Abstract
Ketogenic diets are high in fat and low in carbohydrates, and have long been used as an anticonvulsant therapy for drug-intractable and pediatric epilepsy. Additionally, ketogenic diets have been shown to provide neuroprotective effects against acute and chronic brain injury, including beneficial effects in various rodent models of neurodegeneration. Huntington’s disease is a progressive neurodegenerative disease characterized by neurological, behavioral and metabolic dysfunction, and ketogenic diets have been shown to increase energy molecules and mitochondrial function. We tested the effects of a ketogenic diet in a transgenic mouse model of Huntington’s disease (R6/2 1J), with a focus on life-long behavioral and physiological effects. Matched male and female wild-type and transgenic mice were maintained on a control diet or were switched to a ketogenic diet fed ad libitum starting at six weeks of age. We found no negative effects of the ketogenic diet on any behavioral parameter tested (locomotor activity and coordination, working memory) and no significant change in lifespan. Progressive weight loss is a hallmark feature of Huntington’s disease, yet we found that the ketogenic diet - which generally causes weight loss in normal animals - delayed the reduction in body weight of the transgenic mice. These results suggest that metabolic therapies could offer important benefits for Huntington’s disease without negative behavioral or physiological consequences.
Keywords: body weight, ketones, lifespan, motor coordination, spontaneous alternation, sex differences, working memory
1. Introduction
Huntington’s disease (HD) is a heritable fatal disease caused by expansion of the glutamine (CAG) repeat region in the huntingtin protein [1]. The progressive motor dysfunction and cognitive/affective degradation were described over 125 years ago [2] and in the central nervous system, the mutated huntingtin protein causes neurodegeneration in the caudate, putamen and, to a lesser extent, the cerebral cortex. Huntingtin is expressed widely in the body, however, and peripheral effects of HD include a loss of body weight due to wasting of skeletal muscle and adipose tissue [3]. This weight loss occurs despite elevated appetite and elevated caloric intake. Both the central and peripheral symptoms of HD are progressive.
Though not a mitochondrial disorder per se, like other neurodegenerative disorders HD is associated with mitochondrial and energy metabolism defects. Numerous clinical studies of HD have found impaired energy metabolism in brain and skeletal muscle [4], deficits in ATP synthesis in skeletal muscle [5], reduced activity and/or levels of mitochondrial electron transport chain components in brain (particularly in the caudate and putamen) [6], increased oxidative stress [7], and abnormal mitochondrial calcium sensitivity and membrane potential [8]. Thus, treatments that target mitochondrial dysfunction or augment mitochondrial function might be helpful in HD.
The ketogenic diet (KD) is a high fat, low carbohydrate regimen that shifts the body’s main energy source from glucose to ketone bodies, and thus mimics metabolic changes found during fasting. Ketogenic strategies are used as treatments for drug-refractory and pediatric epilepsy and they have been used clinically continuously for more than 90 years; nevertheless mechanistic information remains ill-understood [9]. Ketones are an alternate source of acetyl-coenzyme A for the tricarboxylic acid cycle, and ATP is produced from them more efficiently than from pyruvate (the final step of glycolysis and the normal major source of acetyl-coenzyme A) [10]. Evidence is accumulating that ketogenic diets are effective against neural degeneration produced by various insults, including traumatic brain injury [11, 12], cerebral ischemia and hypoglycemia [13–15], mutated superoxide dismutase associated with amyotrophic lateral sclerosis [16], catecholaminergic neurotoxins [17, 18], and excitotoxins [19]. In some studies, treatment with ketones themselves (β-hydroxybutyrate, acetoacetate, acetone) protect against degeneration from these insults (for instance, [20, 21].
Ketogenic diets increase cellular energy levels and enhance mitochondrial function. Brain and muscle cells from KD-fed rodents have increased numbers of mitochondria, determined by histology or by proxies for number of mitochondria such as mitochondrial DNA content [22–24], as well as increased levels of high energy molecules [22, 25–27]. Mitochondrial respiration is increased [28] as is the expression of a number of mitochondrial proteins/genes [22, 28, 29]. The KD reverses age-related decrements in mitochondrial number and function [30–32], and is used to treat clinical and experimental mitochondriopathies [23, 33–36]. In mitochondria, the KD also diminishes oxidative stress [28, 32, 37–39]. Therefore, some aspects of neuroprotection offered by a KD might relate to the promotion of mitochondrial function and augmentation of high energy molecules.
Given that the KD offers neuroprotection, we hypothesized that it might reduce or delay some of the progressive central and peripheral symptoms of HD. Any promising HD therapy would need to demonstrate that it not worsen motor or cognitive functions, and does not shorten lifespan. Using male and female littermates that were wild-type (WT) or HD transgenic mice (R6/2 1J) we measured the effects of chronic ad libitum feeding of a control diet versus a KD on locomotor activity and coordination, working memory, and body weight at multiple time points across the lifespan, as well as lifespan itself.
