use disorder (age 22) (b=−.03, t=−1.98, p=.048).
Affiliation with deviant peers (age 16) is directly predicted by quality of parent-child relationship 4–6 years earlier. Peer environment does not mediate the association between quality of parent-child relationship and use of illegal drugs on development of cannabis use disorder. Peer environment does, however, mediate the association between cooperative behavior in childhood and use of illegal drugs (b=.02, t=2.37, p=.018) and cannabis use disorder (b=.02, t=2.16, p=.031).
Both normative attitudes (b=−.02, t=−2.28, p=.023) and peer environment (b=.04, t=3.00, p=.003) in mid-adolescence mediate the association between transmissible liability in childhood and use of illegal drugs in young adulthood. Peer environment, but not normative attitudes in mid-adolescence, mediate the association between transmissible liability in childhood and cannabis use disorder at age 22 (b=.04, t=2.56, p=.01).
An alternative model was also tested in which the variable measuring cooperatively performing household tasks was deleted. A reasonable model data fit was obtained (χ2=4.40, df=2, p=.11, RMSEA=.049, CFI=.99, TLI=.97). However, the modal data fit was not as good as the full model, thus underscoring the informativeness of child’s cooperative behavior for understanding the development of cannabis use disorder. The results of the alternative model nevertheless reveal that traditional attitudes (b =.17, z=3.65, p<.001; b =−.10, z=−2.18, p=.03) and peer environment (b =.26, z=5.44, p<.001; b =−.20, z=−4.52, p<.001) at age 16 are predicted by child’s quality of relationship with parents and transmissible liability. Moreover, consumption of illegal drugs is predicted by peer environment (b =.53, z=13.30, p<.001), and traditional attitudes (b =−.08, z=−2.11, p=.03) which, in turn, predicts cannabis use disorder diagnosis (b =.39, z=5.67, p<.001). Consumption of illegal drugs mediates the relation of traditional attitudes (b =−.03, z=−2.07, p=.04) as well as peer environment (b =.20, z=5.38, p<.001) with cannabis use disorder diagnosis. In addition, peer environment mediates the association between transmissible liability and consumption of illegal drugs (b =.14, z=4.90, p<.001).
Competing models were also tested using directed acyclic graph (DAG) analysis. The results of this analysis point to the importance of indirect effects on development of cannabis use disorder. Specifically, development of cannabis use disorder at age 22 is contingent on normative social attitudes and peer environment given the presence of illicit drug use at age 19 (likelihood ratio = 6.35, df = 2, p = .04), In addition, cannabis use disorder at age 22 is contingent on all the variables measured at age 10–12 given the presence of social attitudes and peer affiliations at age 16 and use of illegal drugs at age 19 (likelihood ratio =7.74, df=3, p=.05). Considering these findings in aggregate, it can be concluded that a model taking into account direct and indirect effects is better fitting than a model consisting only of direct effects.
Discussion
To briefly summarize, transmissible liability for SUD correlates with quality of parent child-relationship and cooperative performance of household tasks. Moreover, transmissible liability and quality of parent-child relationship, but not child’s cooperative behavior, predict normative social attitudes at age 16 which, in turn, predicts use of illegal drugs at age 19 leading to cannabis use disorder at age 22. Transmissible liability, quality of parent-child relationship, and cooperative behavior at home predict affiliation with deviant peers which in turn predicts using illegal drugs leading to cannabis use disorder. The full model has excellent fit to the data. Quality of model data fit is, however, reduced when cooperative behavior is excluded from the model. Thus, a low propensity for cooperatively performing tasks at home during childhood, a harbinger of deviant socialization, is integral to development of cannabis use disorder. This finding complements results obtained by Eiden et al. [43] showing that low social competence in children is associated with both deficient parenting and psychological dysregulation.
Promoting cooperative behavior in children would thus appear to be an important component of family-based interventions directed at preventing SUD. However, considering that cooperative behavior is correlated with the psychological characteristics comprising transmissible risk for SUD as well as quality of parent-child relationship, it is essential to deploy a multifaceted intervention strategy. Family-oriented SUD prevention is, however, complicated by the fact that by definition the severity of transmissible risk is correlated between children and parents. Hence, parents evincing high transmissible risk for SUD (e.g. behavior undercontrol, emotion volatility, poor attention control) not only are likely to have children with the same disposition, but they also are likely to qualify for SUD diagnosis and other axis I and II psychiatric disorders. Thus, consolidating a positive relationship with their children is impeded by the conjoint influence of SUD and comorbid disorders in addition to psychological dysregulation intrinsic to transmissible risk. Accordingly, high transmissible risk for SUD in parents and children potentiates a poor quality relationship, thereby biasing the child’s development toward non-normative socialization.
