Abstract
Aims
Naltrexone can be used to treat opioid dependence, but patients refuse to take it. Extended-release depot formulations may improve adherence, but long-term adherence rates to depot naltrexone are not known. This study determined long-term rates of adherence to depot naltrexone and whether employment-based reinforcement can improve adherence.
Design
Participants who were inducted onto oral naltrexone were randomly assigned to Contingency (n=18) or Prescription (n=17) groups. Participants were offered six depot naltrexone injections and invited to work at the therapeutic workplace weekdays for 26 weeks where they earned stipends for participating in job skills training. Contingency participants were required to accept naltrexone injections to maintain workplace access and to maintain maximum pay. Prescription participants could work independent of whether they accepted injections.
Setting
The therapeutic workplace, a model employment-based intervention for drug addiction and unemployment.
Participants
Opioid-dependent unemployed adults.
Measurements
Depot naltrexone injections accepted and opiate-negative urine samples.
Findings
Contingency participants accepted significantly more naltrexone injections than Prescription participants (81% versus 42%), and were more likely to accept all injections (66% versus 35%). At monthly assessments (with missing urine samples imputed as positive), the groups provided similar percentages of samples negative for opiates (74% versus 62%) and for cocaine (56% versus 54%). Opiate positive samples were more likely when samples were also positive for cocaine.
Conclusions
Employment-based reinforcement can maintain adherence to depot naltrexone. Future research should determine whether persistent cocaine use compromises naltrexone's effect on opiate use. Workplaces may be useful for promoting sustained adherence to depot naltrexone.
Keywords: depot naltrexone, contingency management, heroin, substance abuse, employment-based reinforcement
Opioid addiction is a chronic disorder (1) that can persist throughout a lifetime (2-4). Many patients relapse after treatment (5, 6), suggesting that opioid addiction may require ongoing treatment. Methadone is an effective opioid agonist treatment medication (7), particularly when used for long-term maintenance (8). However, because of its agonist effects, methadone is highly regulated; its availability is restricted; it is illegal in some countries; its use is often discouraged (e.g., by employers); and many opioid-dependent individuals simply do not want agonist treatment (7). Antagonist treatments may be useful alternatives.
Naltrexone is an antagonist that blocks the reinforcing, subjective, and physiological effects of opioids (9-12). Because naltrexone is nonaddicting and without agonist effects, there is no risk of abuse and it is subject to little regulation. Despite these attributes, the utility of naltrexone treatment has been limited because most opioid-dependent individuals refuse it (13-16). Clinicians and researchers throughout the world have been interested in promoting effective use of naltrexone (15).
Oral naltrexone can require daily dosing, which may limit adherence. Extended-release depot formulations have been developed to reduce the frequency of dosing and improve adherence (14). Depot naltrexone is safe and effective in blocking opioid effects for several weeks (17, 18). The depot formulation could be an ideal means of delivering naltrexone as a maintenance intervention, although we do not know whether individuals will maintain its use over extended periods of time.
If naltrexone is to be used as a maintenance intervention, effective means might be needed to promote its long-term use. Behavioral interventions hold promise for enhancing adherence to naltrexone treatment (19). Adherence to oral naltrexone treatment can be promoted through explicit reinforcement of naltrexone ingestion (20-23). Given the positive results with oral naltrexone, reinforcement might also be effective in promoting adherence to depot naltrexone. However, practical means of administering and financing long-duration reinforcement of naltrexone use are needed.
Workplaces have features that could make them ideal vehicles for administering and financing reinforcement of naltrexone use (24). First, individuals maintain regular and extended contact with their places of employment, which could facilitate long-duration treatment. Second, wages could be used to reinforce naltrexone ingestion, which could facilitate the financing of the intervention. Third, through Employee Assistance Programs, workplaces have become common and accepted providers of substance abuse services. Finally, workplaces are everywhere, a feature that could facilitate the dissemination of employment-based reinforcement of depot naltrexone acceptance.