2. Methods
2.0 Subjects and diet protocols
Male and female WT and R6/2 1J mice were used for these studies. The R6/2 1J mouse strain is a transgenic model of HD made by insertion of the mutation-bearing exon of human huntingtin [40]. Mice were bred at Trinity College from breeding pairs purchased from Jackson Laboratories (stock #002810; Bar Harbor, Maine, USA). At weaning (~21 d of age), all pups were tail-biopsied for in-house genotyping by standard techniques, and CAG repeat length analysis of transgenic mice was performed by Laragen (Los Angeles, California, USA); transgenic mice in this study had 172 ± 7 repeats (mean ± standard deviation). Weaned pups were housed in same-sex sibling cohorts (typically containing both wild-type (WT) and transgenic animals), and given a standard rodent pellet diet (Purina 5001) and water ad libitum until 6 wk of age. Water bottles were equipped with long, angled spouts to accommodate motor-impaired transgenic mice. Cages were supplied with wood chip bedding, cotton nesting material and plastic tubes and domes for enrichment. Mice were weighed regularly from 4 wk of age until the end of the experiment. Death dates of R6/2 1J mice were recorded; no WT mice died except at sacrifice. A subset of mice were not included in behavioral tests but were part of the lifespan analysis component of this study. Although females were likely vaginally patent by 4 wk of age, mice at this age are not fully sexually mature and are noted for irregular cycling. Therefore, we did not monitor estrus cycle in females. Also, testing times were assumed to occur randomly in relation to cycle stage across the many female squads, thus any experimental effects of diet and the transgene are unlikely to have been influenced by cycle stage.
At 6 wk of age, approximately half of the mice were changed to a KD (8% protein, #F3666; Bio-Serv, Frenchtown, New Jersey, USA). Both diets were provided ad libitum on the wire cage lid as well as in a dish on the cage floor. Also beginning at 6 wk of age, mice remaining on the control diet (CD) were provided with mash (water-softened pellet diet; provided in a dish in addition to normal pellets on the wire cage lid) to accommodate motor-impaired R6/2 1J mice. The KD is a soft formulation and was replaced daily on the wire cage lid and in a dish on the cage floor.
2.1 Behavioral Testing
Locomotor coordination was tested on an accelerating Rotarod (IITC Life Science, Woodland Hills, California, USA) at 4, 6, 8, 12 and 16 wk of age. The 6 wk test occurred just prior to any diet change. Animals were habituated in the testing room for 30 min and then received a 300 s warm-up trial at 4 rpm. Thirty min later, animals underwent 3 testing trials with linear acceleration from 4 to 40 rpm over 300 s with a 30 min intertrial interval. Latency to fall was recorded electronically. Testing occurred on two consecutive days. Results from all six test trials were averaged.
Spontaneous alternation and locomotor activity were tested one day after Rotarod testing using a custom Y-maze. The Y-maze consisted of three equidistant 45-x-10 cm arms with 21 cm-high walls surrounding a triangular center. Animals were habituated in the testing room for 30 min. Testing began once the animal was placed into the start arm and continued for five minutes; the order of arm entries was recorded. Total entry number was used as a measure of locomotor activity. Spatial working memory was measured by the percentage of possible spontaneous alternations (sequential entry into all three arms). As the maximum number of groups of three in a series is n-2, the percent spontaneous alternations is calculated as (number of spontaneous alternations/(total number of arm entries-2))*100. Data from mice that had two or fewer arm entries were not used for working memory analysis.
2.3 Physiological measures
As noted above, lifespan was recorded and body weights were measured regularly. A subset of mice from all groups, of which behavioral testing and body weights were not included in the overall analyses, were sacrificed at 12 wk of age for blood ketone (β-hydroxybutyrate) measurement. In addition, all WT mice were sacrificed at 16 wk of age for ketone measurement. Trunk blood was collected into ethylenediaminetetraacetic acid-containing tubes and centrifuged for plasma isolation. Plasma β-hydroxybutyrate was measured with a Precision Xtra monitor and ketone test strips (Abbott Laboratories, Abbott Park, Illinois, USA).
Body weight and behavioral data were assessed by two-way analysis of variance of the factors diet and time, and/or genotype and time, with Newman-Keuls post-hoc comparisons. Due to the progressive nature of the peripheral and central symptoms, effects of the time factor were common in the analyses, but were omitted from the Results for brevity and clarity. Plasma ketone levels were assessed with one-way analysis of variance or t-test. Lifespan was assessed by Kaplan-Meier log rank analysis, with Holm-Sidak post-hoc comparisons. Data are presented as mean ± standard error.