Clinical Application
Psychological dysregulation, the cardinal feature of high transmissible SUD liability, has been theorized to originate from a dysfunction of neural systems in the frontal-limbic region [44]. Youths at high risk for SUD exhibit cortical hypoactivation that appears to be circumscribed to the frontal region [45]. Paralleling this finding, Bauer and Hesselbrock [46] demonstrated that attenuation of the P300 wave of the event-related potential in high risk youths is most pronounced over frontal cortex. These findings raise the intriguing possibility that interventions which enhance frontal cortex functioning, thereby augmenting psychological self-regulation, may lower the risk of developing SUD. Significantly, cognitive training augments frontal cortex activity[47,48]and increases density of dopamine receptors [49]. Recently, Vaughn et al. [50] demonstrated that polymorphisms of dopamine transporter (DAT1) and receptor (DRD2) genes predict affiliation with deviant peers as well as low maternal engagement with the child leading to behavior undercontrol and polydrug use. Inasmuch as socialization is a protracted process encompassing manifold interpersonal interactions proceeding concurrently with neuromaturation, it is plausible to speculate that prevention interventions directed at potentiating functioning of the neurological substrate integral to psychological self-regulation also concomitantly facilitates normative socialization. Considering that neuromaturation continues into the third postnatal decade[51] when cannabis use disorder is at peak prevalence [52], there is a limited window of opportunity to modify the neural circuitry underlying psychological self-regulation.
Performing household tasks has historically serendipitiously facilitated normative socialization. However, less help is currently required concomitant to automation, fewer children in the family, routine consumption of prepared food, and eating at quick service restaurants. Parents thus have less need and fewer opportunities to provide direction to their children in the course of everyday routines, thereby allowing children more time for solitary or unsupervised activities. Accordingly, a deliberate effort by parents to assign and oversee age-appropriate household tasks for their children may be an effective in vivo strategy for fostering normative socialization.
Limitations
Several limitations of this study are noted. Importantly, the sample was confined to boys. Girls demonstrate greater willingness for cooperative behavior and are more socially responsive than boys [53]. Hence, the trajectory to cannabis use disorder described herein may not apply to girls. This study also did not take into account the unique aspects of the relationship each parent has with their son. In addition, the factors underlying the quality of parent-child relationship were not investigated. Considering that type and effectiveness of parent discipline behavior predisposing to offspring’s substance abuse is the product of both parent and child characteristics [26], it remains to be determined how the psychological features comprising transmissible liability interacts with parental characteristics to influence overall SUD risk. Furthermore, the outcome variable in this study was circumscribed to cannabis use disorder. Although this is the outcome of the most prevalent illegal drug used by youth, future research needs to examine whether the same developmental trajectory culminates in other types of SUD.
In summary, individual susceptibility (transmissible risk), parent-child relationship, and cooperatively performing household tasks are intercorrelated in pre-adolescent boys. These characteristics are predictors of quality of socialization in mid-adolescence leading to drug use and subsequently cannabis use disorder in adulthood. From the etiological perspective, these findings illustrate that deviant socialization promoting risk for cannabis use disorder results from both psychological characteristics having significant genetic contribution and contextual influences. From the standpoint of prevention, interventions need to attenuate the psychological characteristics comprising transmissible risk while simultaneously improving the quality of parent-child relationship to consolidate prosocial behavior leading to normative socialization.
Footnotes
Declaration of Interest: This research was supported by grants P50DA05605, K02-DA017822, K02-DA018701 from the National Institute on Drug Abuse. The authors do not report any conflict of interest.