Recent clinical trials have shown that workplaces can be used to reinforce therapeutic behavior change in drug-addicted adults (25-29). In these studies, unemployed drug abuse patients were hired and paid as employees in a model therapeutic workplace. To gain access to the workplace and maintain maximum earnings, patients were required to provide routine evidence of recent drug abstinence. The therapeutic workplace intervention has been effective at initiating and maintaining abstinence from opiates and cocaine for as long as three years (26).
The present study assessed long-term rates of adherence to depot naltrexone and determined if employment-based reinforcement could increase acceptance of depot naltrexone injections. Unemployed opioid-dependent adults who completed an opioid detoxification and who were inducted onto oral naltrexone were invited to attend the therapeutic workplace for six months, randomly assigned to one of two groups, and prescribed one depot naltrexone injection every three weeks for 15 weeks. Contingency group participants were required to accept the injections to attend the workplace and to maintain maximum earnings. Prescription group participants could access the workplace independent of whether they accepted injections. We expected that Contingency participants would accept more naltrexone injections than Prescription participants.
Method
Participants
Volunteers were recruited from detoxification programs in Baltimore, MD and through street outreach between October, 2006 and April, 2008. Individuals were eligible if they met the DSM-IV(30) criteria for opioid dependence, reported using heroin at least 21 of the last 30 days while living in the community, were unemployed, were 18-65 years old, were medically approved for naltrexone, and lived in or near Baltimore, MD. Individuals were excluded if they had current DSM-IV major axis I disorders, had current suicidal or homicidal ideation, expressed interest in methadone treatment, were required to use opioids for medical purposes, earned over $200 in taxable income over the previous 30 days, had physical limitations that would prevent them from using a keyboard, were pregnant or breastfeeding, or had serum aminotransferase levels over three times normal. Participants provided written consent. The study was approved by the Johns Hopkins Medicine Institutional Review Board.
General Therapeutic Workplace Procedures
The study was conducted in the therapeutic workplace, a model workplace in which employment-based reinforcement contingencies are arranged to promote therapeutic behavior change. Participants could attend the therapeutic workplace for four hours each weekday and work on training programs that were almost fully automated. Participants were paid in vouchers that were exchangeable for goods and services. Earnings were based on hours worked and performance on the training programs. Overall, voucher earnings were arranged so that participants could earn a base pay of $8.00 per hour plus about $2.00 per hour for their performance on training programs. Detailed descriptions of the therapeutic workplace can be found elsewhere (27-29, 31).
Assessments
Assessments were conducted at intake and every 30 days throughout the study. The main assessments included the Addiction Severity Index – Lite (32) (ASI – Lite) for evaluating drug use, educational, employment, family, medical, and legal histories; the Risk Assessment Battery (33) (RAB) for evaluating HIV-risk behaviors; the heroin, cocaine, alcohol, and nicotine sections of the Composite International Diagnostic Interview (34) (CIDI; intake only), a diagnostic tool for psychiatric disorders; and the Wide Range Achievement Test – 4th edition (35) (WRAT4; intake only) for assessing math, reading, and spelling skills. Additional assessments of exploratory measures were collected but are not reported here.
For safety purposes, blood samples for liver function testing were taken prior to each of the first three naltrexone injections and at the sixth month of the study, and females received pregnancy tests prior to each naltrexone injection. Naltrexone was discontinued for participants with three times the normal levels of serum aminotransferases or who were pregnant. Naltrexone was discontinued for one Contingency participant due to abnormal serum aminotransferase levels and one Prescription participant due to pregnancy. To minimize opioid overdose risk, risk reminders were provided routinely throughout treatment and at monthly lunch-time overdose prevention seminars; free pizza was provided to encourage seminar attendance.
