Abstract
The incidences of esophageal adenocarcinoma and squamous cell carcinoma (SCC) are higher in males than in females. We investigated whether female-related hormonal factors are associated with risks of these two types of esophageal cancer. We examined the association between use of hormone therapy (HT) and the risks of esophageal adenocarcinoma and SCC in postmenopausal women enrolled in the Women's Health Initiative (WHI) clinical trials and observational studies. Twenty-three esophageal adenocarcinoma and 34 esophageal SCC cases were confirmed among the 161,080 participants, after a median of 11.82 years of follow-up. Risk of esophageal SCC was lower among HT users (past users: Hazard Ratio [HR]=0.25, 95% Confidence Interval [CI]: 0.06–1.10 in 2 cases; current users: HR=0.41, 95% CI: 0.18–0.94 in 9 cases). A decreased esophageal SCC risk was observed for current users of estrogen plus progestin (E+P) therapy (HR=0.25, 95% CI: 0.07–0.86 in 3 cases) but not for current users of estrogen-only therapy (HR=0.96, 95% CI: 0.28–3.29 in 6 cases). No association was observed between the use of HT and the risk of esophageal adenocarcinoma. No other reproductive or hormonal factors were significantly associated with the risk of either SCC or adenocarcinoma. Current use of E+P therapy was found to be associated with a decreased risk of esophageal SCC, but no association was observed with esophageal adenocarcinoma. To provide more definitive evidence, a pooled analysis of all available studies or a much larger study would be needed.
Introduction
Esophageal adenocarcinoma has increased dramatically in incidence in many western countries during the last four decades (1, 2), and is now the most common histological type of esophageal cancer in the U.S. (3). Esophageal adenocarcinoma is about seven times more common in males than females (4), for reasons that are largely unknown. This has led to speculation that sex hormones might play an important role in the disease. Support for this notion comes from studies which observed overexpression of estrogen receptors alpha and beta in esophageal malignancies (5, 6).
Few epidemiological studies have explored the association of hormonal-related risk factors and esophageal adenocarcinoma (7–12), and results are conflicting. The most recent study conducted in a cohort of 201,506 women observed a 19% (Hazard Ratio [HR]=0.81, 95% Confidence Interval [CI]: 0.59–1.12) lower risk of gastric adenocarcinoma, which included esophageal adenocarcionma, among subjects who used hormone therapy (HT), with a 48% (HR=0.52, 95% CI: 0.26–1.07) lower risk in a subset of women with intact uterus who were users of estrogen plus progestin (E+P) HT (9). These findings contrast with others that have reported either an increased risk of esophageal adenocarcinoma (7) or no association with HT use (11, 12). In another study, breastfeeding was associated with a significant 59% (95% CI: 18%–80%) lower risk of esophageal adenocarcinoma (8).
Indirect supporting evidence of the relation between hormones and esophageal adenocarcinoma comes from studies of HT and the occurrence of symptomatic gastroesophageal reflux, which is a known risk factor for esophageal adenocarcinoma. In the Women’s Health Initiative (WHI) HT trial, women randomized to estrogen (E), but not to E+P, had a higher incidence of reflux (13). Similar results were found in a study of twins, in which ever-users of estrogen therapy had significantly more reflux symptoms than non-users (14). Finally, a Norwegian study reported that the link between obesity, which is also a strong risk factor for esophageal adenocarcinoma, and reflux was much stronger among women who used HT (15).
Esophageal squamous cell carcinoma (SCC) differs from esophageal adenocarcinoma in its site of origin and etiology (16). Esophageal SCC occurs mainly in the middle and upper portion of the esophagus, as opposed to distally where most esophageal adenocarcinoma cases are found (17). During the last decades, there has been a slight decrease in incidence of esophageal SCC in the US. This histological type of esophageal cancer has a much smaller male-to-female ratio than that of esophageal adenocarcinoma (4). It has been hypothesized that this smaller ratio might partially be explained by the different patterns of smoking and alcohol drinking, the two strongest risk factors for esophageal SCC, between males and females. However, Freedman et al. reported that users of HT had a reduced risk of esophageal SCC compared to never users (9). This inverse association was also observed in an analysis of three small case-control studies which, in addition, observed a significantly lowered risk of esophageal SCC among users of oral contraceptives (18).
From the foregoing discussion, it is clear that a better understanding of the role of hormones in esophageal cancer is needed. Using information from the WHI clinical trials (CTs) and observational study (OS), we examined the association between hormonal factors and the risks of esophageal adenocarcinoma and SCC.
Methods
Details of the WHI study design have been described previously (19, 20). Briefly, postmenopausal women, age 50 to 79, were recruited in 40 clinical centers in the US between October 1, 1993 and December 31, 1998. The WHI included four randomized controlled CTs to test the effects of use of E-alone or E+P (HT trials), calcium plus vitamin D, and a low-fat dietary pattern on several outcomes. All women in the HT trials who were users of HT at the time of recruitment were required to have a 3-month washout period before enrollment in the trial. The WHI OS was designed to obtain detailed information on a full range of lifestyle and medical factors in postmenopausal women and observed the disease outcomes after a follow-up period for comparison with the CT results. Overall, there were 161,808 women enrolled in the WHI, including 27,347 enrolled in the HT trials and 134,461 enrolled in the OS or non-HT trials.
Data collection
All study participants completed baseline questionnaires with detailed information on demographic characteristics, lifestyle, and reproductive and medical history. Medication use was assessed by interviewer-administered questionnaire. Subjects had their weight, height, and waist and hip circumferences measured at baseline by study staff. One of our primary exposures of interest was HT use, which was defined relative to baseline in the OS and non-HT trials and randomization in the HT trial. Specifically, current users of HT were women using HT at baseline in the OS and non-HT trials or assigned to an active intervention arm in the HT trials. Past users of HT were women not using HT at baseline in the OS and non-HT trials but that had done so before enrollment, or women assigned to the placebo arm in the HT trial but who were users of HT before randomization. Never users of HT were women who had not used HT before baseline in the OS and the non-HT trials or women assigned to the placebo arm in the HT trial that had never used HT before randomization. The type of current HT use was defined as the one reported at baseline in the OS and the non-HT trials or the one assigned in the active intervention arm if in the HT trial. Duration of HT use was defined as the number of years using HT before baseline or randomization. All other exposures were analyzed as reported at baseline.