3. Results
3.1 Physiology
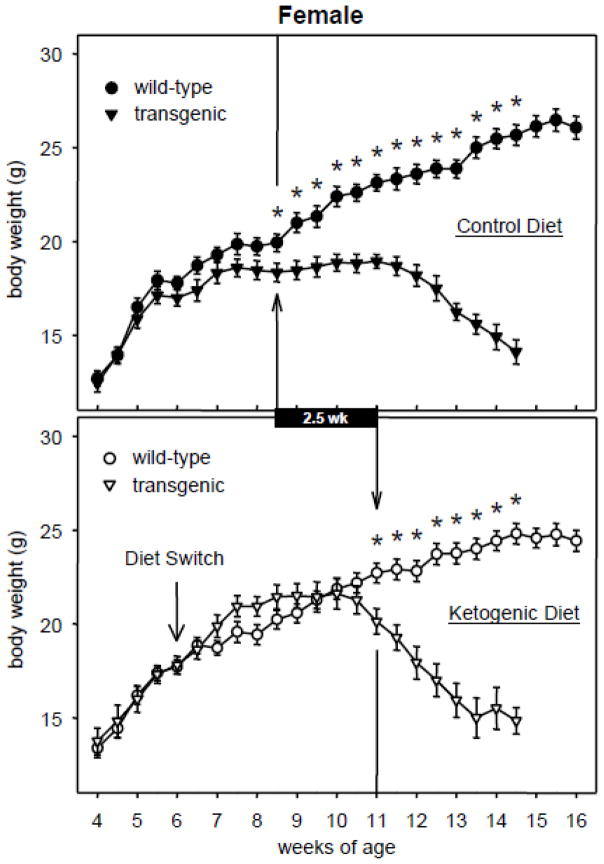
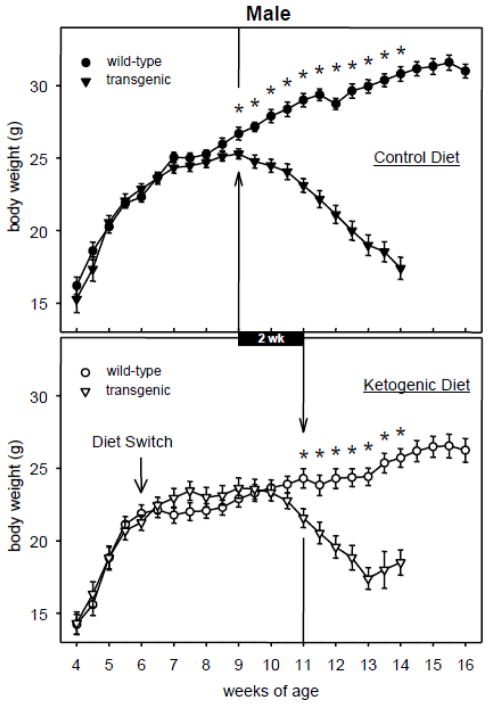
Body weights of male and female transgenic R6/2 1J mice fed either CD or KD rose along with those of WT mice in the earliest weeks after the diet protocol started (6 wk) then slowed their rate of increase and began to decrease until death several weeks later. The KD, however, modified this pattern significantly by delaying the onset of weight loss in transgenic mice (Fig. 1, 2). In females, CD-fed transgenic body weight dropped significantly below CD-fed WT body weight starting at 8.5 wk of age (Fig. 1). The corresponding effect in KD-fed females did not take place until 11 wk of age. Similarly, in males KD feeding delayed the onset of weight loss from 9 until 11 wk of age (Fig. 2). Thus the KD resulted in female and male R6/2 1J mice retaining body weight for an additional 2.5 and 2.0 weeks, respectively.
Fig. 1.
Effects of the KD and R6/2 1J transgene on body weight in female mice. In both panels, the vertical arrow/line indicates the first time point at which transgenic mice significantly differed from WT mice; subsequent time points were also significant (* p<.05). KD feeding delayed the onset of weight loss by 2.5 wk (black bar). WT data beyond 14.5 wk is illustrated but was not analyzed. At week 4, n = 14–21 per group. Number of transgenic mice at the last illustrated time points = 6–9 due to deaths. CD genotype effect F=533.4, p<.001; genotype-x-time effect F=19.7, p<.001. KD genotype effect F=136.1, p<.001; genotype-x-time effect F=17.3, p<.001.
Fig. 2.
Effects of the KD and R6/2 1J transgene on body weight in males. In both panels, the vertical arrow/line indicates the first time point at which transgenic mice significantly differed from WT mice; subsequent time points were also significant (* p<.05). KD feeding delayed the onset of weight loss by 2.0 wk (black bar). WT data beyond 14 wk is illustrated but was not analyzed. At week 4, n = 15–17 per group. Number of transgenic mice at the last illustrated time points = 4–10 due to deaths. CD genotype effect F=669.6, p<.001; genotype-x-time effect F=39.5, p<.001. KD genotype effect F=57.0, p<.001; genotype-x-time effect F=7.8, p<.001.
Plasma β-hydroxybutyrate levels were expectedly low in all CD-fed mice, but were elevated greatly in KD-fed mice of both genotypes, and significantly more so in transgenic mice (Table 1). These data show that KD-induced ketosis was maintained throughout the length of the experiment. The large sample size of the WT groups at 16 wk of age allowed analysis of a sex effect, and KD-fed female WT mice were found to have significantly higher β-hydroxybutyrate levels than male WT mice (1.63 ± 0.10 mM versus 1.13 ± 0.11 mM, p<.01; data not shown).
Table 1.
Plasma β-hydroxybutyrate levels (mM).
| Wild type, Control diet | Wild type, Ketogenic diet | R6/2 1J, Control diet | R6/2 1J, Ketogenic diet | |
|---|---|---|---|---|
| 12 wk of age (6 wk on diet) | 0.14 ± 0.04 | 1.31 ± 0.06§ | 0.22 ± 0.05 | 2.90 ± 0.48* § |
| 16 wk of age (10 wk on diet) | 0.15 ± 0.02 | 1.41 ± 0.08§ | NP | NP |
Plasma β-hydroxybutyrate levels were measured in available groups at 12 and 16 wks. Mice fed the KD showed a significant increase in β-hydroxybutyrate compared to CD, and the R6/2 1J mice showed a significantly higher β-hydroxybutyrate level than WT mice fed the KD. NP: not performed (virtually all transgenic mice died before 16 wk).
p<.001 compared to corresponding control diet group;
p<.001 compared to corresponding WT group. Number of subjects is 8–15 (12 wk) and 30–38 (16 wk).