Urine samples were collected under observation upon arrival at the therapeutic workplace on Mondays, Wednesdays, Fridays, and at each 30-day assessment. Urine samples were screened using an Abbott AxSYM®. All samples were screened for opiates and cocaine. Samples collected at 30-day assessments were also screened for methadone, benzodiazepines, and amphetamines. Samples were considered positive for opiates and cocaine if the concentration of the metabolite (morphine and benzoylecgonine, respectively) was ≥ 300 ng/ml. We use the term “opiate” when referring to the urinalysis testing to reflect the fact that testing covered just a subset of opioids that produced morphine-positive urine samples. Fewer than 5% of samples were positive for methadone, benzodiazepines or amphetamines.
Depot Naltrexone Treatment
Participants were required to complete opioid detoxification and were invited to attend the therapeutic workplace for induction onto oral naltrexone (Depade®; from Mallinckrodt, Inc.). Participants completed opioid detoxifications either through an extended inpatient detoxification program or through a brief inpatient detoxification followed by out an outpatient detoxification. The outpatient portion of the detoxifications was conducted while participants attended the therapeutic workplace. In those cases, participants were required to provide opioid negative urine samples to gain and maintain access to the workplace. All opioid detoxifications and naltrexone inductions were overseen by a physician and were guided solely by clinical considerations.
During induction, participants were required to take scheduled oral naltrexone doses to gain access to the therapeutic workplace. Oral naltrexone induction began with a dose that was determined by clinical judgment. The dose was then increased until a maintenance dose of 100 mg on Monday and Wednesday and 150 mg on Friday was reached. The maintenance routine was continued until three consecutive doses were ingested, after which the induction period ended and oral naltrexone treatment was discontinued. Participants received oral naltrexone for an average of 1.3 weeks (range 1-2 weeks) at the therapeutic workplace.
Following oral naltrexone induction, participants were invited to attend the workplace for 26 weeks and offered an 18-week course of depot naltrexone injections at no cost. Injections were administered at a nearby facility located within walking distance of the therapeutic workplace. The depot injections consisted of 2.4 ml of sterile suspension medium and 352 mg of Depotrex® microcapsules (both from Biotek, Inc.). Once reconstituted in the suspension medium, the 352 mg of naltrexone microcapsules were equivalent to approximately 228 mg of naltrexone base. Based on prior data, this dose should produce lower peak blood levels than a typical daily dose of oral naltrexone (50 mg), but should provide substantial opioid blockade for about 3 weeks (17). The depot naltrexone was injected subcutaneously in the buttocks using a 2.54-cm 18-gauge needle. Participants could receive a total of six injections, once every three weeks. After 18 weeks (the depot naltrexone blockade period), participants were encouraged, but not required, to resume oral naltrexone treatment.
Experimental Design and Groups
Prior to group assignment, participants were stratified according to whether or not participants 1) attended the workplace every day of the last three workdays; 2) submitted one or more opiate-positive urine samples out of the last 3 samples; and 3) submitted one or more cocaine-positive urine samples out of the last 3 samples. Participants were randomly assigned, via computer, to either a Prescription group or a Contingency group in a manner that ensured that the levels of each stratification variable were evenly distributed among the groups (36). All participants were invited to attend the therapeutic workplace for 26 weeks. Prescription group participants were offered depot naltrexone injections, but were allowed access to the therapeutic workplace independent of whether the injections were accepted. Contingency group participants were required to accept the depot injections to gain and maintain access to the workplace. If a Contingency participant missed a scheduled naltrexone injection (more than 3 days from the scheduled date of administration), the participant was not allowed to work in the workplace until the injection was accepted. Additionally, missing a scheduled injection resulted in a base pay reset from $8 per hour to $1 per hour. After the reset, the participant's base pay increased by $1 per hour to the maximum of $8 per hour for every day that the participant attended the workplace at least 5 minutes.
Sample Size
Sample size was determined by a power analysis based on the magnitude of the effect on the percentage of doses taken in a similar study of voucher reinforcement of oral naltrexone (21) assuming an alpha of .05 and power of .80. The resulting sample size was 40 participants per group. Midway through the study, the supply of Depotrex® became unavailable, and the study was ended. At the end of the study, there were 17 participants in the Contingency group and 18 in the Prescription group.