Original reports of clinical outcomes including cancer were obtained by self-administered questionnaires, annually in the OS and biannually in the CTs. All cases were confirmed by medical record and pathology report review and subsequently adjudicated at the clinical coordinating center according to SEER guidelines (National Cancer Institute. Surveillance, Epidemiology, and End Results program. http://seer.cancer.gov/. Accessed October 4, 2010). Deaths were verified and cause of death was attributed following medical record review at the clinical coordinating center. In addition, the National Death Index (NDI) was run on participants at 2 to 3 year intervals.
Seven hundred and twenty-two women had missing follow-up time and were excluded from the analysis, leaving 161,086 women (Figure 1). As of 14 August 2009, 63 esophageal cancer cases have been confirmed in the WHI dataset. Thirty-four of them were classified according to the International Classification of Disease for Oncology (ICD-O) as SCC (ICD-O codes: 8070–8083), 23 as adenocarcinomas (ICD-O code: 8140) and 6 had an unspecified histology (ICD-O codes: 8000–8033). See Figure 1. The 6 cases with unspecified histology were removed from the analyses.
Figure 1.
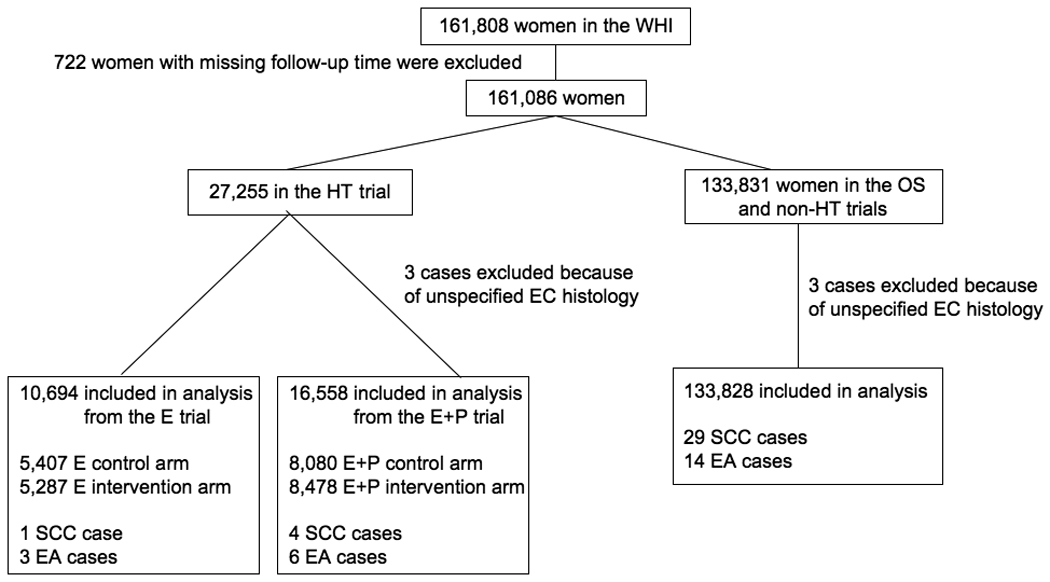
Flow diagram of study participants included in the analysis. EC: Esophageal cancer; E: Estrogen alone; E+P: estrogen plus progestin.
Statistical analysis
Cox regression was used to compute HRs and corresponding 95% CI as a measure of association between potential risk factors and incidence of esophageal adenocarcinoma or SCC separately. Time to diagnosis was computed from randomization in the HT trial or from enrollment in the OS and non-HT trials to diagnosis, with censoring defined by last follow-up contact, death, or 14 August 2009, whichever came first. In analysis regarding esophageal adenocarcinoma risk, SCC cases were censored at the time of diagnosis and vice versa. Similarly, in analyses of mortality, time to death was computed from date of randomization in the HT trial or enrollment in the OS and non-HT trials, with censoring defined by date of loss-to-follow-up, or 14 August 2009, whichever came first. For some subjects who stopped follow-up, death information was obtained from the NDI search. Death after esophageal cancer diagnosis was assumed to be an esophageal cancer death. All other deaths were considered to be censored observations.
All analyses were adjusted for age at baseline, hysterectomy status and study type (HT trials/OS or non-HT trials) by stratifying the baseline hazard function in the Cox model. Analyses regarding the risk of esophageal adenocarcinoma were also adjusted for BMI, heartburn and white race (white/non-white). Analyses of the risk of esophageal SCC were adjusted for pack-years of smoking and drinking. Age at menopause was defined as the age at which a woman had the last menstrual bleeding, a bilateral oophorectomy, or began using HT. Other potential confounders analyzed did not change the risk estimates by more than 10% and were not included in the models. Numbers presented in the tables are for women with no-missing values for the exposure of interest and corresponding adjusting variables. Trend p-values were obtained as the p-value associated with the corresponding continuous variable in the Cox model. All analyses were done by using STATA, version 10.1, software (StataCorp LP, College Station, Texas) for Macintosh.
Results
A total of 161,080 women were included in this analysis with a median follow-up time of 11.82 years (inter-quartile range of 9.02–12.89 years) and person-years of 1,730,836.8. During the follow up period, 23 women were diagnosed with esophageal adenocarcinoma and 34 with esophageal SCC.
Characteristics of the study participants are shown in Table 1. Compared with never users, current users of HT were younger, of white race, more likely to have a college degree and lower body mass index (BMI). These two groups of women were similar in symptomatic heartburn, cigarette and alcohol use. Past users were more likely to be white and have lower BMI than never users.
Table 1.