References
- 1.Patterson GR, DeBaryshe BD, Ramsey E. A developmental perspective on antisocial behavior. Am Psychol. 1989;44:329–335. doi: 10.1037//0003-066x.44.2.329. [DOI] [PubMed] [Google Scholar]
- 2.Forsman M, Larsson H, Andershed H, Lichtenstein P. The association between persistent disruptive childhood behavior and the psychopathic personality constellation in adolescence: A twin study. Br J Develop Psychiat. 2007;25:383–398. [Google Scholar]
- 3.Crowley TJ, Riggs PD. Adolescent substance use disorder with conduct disorder and comorbid conditions. NIDA Res Mono. 1995;156:49–111. [PubMed] [Google Scholar]
- 4.Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use general or specific? Behav Genetics. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 5.Pederson W, Mastekaasa A, Wichstrom L. Conduct problems and early cannabis use initiation: A longitudinal study of gender differences. Addiction. 2001;96:415–431. doi: 10.1046/j.1360-0443.2001.9634156.x. [DOI] [PubMed] [Google Scholar]
- 6.Button TMM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genetics. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- 7.Button TMM, Rhee SH, Hewitt JK, Young SE, Corley RP, Stallings MC. The role of conduct disorder in explaining the comorbidity between alcohol and illicit drug dependence in adolescence. Drug Alc Depend. 2007;87:46–53. doi: 10.1016/j.drugalcdep.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Clark DB, Pollock NK, Mezzich AC, Cornelius J, Martin C. Diachronic substance use assessment and the emergence of substance use disorders. J Child Adolesc Sub Abuse. 2001;10:13–22. [Google Scholar]
- 9.Vanyukov M, Tarter R. Genetic studies of substance use. Drug Alc Depend. 2000;59:101–123. doi: 10.1016/s0376-8716(99)00109-x. [DOI] [PubMed] [Google Scholar]
- 10.Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early onset cannabis users versus co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 11.Krueger R, Hicks B, Patrick C, et al. Etiological connections among substance dependence, antisocial behavior, and personality. Modeling the externalizing spectrum. J Abnorm Child Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- 12.Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Psych Res. 2008;42:230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark D. Etiology of early age onset substance abuse: A maturational perspective Develop Psychopath. 1999;11:657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- 14.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psych. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 15.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch of Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 16.Kandel D, Yamaguchi K. In: Developmental stages of involvement in substance use, in Sourcebook on Substance Abuse: Etiology, Epidemiology, Assessment, and Treatment. Ott P, Tarter R, Ammerman R, editors. Boston: Allyn & Bacon; 1999. pp. 50–74. [Google Scholar]
- 17.Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003a;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS, et al. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Rev. 2003b;27:517–526. doi: 10.1016/j.neubiorev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Vanyukov MM, Kirisci L, Moss L, Tarter RE, Reynolds MD, Maher BS, et al. Measurement of the risk for substance use disorders: Phenotypic and genetic analysis of an index of common liability. Behav Genetics. 2009;39:233–244. doi: 10.1007/s10519-009-9269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirisci L, Tarter R, Mezzich A, Ridenour T, Reynolds M, Vanyukov M. Prediction of cannabis use between childhood and young adulthood: Clarifying the phenotype and environtype. Am J Addictions. 2009;18:36–47. doi: 10.1080/10550490802408829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridenour TA, Tarter RE, Kirisci L, Vanyukov MM. Could a continuous measure of individual transmissible risk be useful in clinical assessment of substance use disorder? Under review. doi: 10.1016/j.drugalcdep.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks BM, Iacono WG, McGue M. Index of the transmissible common liability to addiction: Heritability and prospective associations with substance abuse and related outcomes. Addiction. doi: 10.1016/j.drugalcdep.2011.12.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirisci L, Tarter R, Vanyukov M, Ridenour R, Reynolds M. Quantifying Transmissible Risk for Substance Use Disorder from Childhood to Adulthood. Journal of Abnormal Child Psychology submitted. [Google Scholar]
- 24.Clark DB, Kirisci l, Mezzich A, Chung T. Parental supervision and alcohol use in adolescence: Developmentally specific interactions. J Develop Behav Pediat. 2008;29:285–292. doi: 10.1097/DBP.0b013e31816e22bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laird RD, Criss MM, Petti GS, Dodge KA, Bates JE. Parent’s monitoring knowledge attenuates the link between antisocial friends and adolescent delinquent behavior. J Abnorm Child Psychol. 2008;36:299–310. doi: 10.1007/s10802-007-9178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske U, Tarter TE, Kirisci L, Gao Z, Reynolds M, Vanyukov M. Peer Environment Mediates Parental History and Individual Risk in the Etiology of Cannabis Use Disorder in Boys: A 10-Year Prospective Study. Am J Drug Alc Abuse. 2008;34:307–320. doi: 10.1080/00952990802013631. [DOI] [PubMed] [Google Scholar]