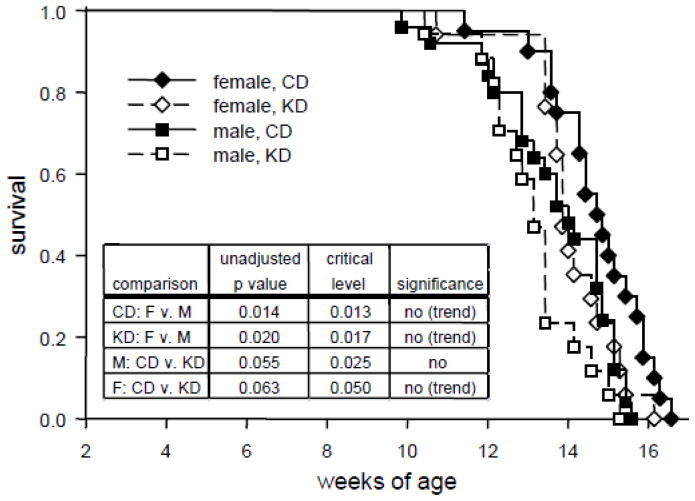
Consistent with previous reports [40–42], most transgenic mice died between 12 and 16 wk of age. Analysis of survival curves indicated that there was a significant overall effect of treatment groups, although effects were subtle and post hoc comparisons did not reach significance (Fig. 3). There were trends for earlier male mortality (as found in some prior reports: [41, 43]), regardless of diet treatment. There was a trend for the KD to reduce lifespan in females, but not males (Fig. 3).
Fig. 3.
Survival curves for all R6/2 1J transgenic mouse groups. Although there was a trend for females and males on the KD to die earlier than on the CD, and a general trend for males to die younger than females, none of these effects reached significance. Female CD n = 20; female KD n = 17; male CD n = 25; male KD n = 17. Overall log rank Z = 17.3, p<.001. Post-hoc p values and multiple comparison-adjusted critical levels are given in the inset table; p values must be lower than the corresponding critical p level to be considered significant in the Holm-Sidak test.
3.2 Behavior
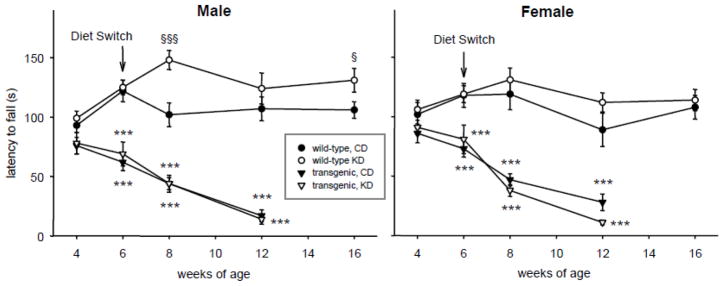
Motor coordination on the accelerating Rotarod was not different between WT and R6/2 1J at 4 wk of age. It was impaired significantly in transgenic animals by 6 wk of age and degraded steadily thereafter (Fig. 4). KD treatment neither alleviated nor worsened this progressive impairment significantly. In WT males, however, KD treatment enhanced motor coordination at some time points. This effect was not seen in WT females (Fig. 4).
Fig. 4.
Effects of the KD and R6/2 1J transgene on Rotarod performance. Transgenic mice progressively lost motor coordination regardless of diet or sex. The KD increased performance in male (but not female) WT mice at some time points (8 and 16 wk). Males: genotype effect in CD groups: genotype F=103.7, p=.001; genotype-x-time F=7.2, p<.001. Genotype effect in KD groups: genotype F=148.7, p<.001; genotype-x-time F=12.4, p<.001. Diet effect in WT groups: diet F=3.6, ns; diet-x-time F=4.5, p=.002. Diet effect in transgenic groups: diet F=0.1, ns; diet-x-time F=0.2, ns. Week 4 n = 15–17. Females: genotype effect in CD groups: genotype F=48.8, p<.001; genotype-x-time F=3.0, p=.035. Genotype effect in KD groups: genotype F=125.0, p<.001; genotype-x-time F=14.2, p<.001. Diet effects in WT groups: diet F=2.2, ns; diet-x-time F=0.4, ns. Diet effects in transgenic groups: diet F=0.4, ns; diet-x-time F=1.3, ns. Week 4 n = 14–21. *** p<.001 versus corresponding WT group. § p<.05, §§§ p<.001 versus corresponding CD group.
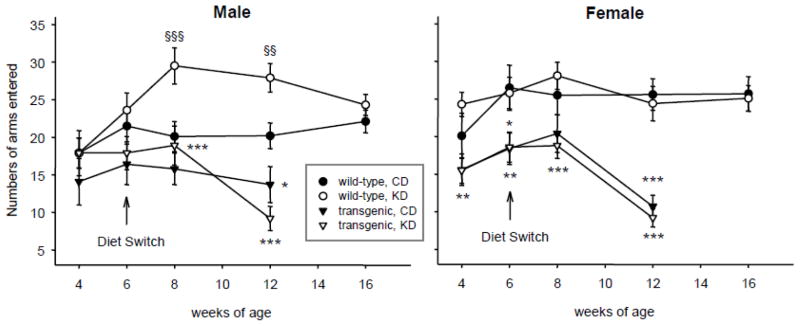
Male and female transgenic mice generally locomoted less during exploration in the Y-maze test. This was evident in females by 4 wk of age, and the decreased locomotor activity became robust in both sexes at 12 wk of age (Fig. 5). KD treatment neither alleviated nor worsened this impairment significantly in the R6/2 1J mice. In WT males, however, KD treatment promoted locomotion at 8 and 12 wk of age; this effect was gone by 16 wk of age, and was not found in WT females (Fig. 5).