Outcome Measures
The primary outcome measure was the percentage of depot naltrexone injections accepted. Secondary outcome measures included the percentage of urine samples negative for opiates and cocaine. Also analyzed were the correlation between naltrexone adherence and opiate use, the percentage of days participants attended the therapeutic workplace, voucher earnings, retention in naltrexone treatment and the therapeutic workplace, and the relationship between opiate urinalysis results and group, naltrexone blockade, and cocaine urinalysis results.
Data Analyses
Participant characteristics at intake were analyzed for group differences using Fisher's Exact tests for dichotomous variables and t-tests for continuous variables. The main outcome analyses were based on data collected during the first 18 weeks after random assignment, the weeks that the depot naltrexone could block the effects of opioids. Analyses of urine samples were based on the first four monthly assessments and the first 54 thrice weekly urine samples after random assignment. Missing samples were treated as positive for opiates and cocaine (missing positive). Two alternative methods of handling missing urine samples were analyzed in which missing samples were not replaced (missing missing) and in which missing samples were interpolated based on the values before and after the missing sample or missing group of samples. All methods of handling missing samples produced essentially the same results. Only the missing-positive and missing-missing analyses are reported. Dichotomous measures were analyzed using generalized estimating equations (37) (GEE). Mean voucher earnings were analyzed using a linear mixed-model analysis (38). Retention in depot naltrexone treatment and the therapeutic workplace were analyzed using a Cox proportional hazards model. All analyses were intent-to-treat. Two-tailed tests were used and results were considered statistically significant if P ≤ .05. Statistical analyses were conducted using SAS software version 9.1.
Results
Participant Characteristics and Flow through the Study
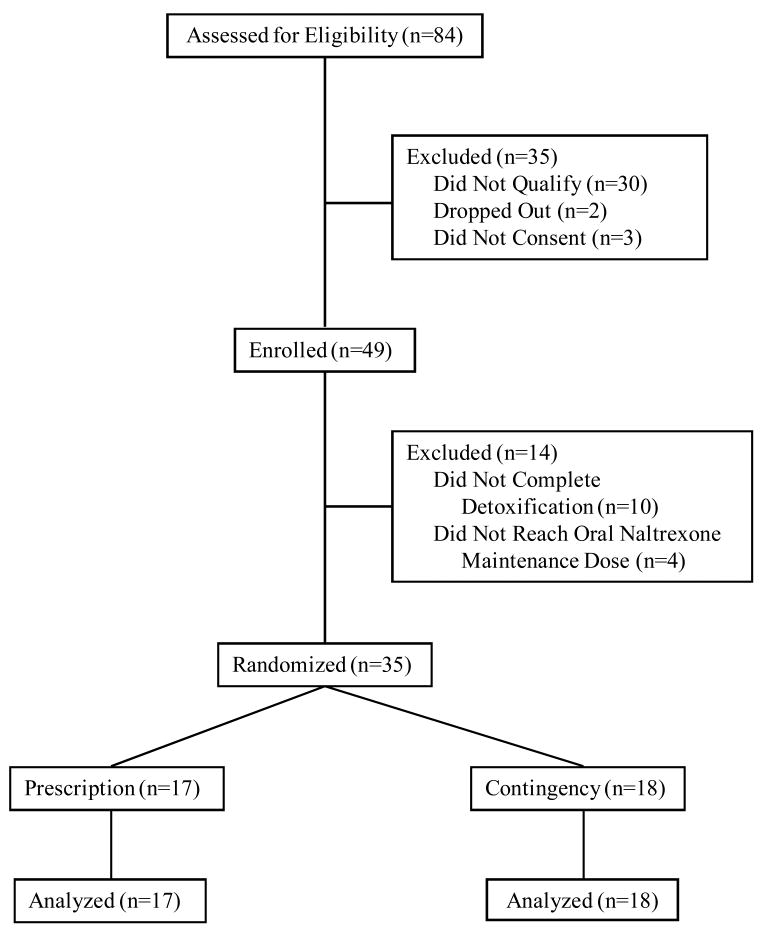
Table 1 shows that there were no significant differences between the groups on any of the characteristics assessed at intake. Figure 1 shows the flow of participants.