- 27.Laird RD, Pettit GS, Dodge KA, Bates JE. Change in parents monitoring knowledge: Links with parenting, relationship quality, adolescent beliefs, and antisocial behavior. Soc Develop. 2003;12:401–419. doi: 10.1111/1467-9507.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeVore ER, Ginsburg KR. The protective effects of good parenting on adolescents. Curr Opinion Pediat. 2005;17:460–465. doi: 10.1097/01.mop.0000170514.27649.c9. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd JJ, Anthony JC. Hanging out with the wrong crowd: how much difference can parents make in an urban environment? J Urban Health. 2003;80:383–399. doi: 10.1093/jurban/jtg043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newcomb MD, Bentler PM. The impact of family context, deviant attitudes, and emotional distress on adolescent drug use: Longitudinal latent-variable analyses of mothers and their children. J Res Person. 1988;22:154–176. [Google Scholar]
- 31.Newcomb MD, Loeb TB. Poor parenting as an adult problem behavior: General deviance, deviant attitudes, Inadequate family support and bonding, or just bad behavior? J Fam Psych. 1999;13:175–193. [Google Scholar]
- 32.Spitzer R, Williams B, Gibbon M. Manual for the Structured Clinical Interview for DSM-III-R (SCID, 4/1/87 revision) Biometrics Research Department, New York: New York State Psychiatric Institute; 1987. [Google Scholar]
- 33.Leckman J, Sholomaskas D, Thompson W. Best estimate of lifetime psychiatric diagnosis: A methodological study. Arch Gen Psych. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 34.Schludermann E, Schludermann S. Replicability of factors in children’s report of parent behavior inventory (CRPBI) J Psychol. 1970;76:239–249. [Google Scholar]
- 35.Jacob T, Seilhamer RA. Adaptation of the areas of change questionnaire for parent-child relationship assessment. Am J Fam Ther. 1985;13:28–38. [Google Scholar]
- 36.Loeber R. Supervision/Involvement Scale. Pittsburgh Youth Study. Department of Psychiatry, University of Pittsburgh; 1989. [Google Scholar]
- 37.Skinner HA, Steinhauer PD, Santa Barbara J. The family assessment measure. Canadian J Com Mental Health. 1983;2:91–105. [Google Scholar]
- 38.Tellegen A. A Manual for the Differential Personality Questionnaire. University of Minnesota; 1982. unpublished manuscript. [Google Scholar]
- 39.Feske U, Tarter R, Kirisci L, Gao Z, Reynolds M, Vanyukov M. Peer environment mediates parental history and individual risk in the etiology of cannabis use disorder in boys: A 10-year prospective study. Am J Drug Alc Abuse. 2008;34:367–320. doi: 10.1080/00952990802013631. [DOI] [PubMed] [Google Scholar]
- 40.Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alc Abuse. 1990;16:1–46. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- 41.West G, Aiken L. Toward understanding effects in multi-component prevention programs: design and analyses strategies. In: Bryant K, Windle M, West S, editors. The Science of Prevention. Baltimore, MD: American Psychological Association; 1997. pp. 167–209. [Google Scholar]
- 42.Ghahramani Z. Graphical models: parameter learning. In: Arbib MA, editor. Handbook of Brain Theory and Neural Networks. 2. MIT Press; 2002. [Google Scholar]
- 43.Eiden RD, Colder C, Edwards EP, Leonard KE. A longitudinal study of social competence among children of alcoholic and non alcoholic parents: Role of parental psychopathology, parental warmth and dysregulation. Psychol Addict Behav. 2009;23:36–46. doi: 10.1037/a0014839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: a maturational perspective. Develop Psychopath. 1999;11:657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- 45.McNamee R, Dunfee K, Luna B, Clark D, Eddy W, Tarter R. Neural activation, response inhibition, and increased risk for substance use disorder: A pilot fMRI study. Alc: Clin Exp Res. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 46.Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents “at risk”: Evidence of frontal cortex dysfunction in conduct disorder. Soc Biol Psychiat. 2001;50:600–608. doi: 10.1016/s0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- 47.Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, Mielke M, McGill S, Rebok GW, Seeman T, Fried LP. Evidence for neurocognitive plasticity in at-risk older adults: The Experience Corps Program. J Geron, Bio Science Med Science. 2009;10:1093–1099. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63:127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNab F, Varrone A, Farde L, Jucaite A, Bystrisky HF, Klinberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 50.Vaughn MG, Beaver KM, DeLisi M, Perron BE, Schelbe L. Gene-environment interplay and the importance of self-control in predicting polydrug use and substance-related problems. Addict Behav. 2009;34:112–116. doi: 10.1016/j.addbeh.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Giedd J, Coffey C. Neuroimaging 1: Anatomic imaging of the developing human brain. In: Coffey C, Brumbach R, editors. Textbook of Pediatric Neuropsychiatry. American Psychiatric Press; Washington, DC: 1998. [Google Scholar]
- 52.Wagner RA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alc Depend. 2007;86:191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Forman DR, Kochanska G. Viewing imitation as child responsiveness: A link between teaching and discipline domains of socialization. Develop Psych. 2001;37:198–206. doi: 10.1037/0012-1649.37.2.198. [DOI] [PubMed] [Google Scholar]