Fig. 5.
Effects of the KD and R6/2 1J transgene on locomotion. Both male and female transgenic mice showed progressively reduced locomotion in the Y-maze regardless of diet. The KD increased locomotion in male (but not female) WT mice at early time points after the diet switch. Male: Genotype effects in CD groups: genotype F=103.7, p<.001; genotype-x-time F=7.2, p<.001. Genotype effects in KD groups: genotype F=106.2, p<.001; genotype-x-time F=8.0, p<.001. Diet effects in WT groups: diet F=12.1, p<.001; diet-x-time F=2.1, ns. Diet effects in transgenic groups: diet F=0.3, ns; diet-x-time F=1.0, ns. Females: genotype effects in CD groups: genotype F=48.8, p<.001; genotype-x-time F=3.0, p=.035. Genotype effects in KD groups: genotype F=125.0, p<.001; genotype-x-time F=14.2, p<.001. Diet effects in WT groups: diet F=0.4, ns; diet-x-time F=0.6, ns. Diet effects in transgenic groups: diet F=0.6, ns; diet-x-time F=1.0, ns. Number of subjects as in Fig. 4. * p<.05, ** p<.01, *** p<.001 versus corresponding WT group. §§ p<.01, §§§ p<.001 versus corresponding CD group.
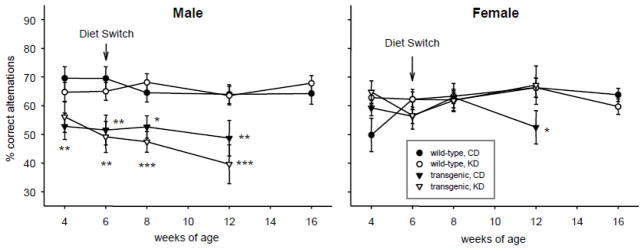
We found sex differences in spatial working memory in the R6/2 1J mice, as measured by spontaneous alternation in the Y-maze, and the effect of KD on working memory also differed between males and females. Male R6/2 1J mice had poor working memory in the Y-maze test which was evident starting at the first time point tested (4 wks). Female transgenic mice did not demonstrate significantly impaired working memory until 12 wks (Fig. 6). Feeding with KD did not alter working memory in R6/2 1J males, but completely reversed the impairment found at 12 wk in R6/2 1J females. KD treatment had no effect on working memory in WT mice of either sex (Fig. 6).
Fig. 6.
Effects of the KD and R6/2 1J transgene on spontaneous alternation. Male transgenic mice had impaired spontaneous alternation consistently from 6 wk of age, regardless of diet. Spontaneous alternation in female transgenic mice was impaired only at 12 wk, and this impairment was not found in KD-fed females. There was no effect of diet on the performance of WT mice. Males: Genotype effects in CD groups: genotype F=25.8, p<.001; genotype-x-time F=0.2, ns. Genotype effects in KD groups: genotype F=32.5, p<.001; genotype-x-time F=1.2, ns. Diet effects in WT groups: diet F=0.1, ns; diet-x-time F=0.8, ns. Diet effects in transgenic groups: diet F=0.8, ns; diet-x-interaction F=0.5, ns. Females: Genotype effects in CD groups: genotype F=0.7, ns; genotype-x-time F=3.0, p=.035. Genotype effects in KD groups: genotype F=0.5, ns; genotype-x-time F=0.2, ns. Diet effects in WT groups: diet F=1.0, ns; diet-x-time F=1.8, ns. Diet effects in transgenic groups: diet F=2.3, ns; diet-x-interaction F=1.2, ns. * p<.05, ** p<.01, *** p<.001 versus corresponding WT group.
4. Discussion
Here we report the effects of a KD on body weight, lifespan, locomotor behaviors and working memory in the R6/2 1J mouse model of HD. Overall, there were no adverse effects and some positive effects of the KD. There was no significant change in lifespan, no decrement in working memory, and no loss of motor activity or coordination in transgenic mice fed a KD vs. a control diet. Rather, there was a delayed onset of significant weight loss, a clinically relevant endpoint found consistently in male and female R6/2 1J mice, and reversal of a working memory deficit in females. In addition, we describe a novel sex disparity in working memory in R6/2 1J mice, and a KD-related promotion of motor function in male wild-type (WT) mice.
Apart from motor problems, progressive weight loss is a striking symptom of HD. Persons with HD have higher calorie intake but lower body weight and muscle and fat mass [44, 45], even early in the disease process [46]. Slow HD progression is related to higher body weight [47], and thus maintaining weight is important; some patients die from nutritional deficiencies [48]. In this study, the delayed onset of progressive weight loss (delayed by 2–2.5 wk) is particularly striking: KD treatment was started at 6 weeks, and R6/2 1J animals in this study died on average at 14 wk of age. Therefore, an additional 2–2.5 wk of maintained body weight is a substantial portion of the timeframe of disease progression in this mouse model of HD. Here, while a KD alleviated weight loss, the prolonged weight maintenance did not translate to extended lifespan.