Distribution of characteristics of subjects by use of HT.
| Characteristics | Never users (N=61,473) n (%) |
Past users only (N=23,482) n (%) |
Current users (N=75,986) n (%) |
|---|---|---|---|
| Age (years) | |||
| 50–59 | 17,084 (27.8) | 6,348 (27.0) | 29,783 (39.2) |
| 60–69 | 28,185 (45.9) | 10,376 (44.2) | 33,670 (44.3) |
| 70–79 | 16,204 (26.4) | 6,758 (28.8) | 12,533 (16.5) |
| Race | |||
| White | 48,373 (78.7) | 19,680 (83.8) | 64,975 (85.5) |
| Black | 7,736 (12.6) | 1,994 (8.5) | 4,744 (6.2) |
| Other | 5,200 (8.5) | 1,733 (7.4) | 6,098 (8.0) |
| Missing | 164 (0.3) | 75 (0.3) | 169 (0.2) |
| Education | |||
| High school or less | 15,672 (25.5) | 5,416 (23.1) | 14,896 (19.6) |
| Some college/vocational | 22,269 (36.2) | 9,476 (40.4) | 28,866 (38.0) |
| College graduate or more | 23,025 (37.5) | 8,417 (35.8) | 31,703 (41.7) |
| Missing | 507 (0.8) | 173 (0.7) | 521 (0.7) |
| Income | |||
| Less than $19,999 | 12,070 (19.6) | 4,075 (17.4) | 9,049 (11.9) |
| $20,000 to $34,999 | 14,804 (24.1) | 5,867 (25.0) | 15,820 (20.8) |
| $35,000 to $49,999 | 11,419 (18.6) | 4,439 (18.9) | 14,940 (19.7) |
| $50,000 or more | 18,364 (29.9) | 7,518 (32.0) | 31,817 (41.9) |
| Missing | 4,816 (7.8) | 1,583 (6.7) | 4,360 (5.7) |
| BMI (Kg/m2) | |||
| < 18.5 | 582 (1.0) | 203 (0.9) | 608 (0.8) |
| 18.5–24.9 | 18,439 (30.0) | 7,754 (33.0) | 28,514 (37.5) |
| 25.0–29.9 | 20,722 (33.7) | 8,360 (35.6) | 26,342 (34.7) |
| 30.0–34.9 | 12,343 (20.1) | 4,329 (18.4) | 12,884 (17.0) |
| ≥ 35.0 | 8,755 (14.2) | 2,633 (11.2) | 7,093 (9.3) |
| Missing | 632 (1.0) | 203 (0.9) | 545 (0.7) |
| Symptoms of heartburn | |||
| Did not occur | 39,937 (65.0) | 14,395 (61.3) | 48,417 (63.7) |
| Mild | 15,558 (25.3) | 6,401 (27.3) | 19,957 (26.3) |
| Moderate | 4,177 (6.8) | 1,961 (8.4) | 5,622 (7.4) |
| Severe | 1,150 (1.9) | 528 (2.3) | 1,458 (1.9) |
| Missing | 651 (1.1) | 197 (0.8) | 532 (0.7) |
| Pack-years of cigarette use | |||
| Never | 31,838 (51.8) | 11,483 (48.9) | 37,680 (49.6) |
| < 4.9 | 8,226 (13.4) | 3,291 (14.0) | 11,296 (14.9) |
| 5–19.9 | 8,194 (13.3) | 3,350 (14.3) | 10,822 (14.2) |
| 20–39.9 | 6,342 (10.3) | 2,643 (11.3) | 8,225 (10.8) |
| ≥ 40 | 4,859 (7.9) | 1,979 (8.4) | 5,497 (7.2) |
| Missing | 2,014 (3.3) | 736 (3.1) | 2,466 (3.3) |
| Alcohol use | |||
| Non-drinker | 7,647 (12.4) | 2,519 (10.7) | 7,343 (9.7) |
| Past drinker | 12,467 (20.3) | 4,684 (20.0) | 12,769 (16.8) |
| < 1 drink/week | 19,999 (32.5) | 7,537 (32.1) | 25,067 (33.0) |
| 1–7 drinks/week | 14,293 (23.3) | 5,784 (24.6) | 20,934 (27.6) |
| > 7 drinks/week | 6,533 (10.6) | 2,782 (11.9) | 9,378 (12.3) |
| Missing | 534 (0.9) | 176 (0.8) | 495 (0.7) |
Data might not add up to 100% because of rounding.
139 women had missing information on the use of HT and they were excluded from this table.
There was no evidence that HT use or other reproductive factors were associated with altered risk of esophageal adenocarcinoma (Table 2), with the exception of breastfeeding that was associated with a non-significant reduction of risk (HR=0.44, 95% CI: 0.18–1.07). In contrast with esophageal adenocarcinoma, women who had ever used HT were at a lower risk of esophageal SCC compare to never users. Specifically, past use of HT was associated with a 75% lower risk in 2 cases (HR=0.25, 95% CI: 0.06–1.10) and current use of HT was associated with a significant 59% lower risk in 9 cases (HR=0.41, 95% CI: 0.18–0.94). The reduced risk appears to be concentrated among baseline E+P users (HR=0.25, 95% CI: 0.07–0.86 in 3 cases) as no association was observed for E-alone (HR=0.96, 95% CI: 0.28–3.29, in 6 cases). A non-significant reduction of risk of esophageal SCC was also observed with the duration of use of E-alone and E+P (data not shown). No statistically significant evidence of effects on SCC risk was observed for other hormonal/reproductive factors (Table 2).
Table 2.
Adjusted hazard ratios of hormonal related risk factors by outcome.