Table 1.
Participant characteristics at intake.
| Characteristica | Naltrexone | Naltrexone | Fisher's | t-test |
|---|---|---|---|---|
| Prescription (n=17) | Contingency (n=18) | Exact (P) | (P) | |
| Age, mean (SEM), years | 42 (2) | 43 (2) | 0.70 | |
| Female, % | 53 | 44 | 0.74 | |
| Black/white, % | 94/6 | 89/11 | 1.00 | |
| Married, % | 24 | 18 | 1.00 | |
| HIV positive, %b | 6 | 6 | 1.00 | |
| High school diploma or GED, % | 77 | 61 | 0.47 | |
| Opioid dependent, %c | 100 | 100 | - | |
| Opiate positive, % | 100 | 100 | - | |
| Cocaine dependent, %c | 71 | 61 | 0.73 | |
| Cocaine positive, % | 94 | 83 | 0.60 | |
| Usually unemployed past 3 years, % | 59 | 72 | 0.49 | |
| Past 30 days income, mean (SEM), $ | ||||
| Employment | 4 (4) | 0 (0) | 0.33 | |
| Welfare | 167 (56) | 118 (48) | 0.52 | |
| Pension, benefits, Social Security | 100 (70) | 61 (44) | 0.64 | |
| Mate, family, friends | 63 (59) | 737 (556) | 0.24 | |
| Illegal | 753 (444) | 667 (350) | 0.88 | |
| Total income | 1086 (452) | 1583 (648) | 0.54 | |
| $ spent on drugs, mean (SEM), past 30 days | 1539 (320) | 1778 (359) | 0.62 | |
| Currently on parole/probation, % | 53 | 61 | 0.74 | |
| Lifetime felony conviction, % | 88 | 89 | 1.00 | |
| Grade levels, mean (SEM)d | ||||
| Reading | 8 (1) | 8 (1) | 0.72 | |
| Spelling | 8 (1) | 8 (1) | 0.87 | |
| Arithmetic | 6 (0) | 5 (0) | 0.07 |
Note. Results for Fisher's Exact tests and t-tests are based on two-tailed tests with an alpha of .05.
Unless otherwise noted, characteristics are taken from the Addiction Severity Index – Lite.
Taken from the Risk Assessment Battery.
Taken from the Composite International Diagnostic Interview.
Taken from the Wide Range Achievement Test. Due to missing data for one participant, analyses for the Naltrexone Prescription group were based on n=16.
Figure 1.
The flow of participants through the study.
Naltrexone Adherence and Drug Use
Contingency participants took significantly more naltrexone injections than Prescription participants (Figure 2 and Table 2). Figure 3 (top panel) shows that 65% of Prescription participants took their first scheduled injection, whereas 100% of Contingency participants took their first scheduled injection. Only 35% of Prescription participants took all injections, whereas 66% of Contingency participants took all injections, representing significantly greater retention in depot naltrexone treatment for Contingency than Prescription participants [χ2 (1) = 4.94, P = .026; HR = 0.32; 95% CI = 0.117 - 0.874]. This difference in naltrexone retention occurred despite identical retention in the workplace (77% of participants; Fig. 3 bottom panel).
Figure 2.