Cognitive problems in HD are a major contributor to disability early in disease progression [49], and include working memory deficits [50, 51]. Concerning working memory in animals, spontaneous alternation is impaired in R6/1 mice [52], and deficits in rewarded alternation are found in R6/2 mice and a transgenic rat HD model [42, 53, 54]. Thus, working memory is impaired by mutant huntingtin. Whereas prior studies have found effects on alternation, these have rarely investigated males and females separately. Our study showed a strong impairment in R6/2 1J males, which was unaffected by the KD, and a mild impairment in R6/2 females, which was reversed by the KD. Our finding is a novel sex disparity in the R6/2 strain. The present results are encouraging in that we found no adverse effects and some positive effects on cognitive function. Clinical studies of Huntington’s disease patients in which men and women are analyzed separately for working memory problems (and weight loss) should be encouraged.
We did not find any significant effects of the KD on locomotor activity in the R6/2 1J mice - there was no worsening of motor and locomotor deficits. Overall this is a positive result - motor problems are severely disabling to HD patients (and to this mouse model of HD). A worsening of motor problems would not be tolerated in any emerging HD therapy or treatment strategy.
It should be noted that the transgenic mouse and the KD used in this study were quite stringent - a severe genetic model and a highly restrictive diet formulation. The transgenic R6/2 1J mice have a high CAG repeat length. To compare to humans, a CAG repeat length over ~39 produces HD (over ~55 is considered severe, and higher CAG repeat length corresponds with an earlier onset [55, 56]). R6/2 1J mice exhibit a dramatically progressive deterioration until a very premature death, usually before 16 wk of age [40], consistent with our observations. Coupled with the severity of this transgenic mouse model, the KD formulation used here is stricter than the most common clinical formulas, but is used often for research. Even though the KD was fed ad libitum, it contains a >6:1 ratio of fat:(carbohydrate + protein). For comparison, a typical clinical application of a KD is no higher than a 4:1 ratio, but could be as low as 2:1. Nevertheless, using this severe mouse model/strict diet combination we found no worsening of progressive cognitive and locomotor effects induced by the transgene and found positive effects on working memory and body weight.
In addition to novel findings of the KD in the R6/2 1J model of HD - some effects were consistent between males and females and others only in females - we found novel sex-dependent effects of the KD in the WT mice. Increased locomotion and Rotarod performance were observed in male WT mice at some time points. Prior studies suggest that the KD produces locomotor hypoactivity initially in male mice, which switches to hyperactivity by 4 wk of diet treatment [57, 58]. Our study shows that hyperactivity appears by 2 wk of diet treatment, and that it is restricted to males. Motor coordination was also enhanced in KD-fed WT male mice at some time points. The lack of KD-related increased locomotor activity or enhanced motor coordination in females was not due to lower ketosis (in fact, higher blood ketones were measured in KD-fed WT females vs. WT males). Also, the lack of KD-enhanced locomotor activity or coordination in male transgenic mice was not due to lower ketosis (KD-fed R6/2 1J had higher plasma ketones than WT). The mechanism of the stronger ketosis in the transgenic mice remains speculative, but presumably are likely to relate to the mechanisms that produce the striking cachexia in these mice.
Given its clinical use, which appears to be increasing and broadening beyond its initial use in epilepsy, more work on the effects of KD on behavior and cognition is needed. Reported cognitive effects of the KD in animal models range from positive [59, 60] to negative [61, 62] to a majority reporting no significant effects as in our WT mice (for instance [63–65]). Some of these disparities are likely due to use of differing tasks, species, or diet treatment length. Yet it seems clear that a KD does not indiscriminately impair cognitive function. Reported effects of the KD in non-epileptic humans are also mixed: One study found a transient, moderate impairment in one of three cognitive tasks present at one week of diet treatment but not at later time points [66]. Yet two other studies examining chronic KD treatments reported improved processing speed and working memory lasting up to one year [67, 68]. In general, characterizing the relationship between a KD and cognition is vital, particularly as its main target group is pediatric patients.
Based on this study we are unable to determine how the KD delays weight loss or reverses mild cognitive deficits in this mouse model of HD. However, the diverse evidence for improved mitochondrial function and levels of high energy molecules, corresponding to similar energy-related deficits in HD, make these mechanisms likely candidates. Other neuroprotective mechanisms associated with a KD are increased purines, particularly adenosine, and decreased oxidative stress [9, 25, 28]. Ultimately a combination of these mechanisms mobilized by a KD could delay progression of a neurodegenerative disease. The present results are particularly promising in that even using a severe transgenic model (with high CAG repeat length and rapid disease progression) and a very strict KD we did not see any detrimental effects. The positive effects observed here (particularly on body weight) might be even more significant, or found at more time points, and additional positive effects might emerge with a more clinically-relevant combination – i.e. a less severe HD model and a more liberal KD formulation. Additional research into the benefits of metabolic therapy in HD should parallel the extension of the usefulness of this emerging therapy into a host of disorders, such as epilepsy, cancer, pain and brain injury.