| Esophageal adenocarcinoma | Esophageal SCC | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | Total number of women (N=161,080) n (%) |
Number of cases (N=23) n (%) |
HR* | [95% CI] | Total number of women (N=161,080) n (%) |
Number of cases (N=34) n (%) |
HR** | [95% CI] |
| Hormone therapy use | ||||||||
| Never users | 60,031 (38.1) | 9 (39.1) | 1.00 | ref. | 59,091 (38.2) | 21 (65.6) | 1.00 | ref. |
| Past users only | 22,998 (14.6) | 3 (13.0) | 0.74 | [0.20, 2.82] | 22,622 (14.6) | 2 (6.3) | 0.25 | [0.06, 1.10] |
| Current users | 74,704 (47.4) | 11 (47.8) | 0.87 | [0.35, 2.17] | 73,132 (47.2) | 9 (28.1) | 0.41 | [0.18, 0.94] |
| Type of hormone therapy (in current users) | ||||||||
| Never users | 60,031 (44.6) | 9 (45.0) | 1.00 | ref. | 59,109 (38.2) | 21 (70.0) | 1.00 | ref. |
| E-alone | 39,630 (29.4) | 5 (25.0) | 0.57 | [0.15, 2.13] | 38,846 (29.4) | 6 (20.0) | 0.96 | [0.28, 3.29] |
| E+P | 35,068 (26.0) | 6 (30.0) | 1.12 | [0.35, 3.59] | 34,280 (25.9) | 3 (10.0) | 0.25 | [0.07, 0.86] |
| Duration of hormone therapy use (years) | ||||||||
| Never users | 60,031 (40.3) | 9 (45.0) | 1.00 | ref. | 59,091 (40.4) | 21 (67.7) | 1.00 | ref. |
| < 10 | 54,215 (36.4) | 7 (35.0) | 0.94 | [0.34, 2.62] | 53,133 (36.4) | 3 (9.7) | 0.19 | [0.06, 0.64] |
| ≥ 10 | 34,623 (23.3) | 4 (20.0) | 0.72 | [0.20, 2.57] | 33,950 (23.3) | 7 (22.6) | 0.62 | [0.24, 1.56] |
| Ptrend=0.632 | Ptrend=0.093 | |||||||
| Use of oral contraceptives | ||||||||
| No | 92,222 (58.4) | 15 (65.2) | 1.00 | ref. | 90,630 (58.5) | 25 (78.1) | 1.00 | ref. |
| Yes | 65,641 (41.6) | 8 (34.8) | 0.92 | [0.37, 2.28] | 64,345 (41.5) | 7 (21.9) | 0.47 | [0.19, 1.15] |
| Number of term pregnancies | ||||||||
| None | 18,638 (11.9) | 2 (8.7) | 0.91 | [0.17, 5.02] | 18,351 (11.9) | 5 (15.6) | 0.65 | [0.22, 1.97] |
| 1–2 | 53,250 (33.9) | 9 (39.1) | 1.39 | [0.42, 4.59] | 52,160 (33.8) | 7 (21.9) | 0.34 | [0.12, 0.92] |
| 3–4 | 61,984 (39.5) | 8 (34.8) | 0.87 | [0.26, 2.90] | 60,889 (39.5) | 11 (34.4) | 0.46 | [0.19, 1.10] |
| ≥ 5 | 23,094 (14.7) | 4 (17.4) | 1.00 | ref. | 22,754 (14.8) | 9 (28.1) | 1.00 | ref. |
| Ptrend=0.681 | Ptrend=0.239 | |||||||
| Ever breastfed§ | ||||||||
| No | 57,194 (41.8) | 13 (61.9) | 1.00 | ref. | 56,018 (41.7) | 10 (37.0) | 1.00 | ref. |
| Yes | 79,709 (58.2) | 8 (38.1) | 0.44 | [0.18, 1.07] | 78,444 (58.3) | 17 (63.0) | 1.32 | [0.60, 2.89] |
| Number of months breastfed§ | ||||||||
| Never breastfed | 57,194 (41.9) | 13 (61.9) | 1.00 | ref. | 56,018 (41.8) | 10 (37.0) | 1.00 | ref. |
| 1–6 | 40,174 (29.4) | 3 (14.3) | 0.32 | [0.09, 1.13] | 39,483 (29.5) | 12 (44.4) | 1.73 | [0.75, 4.02] |
| ≥ 7 | 39,102 (28.7) | 5 (23.8) | 0.57 | [0.21, 1.60] | 38,539 (28.8) | 5 (18.5) | 0.84 | [0.29, 2.48] |
| Ptrend=0.183 | Ptrend=0.960 | |||||||
| Age at menopause (years) | ||||||||
| < 45 | 43,060 (29.0) | 4 (20.0) | 1.00 | ref. | 42,343 (29.0) | 8 (28.6) | 1.00 | ref. |
| 45–49 | 36,185 (24.4) | 7 (35.0) | 2.55 | [0.70, 9.30] | 35,552 (24.4) | 4 (14.3) | 0.51 | [0.14, 1.79] |
| 50–54 | 51,502 (34.7) | 5 (25.0) | 1.33 | [0.31, 5.78] | 50,652 (34.7) | 10 (35.7) | 0.86 | [0.29, 2.53] |
| ≥ 55 | 17,670 (11.9) | 4 (20.0) | 2.63 | [0.54, 12.77] | 17,328 (11.9) | 6 (21.4) | 1.36 | [0.40, 4.62] |
| Ptrend=0.450 | Ptrend=0.527 | |||||||
| Age at menarche (years) | ||||||||
| < 12 | 18,428 (14.0) | 4 (19.1) | 1.00 | ref. | 18,187 (14.0) | 6 (20.7) | 1.00 | ref. |
| 12 | 27,470 (20.8) | 6 (28.6) | 1.07 | [0.30, 3.79] | 27,003 (20.8) | 9 (31.0) | 0.99 | [0.35, 2.80] |
| 13 | 34,365 (26.0) | 4 (19.1) | 0.59 | [0.15, 2.36] | 33,824 (26.0) | 4 (13.8) | 0.35 | [0.10, 1.24] |
| 14 | 22,826 (17.3) | 3 (14.3) | 0.70 | [0.15, 3.15] | 22,474 (17.3) | 5 (17.2) | 0.65 | [0.20, 2.14] |
| ≥ 15 | 29,041 (22.0) | 4 (19.1) | 0.72 | [0.18, 2.91] | 28,532 (21.9) | 5 (17.2) | 0.51 | [0.16, 1.69] |
| Ptrend=0.482 | Ptrend=0.169 | |||||||
| Age at first term pregnancy (years) | ||||||||
| < 20 | 20,112 (16.1) | 4 (21.1) | 1.43 | [0.42, 4.85] | 19,779 (16.1) | 2 (8.0) | 0.44 | [0.10, 1.96] |
| 20–24 | 59,431 (47.7) | 8 (42.1) | 1.00 | ref. | 58,455 (47.7) | 13 (52.0) | 1.00 | ref. |
| 25–29 | 33,407 (26.8) | 5 (36.3) | 1.26 | [0.41, 3.87] | 32,833 (26.8) | 5 (20.0) | 0.67 | [0.24, 1.90] |
| ≥ 30 | 11,751 (9.4) | 2 (10.5) | 1.52 | [0.32, 7.22] | 11,501 (9.4) | 5 (20.0) | 1.91 | [0.67, 5.45] |
| Ptrend=0.883 | Ptrend=0.195 | |||||||
Numbers only for women with no-missing values for the exposure of interest and corresponding adjusting variables.