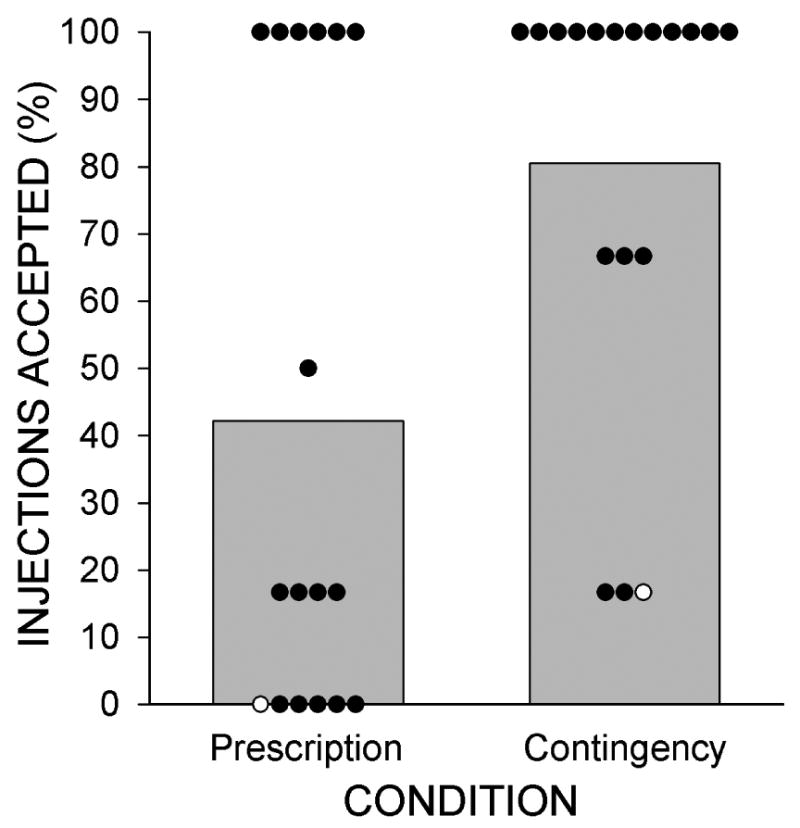
The percentage of depot naltrexone injections accepted by participants in the Prescription and Contingency Groups. Bars show group percentages and circles show individual percentages. Open circles show individuals for whom depot naltrexone treatment was discontinued for medical reasons.
Table 2.
Naltrexone injections, opiate and cocaine urinalysis results, and workplace attendance for participants in the two groups during the depot naltrexone treatment phase of the study.
| Percentage | Chi Squarea | OR (95% CI) | P | ||
|---|---|---|---|---|---|
| Prescription | Contingency | ||||
| Naltrexone Injections Received | 42.2 | 80.6 | 6.97 | 5.68 (1.61-20.02) | 0.008 |
| Monthly Urinalysis Results | |||||
| Opiate Negative (missing positive) | 61.8 | 73.6 | 0.67 | 1.73 (0.47-6.29) | 0.413 |
| Opiate Negative (missing missing) | 76.4 | 88.3 | 1.12 | 2.27 (0.54-9.55) | 0.290 |
| Cocaine Negative (missing positive) | 54.4 | 55.6 | 0.01 | 1.05 (0.32-3.42) | 0.939 |
| Cocaine Negative (missing missing) | 67.3 | 66.7 | 0.00 | 0.98 (0.28-3.41) | 0.973 |
| Collected Urine Samples | 81.0 | 83.3 | 0.04 | 1.18 (0.25-5.51) | 0.830 |
| Thrice Weekly Urinalysis Results | |||||
| Opiate Negative (missing positive) | 51.6 | 72.0 | 2.16 | 3.57 (0.81-15.75) | 0.142 |
| Opiate Negative (missing missing) | 76.1 | 93.0 | 1.25 | 2.24 (0.85-8.60) | 0.260 |
| Cocaine Negative (missing positive) | 48.9 | 55.6 | 0.09 | 1.21 (0.34-4.26) | 0.769 |
| Cocaine Negative (missing missing) | 72.1 | 71.6 | 0.22 | 0.76 (0.24-2.40) | 0.640 |
| Collected Urine Samples | 67.9 | 77.6 | 0.74 | 1.64 (0.53-5.02) | 0.388 |
| Days in Attendance at Workplace | 56.4 | 67.7 | 1.01 | 1.64 (0.67-4.04) | 0.316 |
DF=1
Figure 3.