Acknowledgments
Supported by CHDI Foundation, NSF (IOS0843585), NIH (P20RR017699 from NCRR, R15NS065446, R15NS066392, R01NS065957) and Trinity College. We thank Jenny Nord, David J. Patrick, Tracey Suter and Julia Svedova for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Huntington’s Research Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Huntington G. On chorea. Med Surg Report. 1872;26:317–21. [Google Scholar]
- 3.van der Burg JMM, Björkqvist M, Brundin P. Beyond the brain: widespread pathology in Huntington’s disease. Lancet Neurol. 2009;8:765–74. doi: 10.1016/S1474-4422(09)70178-4. [DOI] [PubMed] [Google Scholar]
- 4.Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann. Neurol. 1997;41:160–5. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- 5.Lodi R, Schapira AHV, Manners D, Styles P, Wood NW, Taylor DJ, et al. Abnormal in vivo skeletal muscle energy metabolism in Huntington’s disease and dentatorubropallidoluysian atrophy. Ann Neurol. 2000;48:72–6. [PubMed] [Google Scholar]
- 6.Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann Neurol. 1996;39:385–9. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 7.Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MMK, et al. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–53. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 8.Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr, et al. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat Med. 1999;5:1194–8. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- 9.Masino SA, Kawamura M, Jr, Wasser CD, Pomeroy LT, Ruskin DN. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7:257–68. doi: 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413–20. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- 12.Hu ZG, Wang HD, Jin W, Yin HX. Ketogenic diet reduces cytochrome c release and cellular apoptosis following traumatic brain injury in juvenile rats. Ann Clin Lab Sci. 2009;39:76–83. [PubMed] [Google Scholar]
- 13.Tai KK, Nguyen N, Pham L, Truong DD. Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J Neural Transm. 2008;115:1011–7. doi: 10.1007/s00702-008-0050-7. [DOI] [PubMed] [Google Scholar]
- 14.Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, et al. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–16. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada KA, Rensing N, Thio LL. Ketogenic diet reduces hypoglycemia-induced neuronal death in young rats. Neurosci Lett. 2005;385:210–4. doi: 10.1016/j.neulet.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci. 2010;42:145–53. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- 18.Cheng B, Yang X, Chen C, Cheng D, Xu X, Zhang X. D-β-hydroxybutyrate prevents MPP+-induced neurotoxicity in PC12 cells. Neurochem Res. 2010;35:444–51. doi: 10.1007/s11064-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 19.Noh HS, Kim YS, Kim YH, Han JY, Park CH, Kang AK, et al. Ketogenic diet protects the hippocampus from kainic acid toxicity by inhibiting the dissociation of Bad from 14–3-3. J Neurosci Res. 2006;84:1829–36. doi: 10.1002/jnr.21057. [DOI] [PubMed] [Google Scholar]
- 20.Izumi Y, Ishii K, Benz AM, Zorumski CF. β-hydroxybutyrate fuels synaptic function during development - Histological and physiological evidence in rat hippocampal slices. J Clin Invest. 1998;101:1121–32. doi: 10.1172/JCI1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97:5440–4. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 23.Ahola-Erkkilä S, Carroll C, Peltola-Mjösund K, Tulkki V, Mattila I, Seppänen-Laasko T, et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Gen. 2010;19:1974–84. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- 24.Al-Zaid NS, Dashti HM, Mathew TC, Juggi JS. Low carbohydrate ketogenic diet enhances cardiac tolerance to global ischemia. Acta Cardiol. 2007;62:381–9. doi: 10.2143/AC.62.4.2022282. [DOI] [PubMed] [Google Scholar]
- 25.DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–7. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 26.Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–80. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- 27.Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40:703–7. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–80. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 29.Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM, et al. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Molec Brain Res. 2004;129:80–7. doi: 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Balietti M, Giorgetti B, Di Stefano G, Casoli T, Platano D, Solazzi M, et al. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron. 2010;41:143–8. doi: 10.1016/j.micron.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Balietti M, Fattoretti P, Giorgetti B, Casoli T, Di Stefano G, Solazzi M, et al. A ketogenic diet increases succinic dehydrogenase activity in aging cardiomyocytes. Ann NY Acad Sci. 2009;1171:377–84. doi: 10.1111/j.1749-6632.2009.04704.x. [DOI] [PubMed] [Google Scholar]
- 32.Studzinski CM, MacKay WA, Beckett TL, Henderson ST, Murphy MP, Sullivan PG, et al. Induction of ketosis may improve mitochondrial function and decrease steady-state amyloid-β precursor protein (APP) levels in the aged dog. Brain Res. 2008;1226:209–17. doi: 10.1016/j.brainres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Kang HC, Kim HD, Lee YM, Han SH. Landau-Kleffner syndrome with mitochondrial respiratory chain-complex I deficiency. Pediatr Neurol. 2006;35:158–61. doi: 10.1016/j.pediatrneurol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Joshi CN, Greenberg CR, Mhanni AA, Salman MS. Ketogenic diet in Alpers-Huttenlocher syndrome. Pediatr Neurol. 2009;40:314–6. doi: 10.1016/j.pediatrneurol.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Seo JH, Lee YM, Lee JS, Kim SH, Kim HD. A case of Ohtahara syndrome with mitochondrial respiratory chain complex I deficiency. Brain Dev. 2010;32:253–7. doi: 10.1016/j.braindev.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Nylen K, Velazquez JLP, Likhodii SS, Cortez MA, Shen L, Leshchenko Y, et al. A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp Neurol. 2008;210:449–57. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106:1044–51. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- 38.Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238–44. doi: 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–64. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 41.Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, et al. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 42.Morton AJ, Hunt MJ, Hodges AK, Lewis PD, Redfern AJ, Dunnett SB, et al. A combination drug therapy improves cognition and reverses gene expression changes in a mouse model of Huntington’s disease. Eur J Neurosci. 2005;21:855–70. doi: 10.1111/j.1460-9568.2005.03895.x. [DOI] [PubMed] [Google Scholar]