Adjusted for BMI (continuous), heartburn (did not occur/mild/moderate/severe), White race (white/non-white) and stratified by age (50–59/60–69/70–79), hysterectomy status and study type (HT trial/OS or non-HT trials).
Adjusted for pack-years of smoking (never/<5/5–19.9/20–39.9/≥40), drinking (non-drinker/past drinker/<1 drink per week/1–7 drinks per week/>7 drinks per week), and stratified by age (50–59/60–69/70–79), hysterectomy status and study type (HT trial/OS or non-HT trials).
Adjusted for past use of HT (defined as non-current users relative to baseline in the OS and non-HT trials but reported use of HT before baseline; and users of HT before randomization in the HT trial but assigned to the placebo arm).
Adjsuted for past use of E (similar definition to past use of HT).
Adjusted for past use of P+E (similar definition to past use of HT).
Only women with at least one live birth.
For esophageal adenocarcinoma, each of the available measures of obesity was strongly associated with incidence (Table 3). Of the 23 cases of esophageal adenocarcinoma, 21 (91.3%) were in the top half of the distribution of the entire WHI cohort with regard to waist-to-hip ratio and waist circumference. Compared to those in the highest quartile, those in the lowest quartile of waist circumference experienced a 92% (95% CI: 37%–99%) lower incidence of esophageal adenocarcinoma (Ptrend<0.001). A similar trend was seen with BMI. Having mild, moderate or severe symptoms of heartburn were associated with approximately 2-fold, 3-fold and 6-fold increased risk of esophageal adenocarcinoma, respectively (Ptrend=0.009). Compared to never smokers, women who smoked over 40 pack-years were at a significantly increased esophageal adenocarcinoma risk (Ptrend=0.023).
Table 3.
Adjusted hazard ratios of other risk factors by outcome.
| Esophageal adenocarcinoma | Esophageal SCC | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | Total number of women (N=161,080) n (%) |
Number of cases (N=23) n (%) |
HR* | [95% CI] | Total number of women (N=161,080) n (%) |
Number of cases (N=34) n (%) |
HR** | [95% CI] |
| BMI (Kg/m2)† | ||||||||
| < 24.9 | 55,559 (35.2) | 2 (8.7) | 0.12 | [0.02, 0.64] | 54,120 (35.2) | 11 (34.4) | 1.00 | ref. |
| 25.0–29.9 | 54,810 (34.7) | 8 (34.8) | 0.42 | [0.14, 1.24] | 53,394 (34.8) | 14 (43.8) | 1.23 | [0.56, 2.72] |
| 30.0–34.9 | 29,227 (18.5) | 7 (30.4) | 0.66 | [0.22, 1.98] | 28,389 (18.5) | 5 (15.6) | 0.86 | [0.30, 2.50] |
| ≥ 35.0 | 18,270 (11.6) | 6 (26.1) | 1.00 | ref. | 17,762 (11.6) | 2 (6.3) | 0.59 | [0.13, 2.72] |
| Ptrend=0.005 | Ptrend=0.529 | |||||||
| Waist-to-hip ratio† | ||||||||
| Quartile 1 (x≤0.76) | 39,750 (25.1) | 1 (4.4) | 0.11 | [0.01, 0.83] | 38,730 (25.1) | 4 (12.9) | 0.50 | [0.16, 1.59] |
| Quartile 2 (0.76<x≤0.80) | 39,653 (25.0) | 1 (4.4) | 0.09 | [0.01, 0.73] | 38,611 (25.0) | 6 (19.4) | 0.64 | [0.23, 1.75] |
| Quartile 3 (0.80<x≤0.86) | 39,717 (25.1) | 9 (39.1) | 0.78 | [0.33, 1.87] | 38,627 (25.0) | 10 (32.3) | 0.99 | [0.42, 2.35] |
| Quartile 4 (x>0.86) | 39,399 (24.9) | 12 (52.2) | 1.00 | ref. | 38,330 (24.8) | 11 (35.5) | 1.00 | ref. |
| Ptrend=0.002 | Ptrend=0.173 | |||||||
| Waist circumference† | ||||||||
| Quartile 1 (x≤76) | 40,043 (25.2) | 1 (4.4) | 0.08 | [0.01, 0.63] | 39,014 (25.3) | 6 (18.8) | 0.65 | [0.23, 1.84] |
| Quartile 2 (76<x≤84.5) | 39,700 (25.0) | 1 (4.4) | 0.07 | [0.01, 0.55] | 38,681 (25.0) | 9 (28.1) | 0.90 | [0.36, 2.26] |
| Quartile 3 (84.5<x≤95) | 40,787 (25.7) | 6 (26.1) | 0.38 | [0.15, 0.98] | 39,675 (25.7) | 7 (21.9) | 0.65 | [0.25, 1.73] |
| Quartile 4 (x>95) | 38,150 (24.0) | 15 (65.2) | 1.00 | ref. | 37,086 (24.0) | 10 (31.3) | 1.00 | ref. |
| Ptrend< 0.001 | Ptrend=0.564 | |||||||
| Symptoms of heartburn‡ | ||||||||
| Did not occur | 101,628 (64.4) | 9 (39.1) | 1.00 | ref. | 99,110 (64.4) | 23 (71.9) | 1.00 | ref. |
| Mild | 41,517 (26.3) | 8 (34.8) | 1.89 | [0.73, 4.90] | 40,459 (26.3) | 7 (21.9) | 0.71 | [0.31, 1.66] |
| Moderate | 11,623 (7.4) | 4 (17.4) | 3.13 | [0.95, 10.26] | 11,273 (7.3) | 2 (6.3) | 0.70 | [0.16, 2.97] |
| Severe | 3,098 (2.0) | 2 (8.7) | 5.63 | [1.19, 26.59] | 3,026 (2.0) | -- | -- | -- |
| Ptrend=0.009 | Ptrend=0.268 | |||||||
| Cigarette use§ | ||||||||
| Never smoker | 79,517 (51.0) | 8 (34.8) | 1.00 | ref. | 80,514 (50.9) | 7 (21.2) | 1.00 | ref. |
| Past smoker | 65,641 (42.1) | 13 (56.5) | 1.92 | [0.79, 4.65] | 66,551 (42.1) | 17 (51.5) | 2.93 | [1.18, 7.32] |
| Current smoker | 10,860 (7.0) | 2 (8.7) | 2.49 | [0.52, 11.98] | 11,014 (7.0) | 9 (27.3) | 12.67 | [4.55, 35.28] |
| Pack-years of cigarette use§ | ||||||||
| Never smoker | 79,517 (51.9) | 8 (36.4) | 1.00 | ref. | 80,514 (51.9) | 7 (21.9) | 1.00 | ref. |
| < 20 | 44,519 (29.1) | 6 (27.3) | 1.42 | [0.49, 4.12] | 45,147 (29.1) | 7 (21.9) | 1.89 | [0.64, 5.56] |
| 20–39.9 | 16,924 (11.1) | 2 (9.1) | 1.16 | [0.25, 5.48] | 17,136 (11.1) | 10 (31.3) | 7.27 | [2.67, 19.83] |
| ≥ 40 | 12,124 (7.9) | 6 (27.3) | 4.12 | [1.42, 11.99] | 12,299 (7.9) | 8 (25.0) | 7.98 | [2.79, 22.85] |
| Ptrend=0.023 | Ptrend< 0.001 | |||||||
| Alcohol use¶ | ||||||||
| Non-drinker | 17,091 (10.9) | 1 (4.4) | 0.38 | [0.05, 2.87] | 17,291 (11.2) | 2 (6.3) | 1.28 | [0.28, 5.82] |
| Past drinker | 29,318 (18.7) | 5 (21.7) | 1.01 | [0.36, 2.77] | 29,016 (18.7) | 10 (31.3) | 1.96 | [0.92, 4.20] |
| Current drinker | 110,422 (70.4) | 17 (73.9) | 1.00 | ref. | 108,670 (70.1) | 20 (62.5) | 1.00 | ref. |
| Alcohol use¶ | ||||||||
| Non-current drinker | 46,409 (29.6) | 6 (26.1) | 1.00 | ref. | 46,307 (29.9) | 12 (37.5) | 1.00 | ref. |
| < 1 drink/week | 51,721 (33.0) | 8 (34.8) | 1.18 | [0.41, 3.42] | 51,124 (33.0) | 6 (18.8) | 0.41 | [0.15, 1.10] |
| 1–7 drinks/week | 40,331 (25.7) | 7 (30.4) | 1.53 | [0.50, 4.64] | 39,579 (25.5) | 11 (34.4) | 0.83 | [0.36, 1.90] |
| > 7 drinks/week | 18,370 (11.7) | 2 (8.7) | 1.00 | [0.20, 5.06] | 17,967 (11.6) | 3 (9.4) | 0.36 | [0.10, 1.29] |
| Ptrend=0.687 | Ptrend=0.247 | |||||||
| Education | ||||||||
| High school or less | 35,112 (22.4) | 8 (23.5) | 1.00 | ref. | 34,586 (22.5) | 7 (21.9) | 1.00 | ref. |
| Some college/vocational | 59,541 (38.0) | 10 (29.4) | 1.01 | [0.39, 2.62] | 58,360 (37.9) | 10 (31.3) | 0.85 | [0.32, 2.24] |
| College graduate or more | 62,019 (39.6) | 16 (47.1) | 0.55 | [0.17, 1.78] | 60,889 (39.6) | 15 (46.9) | 1.32 | [0.53, 3.33] |
| Ptrend=0.324 | Ptrend=0.445 | |||||||
| Income | ||||||||
| Less than $19,999 | 24,473 (16.6) | 10 (30.3) | 1.00 | ref. | 24,160 (16.7) | 10 (32.2) | 1.00 | ref. |
| $20,000 to $34,999 | 35,833 (24.3) | 13 (39.4) | 0.66 | [0.23, 1.88] | 35,192 (24.3) | 11 (35.5) | 0.76 | [0.32, 1.80] |
| $35,000 to $49,999 | 30,254 (20.5) | 4 (12.1) | 0.25 | [0.05, 1.22] | 29,715 (20.5) | 4 (12.9) | 0.34 | [0.10, 1.11] |
| $50,000 or more | 56,832 (38.6) | 6 (18.2) | 0.60 | [0.20, 1.83] | 55,674 (38.5) | 6 (19.4) | 0.30 | [0.10, 0.88] |
| Ptrend=0.282 | Ptrend=0.013 | |||||||
Numbers only for women with no-missing values for the exposure of interest and corresponding adjusting variables.
Adjusted for BMI (continuous), heartburn (did not occur/mild/moderate/severe), White race (white/non-white) and stratified by age (50–59/60–69/70–79), hysterectomy status and study type (HT trial/OS or non-HT trials).
Adjusted for pack-years of smoking (never/<5/5–19.9/20–39.9/≥40), drinking (non-drinker/past drinker/<1 drink per week/1–7 drinks per week/>7 drinks per week) and stratified by age (50–59/60–69/70–79), hysterectomy status and study type (HT trial/OS or non-HT trials).
Not adjusted for BMI.
Not adjusted for heartburn.
Not adjusted for smoking.
Not adjusted for drinking.
For esophageal SCC, as expected, a history of cigarette use was strongly associated with increased risk, particularly among current smokers (Table 3). In contrast, in this population drinking was not associated with risk of esophageal SCC. Increasing income was also associated with decreasing SCC risk (Ptrend=0.013).