- 43.Wood NI, Carta V, Milde S, Skillings EA, McAllister CJ, Ang YLM, et al. Responses to environmental enrichment differ with sex and genotype in a transgenic mouse model of Huntington’s disease. PLoS One. 2010;5:e9077. doi: 10.1371/journal.pone.0009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morales LM, Estévez J, Suárez H, Villalobos R, Chacín de Bonilla L, Bonilla E. Nutritional evaluation of Huntington disease patients. Am J Clin Nutr. 1989;50:145–50. doi: 10.1093/ajcn/50.1.145. [DOI] [PubMed] [Google Scholar]
- 45.Sanberg PR, Fibiger HC, Mark RF. Body weight and dietary factors in Huntington’s disease patients compared with matched controls. Med J Aust. 1981;1:407–9. doi: 10.5694/j.1326-5377.1981.tb135681.x. [DOI] [PubMed] [Google Scholar]
- 46.Djoussé L, Knowlton B, Cupples LA, Marder K, Shoulson I, Myers RH. Weight loss in early stage of Huntington’s disease. Neurology. 2002;59:1325–30. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- 47.Myers RH, Sax DS, Koroshetz WJ, Mastromauro C, Cupples LA, Kiely DK, et al. Factors associated with slow progression in Huntington’s disease. Arch Neurol. 1991;48:800–4. doi: 10.1001/archneur.1991.00530200036015. [DOI] [PubMed] [Google Scholar]
- 48.Lanska DJ, Lanska MJ, Lavine L, Schoenberg BS. Conditions associated with Huntington’s disease at death: a case-control study. Arch Neurol. 1988;45:878–80. doi: 10.1001/archneur.1988.00520320068017. [DOI] [PubMed] [Google Scholar]
- 49.Mayeux R, Stern Y, Herman A, Greenbaum L, Fahn S. Correlates of early disability in Huntington’s disease. Ann Neurol. 1986;20:727–31. doi: 10.1002/ana.410200613. [DOI] [PubMed] [Google Scholar]
- 50.Lange KW, Sahakian BJ, Quinn NP, Marsden CD, Robbins TW. Comparison of executive and visuospatial memory function in Huntington’s disease and dementia of Alzheimer type matched for degree of dementia. J Neurol Neurosurg Psychiatry. 1995;58:598–606. doi: 10.1136/jnnp.58.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington’s disease. Brain. 1996;119:1633–45. doi: 10.1093/brain/119.5.1633. [DOI] [PubMed] [Google Scholar]
- 52.Pang TYC, Stam NC, Nithianantharajah J, Howard JL, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience. 2006;141:569–84. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Lione LA, Carter RJ, Hunt MJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington’s disease mutation. J Neurosci. 1999;19:10428–37. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Hörsten S, Schmitt I, Nguyen HP, Holzmann C, Schmidt T, Walther T, et al. Transgenic rat model of Huntington’s disease. Hum Mol Gen. 2003;12:617–24. doi: 10.1093/hmg/ddg075. [DOI] [PubMed] [Google Scholar]
- 55.Ashizawa T, Wong LJC, Richards CS, Caskey CT, Jankovic J. CAG repeat size and clinical presentation in Huntington’s disease. Neurology. 1994;44:1137–43. doi: 10.1212/wnl.44.6.1137. [DOI] [PubMed] [Google Scholar]
- 56.Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, et al. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat Gen. 1993;4:387–92. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- 57.Mantis JG, Fritz CL, Marsh J, Heinrichs SC, Seyfried TN. Improvement in motor and exploratory behavior in Rett syndrome mice with restricted ketogenic and standard diets. Epilepsy Behav. 2009;15:133–41. doi: 10.1016/j.yebeh.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 58.Oishi K, Sakamoto K, Konishi M, Murata Y, Itoh N, Sei H. FGF21 is dispensable for hypothermia induced by fasting in mice. Neuroendocrinol Lett. 2010;31:101–5. [PubMed] [Google Scholar]
- 59.Pan Y, Larson B, Araujo JA, Lau W, de Rivera C, Santana R, et al. Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br J Nutr. 2010;103:1746–54. doi: 10.1017/S0007114510000097. [DOI] [PubMed] [Google Scholar]
- 60.Xu K, Sun X, Eroku BO, Tsipis CP, Puchowicz MA, LaManna JC. Diet-induced ketosis improves cognitive performance in aged rats. Adv Exp Biol Med. 2010;662:71–5. doi: 10.1007/978-1-4419-1241-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su SW, Sogawa MRCY, Silveira DC, Holmes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Dev Brain Res. 2000;125:131–8. doi: 10.1016/s0165-3806(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]
- 63.Silva MC, Rocha J, Pires CS, Ribeiro LC, Brolese G, Leite MC, et al. Transitory gliosis in the CA3 hippocampal region in rats fed on a ketogenic diet. Nutr Neurosci. 2005;8:259–64. doi: 10.1080/10284150500475032. [DOI] [PubMed] [Google Scholar]
- 64.Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–40. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 65.Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–8. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 66.Wing RR, Vazquez JA, Ryan CM. Cognitive effects of ketogenic weight-reducing diets. Int J Obes Relat Metab Disord. 1995;19:811–6. [PubMed] [Google Scholar]
- 67.Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Int Med. 2009;169:1873–80. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- 68.Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, et al. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. Am J Clin Nutr. 2007;86:580–7. doi: 10.1093/ajcn/86.3.580. [DOI] [PubMed] [Google Scholar]