We examined the relationship between HT use and type of HT use and esophageal cancer mortality. Of the 23 cases of esophageal adenocarcinoma, 17 (74%) died during the study period with a median time from diagnosis to death of 1.02 years (inter-quartile range 0.67–1.83). Use of HT was not associated with the risk of dying from esophageal adenocarcinoma (Table 4). Of the 34 cases of esophageal SCC, 28 (82%) died during the study period with a median time from diagnosis to death of 1.22 years (inter-quartile range 0.60–2.20). Esophageal SCC mortality was inversely related with use of E+P, although non-significantly, consistent with the relationship observed with incidence of esophageal SCC.
Table 4.
Esophageal cancer mortality, assuming deaths after diagnosis of esophageal cancer to be esophageal cancer deaths.
| Esophageal adenocarcinoma | Esophageal SCC | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | Total number of women (N=161,080) n (%) |
Number of deaths (N=17) n (%) |
HR* | [95% CI] | Total number of women (N=161,080) n (%) |
Number of deaths (N=28) n (%) |
HR** | [95% CI] |
| Hormone therapy use | ||||||||
| Never users | 60,031 (38.1) | 6 (35.3) | 1.00 | ref. | 59,091 (38.2) | 16 (59.3) | 1.00 | ref. |
| Past users only | 22,998 (14.6) | 3 (17.7) | 1.03 | [0.25, 4.28] | 22,622 (14.6) | 2 (7.4) | 0.33 | [0.07, 1.43] |
| Current users | 74,704 (47.4) | 8 (47.1) | 0.89 | [0.29, 2.68] | 73,132 (47.2) | 9 (33.3) | 0.55 | [0.23, 1.31] |
| Type of hormone therapy (in current users) | ||||||||
| Never users | 60,031 (44.6) | 6 (42.9) | 1.00 | ref. | 59,091 (44.7) | 16 (64.0) | 1.00 | ref. |
| E-alone | 39,630 (29.4) | 5 (35.7) | 0.72 | [0.17, 3.02] | 38,846 (29.4) | 6 (24.0) | 1.07 | [0.30, 3.88] |
| E+P | 38,068 (26.0) | 3 (21.4) | 0.98 | [0.21, 4.64] | 34,280 (25.9) | 3 (12.0) | 0.38 | [0.11, 1.35] |
Numbers only for women with no-missing values for the exposure of interest and corresponding adjusting variables.
Adjusted for BMI (continuous), heartburn (did not occur/mild/moderate/severe), White race (white/non-white) and stratified by age (50–59/60–69/70–79), hysterectomy status and study type (HT trial/OS or non-HT trials).
Adjusted for pack-years of smoking (never/<5/5–19.9/20–39.9/≥40), drinking (non-drinker/past drinker/<1 drink per week/1–7 drinks per week/>7 drinks per week) and stratified by age (50–59/60–69/70–79), hysterectomy status and study type (HT trial/OS or non-HT trials).
Discussion
No association was observed between use of HT and risk of esophageal adenocarcinoma. In contrast, the use of HT was associated with a lower risk of esophageal SCC in postmenopausal women. This association was observed in current users of E+P but not in current users of E-alone. No other hormonal or reproductive factors were significantly related to risk of either esophageal SCC or adenocarcinoma. Esophageal cancer mortality exhibited a similar pattern of hormone effects but these were not statistically significant.
Our findings of no association between HT use and risk of esophageal adenocarcinoma are similar to the results of two other studies (11, 12). One examined the risk of esophageal adenocarcinoma after initiation of estrogen therapy among prostate cancer patients, and thus is not directly comparable to our study (21). A second examined the short-term risk of esophageal adenocarcinoma among breast cancer survivors treated with tamoxifen, a selective estrogen receptor modulator, reporting a 1.6-fold increase in esophageal adenocarcinoma risk (95% CI=0.8–3.1) (7). However, their study did not provide information on whether these women had also been treated with radiation, which may increase increase of esophageal risk in breast cancer patients (22–24). A more recent study did not find an association between the risk of gastric adenocarcinoma, which included esophageal adenocarcinoma, and the use of HT when the most distal cases of stomach cancer were removed from the analysis (HR=0.90, 95% CI: 0.54–1.49) (9). Interestingly, we found that breastfeeding was associated with a non-significant 54% reduction of risk of esophageal adenocarcinoma (HR=0.44, 95% CI: 0.18–1.07), similar to the 59% reduction (95% CI: 18–80%) found in a case-control study from the UK (8).
Some of our findings regarding the risk of esophageal SCC are consistent with results reported previously (9, 18). In a pooled analysis of three case-control studies in Italy and Switzerland, ever use of HT was associated with a reduction in risk of esophageal SCC (OR=0.32, 95% CI: 0.09–1.13) (18). Results from Freedman et al. also suggest that users of HT were at slightly lower risk of esophageal SCC (HR=0.74, 95% CI: 0.42–1.28) (9). This study found that use of E+P among women with intact uterus was associated with a decreased risk (HR=0.41, 95% CI: 0.15–1.14) (9). Our findings are consistent with the notion that an inverse association may be limited to users of E+P. It could be that those women not taking HT have less access to health care due to low socio-economic status. Although socio-economic status is a risk factor for esophageal SCC (25, 26), our results were qualitatively similar after adjustment for education and income.
Most relative risk estimates associated with the established risk factors for esophageal adenocarcinoma and SCC, such as obesity, symptomatic reflux smoking and drinking, have been studied in populations of men only (27, 28) or predominantly (29, 30). In our cohort of women, we found that smoking, symptomatic reflux and BMI were associated with esophageal adenocarcinoma in similar patterns to other studies (29–32). For esophageal SCC, smoking was associated with a large increase in risk, as previously reported (18, 28). In contrast, we did not observe alcohol use to be associated with the risk of esophageal SCC, but it is worth noting that these women were not heavy drinkers; over 88% of women reported having 7 or fewer drinks per week. In other studies, associations with drinking were found in much heavier drinkers (18, 28).
Several limitations should be noted. The major one is the small number of esophageal cancer cases observed. This is a problem that most studies of esophageal cancer in women suffer, particularly prospective studies, due to the low incidence of this disease among women (7–12, 18). The small number of events observed in the HT trials did not allow for a reliable estimate of HT effects using only clinical trial information so these data were pooled with the larger observational study to increase study power. These analyses used the randomized assignment for the trial participants as well as the baseline use status in the remaining WHI participants, in a manner related to Prentice et al. (2005) (33). Even in the pooled dataset, the numbers of cases were small and might have precluded us from observing statistically significant associations, especially with the risk of esophageal adenocarcinoma. Another limitation of our study was that our estimates rely on information collected at study enrollment for the exposures of interest and did not take into account changes that might have occurred during the follow-up period. We also tested for multiple variables, which might have increased the likelihood of finding a false association by chance. Finally, we attempted to control for known confounders, but it is possible that unknown or unmeasured variables might have influenced our results.
Our study has several notable strengths, such as a prospective design, high quality assessment of HT use as well as other reproductive variables, and high quality assessment of endpoints. We were also able to adjust for a majority of the known risk factors for the two types of cancer.
In summary, the use of HT was found to be associated with a decreased risk of esophageal SCC, but no association was observed with esophageal adenocarcinoma. Due to the small numbers of cases, a pooled analysis of all available studies or a much larger study would be needed to provide more definitive and reliable evidence of the use of HT, reproductive factors and the risks of esophageal adenocarcinoma and SCC.
Acknowledgments
Funding: CB was supported by grant number T32 CA009168 from the National Institutes of Health. TLV was supported by grant number K05CA124911 from the National Institutes of Health. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Footnotes
Conflict of interest: None.
References
- 1.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973𒀓1995. Int J Cancer. 2002;99:860–868. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kalayarasan R, Ananthakrishnan N, Kate V, Basu D. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus. 2008;21:298–303. doi: 10.1111/j.1442-2050.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 6.Nozoe T, Oyama T, Takenoyama M, Hanagiri T, Sugio K, Yasumoto K. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2007;13:4046–4050. doi: 10.1158/1078-0432.CCR-07-0449. [DOI] [PubMed] [Google Scholar]
- 7.Chandanos E, Lindblad M, Jia C, Rubio CA, Ye W, Lagergren J. Tamoxifen exposure and risk of oesophageal and gastric adenocarcinoma: a population-based cohort study of breast cancer patients in Sweden. Br J Cancer. 2006;95:118–122. doi: 10.1038/sj.bjc.6603214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng KK, Sharp L, McKinney PA, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. Br J Cancer. 2000;83:127–132. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman ND, Lacey JJV, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer. 2010;116:1572–1581. doi: 10.1002/cncr.24880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagergren J, Jansson C. Sex hormones and oesophageal adenocarcinoma: influence of childbearing? Br J Cancer. 2005;93:859–861. doi: 10.1038/sj.bjc.6602810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagergren J, Nyren O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:913–915. [PubMed] [Google Scholar]
- 12.Lindblad M, Garcia Rodriguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94:136–141. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Z, Margolis KL, Liu S, Tinker LF, Ye W. Effects of estrogen with and without progestin and obesity on symptomatic gastroesophageal reflux. Gastroenterology. 2008;135:72–81. doi: 10.1053/j.gastro.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordenstedt H, Zheng Z, Cameron AJ, Ye W, Pedersen NL, Lagergren J. Postmenopausal hormone therapy as a risk factor for gastroesophageal reflux symptoms among female twins. Gastroenterology. 2008;134:921–928. doi: 10.1053/j.gastro.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 16.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 17.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Gallus S, Bosetti C, Franceschi S, et al. Oesophageal cancer in women: tobacco, alcohol, nutritional and hormonal factors. Br J Cancer. 2001;85:341–345. doi: 10.1054/bjoc.2001.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 20.The Women’s Health Initiative Study Group. Design of the Women's Health Initiative Clinical Trial and Observational Study. Controlled Clinical Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 21.Cooper SC, Croft S, Day R, Thomson CS, Trudgill NJ. Patients with prostate cancer are less likely to develop oesophageal adenocarcinoma: could androgens have a role in the aetiology of oesophageal adenocarcinoma? Cancer Causes Control. 2009;20:1363–1368. doi: 10.1007/s10552-009-9359-2. [DOI] [PubMed] [Google Scholar]
- 22.Ahsan H, Neugut AI. Radiation therapy for breast cancer and increased risk for esophageal carcinoma. Ann Intern Med. 1998;128:114–117. doi: 10.7326/0003-4819-128-2-199801150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Cooper SC, Croft S, Day R, Thomson CS, Trudgill NJ. The risk of oesophageal cancer is not affected by a diagnosis of breast cancer. European Journal of Cancer Prevention. 2010;19 doi: 10.1097/CEJ.0b013e3283372137. [DOI] [PubMed] [Google Scholar]
- 24.Salminen EK, Pukkala E, Kiel KD, Hakulinen TT. Impact of radiotherapy in the risk of esophageal cancer as subsequent primary cancer after breast cancer. Int J Radiat Oncol Biol Phys. 2006;65:699–704. doi: 10.1016/j.ijrobp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansson C, Johansson AL, Nyren O, Lagergren J. Socioeconomic factors and risk of esophageal adenocarcinoma: a nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1754–1761. doi: 10.1158/1055-9965.EPI-05-0140. [DOI] [PubMed] [Google Scholar]
- 27.Brown LM, Hoover R, Silverman D, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001;153:114–122. doi: 10.1093/aje/153.2.114. [DOI] [PubMed] [Google Scholar]
- 28.Brown LM, Hoover RN, Greenberg RS, et al. Are racial differences in squamous cell esophageal cancer explained by alcohol and tobacco use? J Natl Cancer Inst. 1994;86:1340–1345. doi: 10.1093/jnci/86.17.1340. [DOI] [PubMed] [Google Scholar]
- 29.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 30.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–732. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 31.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette Smoking and Adenocarcinomas of the Esophagus and Esophagogastric Junction: A Pooled Analysis From the International BEACON Consortium. J Natl Cancer Inst. 2010 doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrow DC, Vaughan TL, Sweeney C, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–238. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 33.Prentice RL, Langer R, Stefanick ML, et al. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women's Health Initiative clinical trial. Am J Epidemiol. 2005;162:404–414. doi: 10.1093/aje/kwi223. [DOI] [PubMed] [Google Scholar]