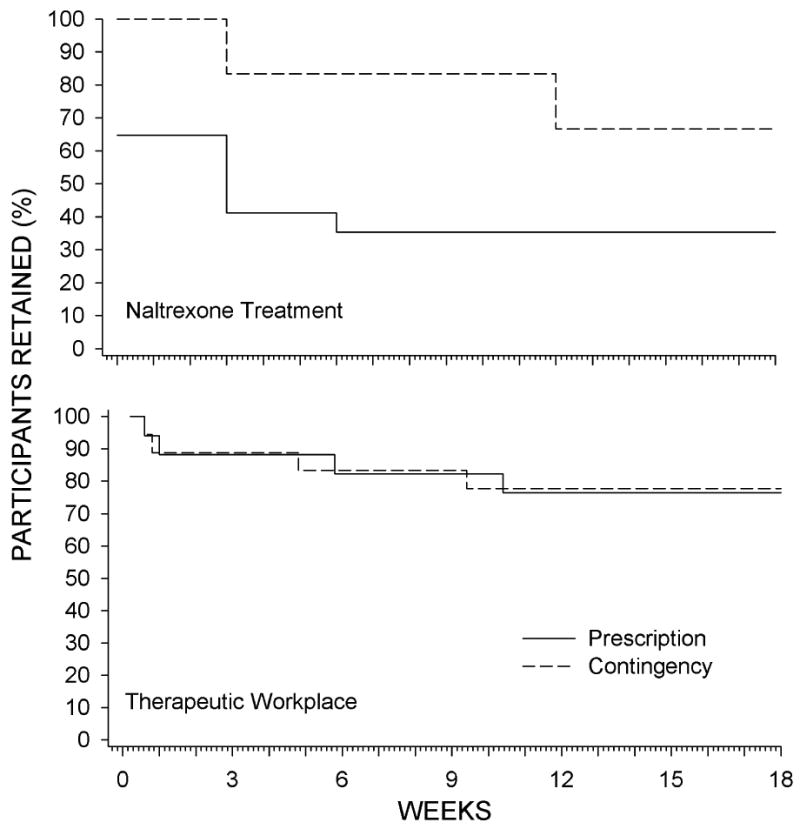
The percentage of participants retained in depot naltrexone treatment (continued to take scheduled depot naltrexone injections; top panel) and the therapeutic workplace (continued attending the workplace; bottom panel) across study weeks.
Urinalysis test results
As shown in Table 2, at least two-thirds of scheduled workplace-attendance urines and 80% of monthly-assessment urines were collected, with no differences in collection rates between groups. Importantly, there were no significant between group differences on percentage of negative urine tests for opiates or for cocaine based on either thrice weekly or monthly urinalysis test results.
Table 2 shows that fifty percent or more of urines delivered were negative for opiates and for cocaine. Overall, the percentage of urines testing negative for cocaine was consistently lower than the percentage testing negative for opiates, indicating more use of cocaine than of opiates by study participants. This gap between opiate and cocaine negative tests was wider for the Contingency than for the Prescription group. This was because the percentage of samples negative for opiates was consistently, though not significantly, higher in the Contingency than the Prescription groups (74% vs 62% opiate negative, respectively in monthly testing) while rates of cocaine use (56% vs 54%) were virtually identical for the two groups.
Blockade effects and opiate use
Although the increase in naltrexone adherence in the Contingency group was not associated with a significant increase in opiate negative urine samples, there was nevertheless a statistically significant correlation between naltrexone adherence and opiate abstinence. Monthly urinalysis results (excluding missing samples) from both groups were combined to assess the association between naltrexone adherence and opiate abstinence using Spearman's rho. Data from three participants were excluded because all four urine samples were missing. There was a significant correlation between naltrexone adherence and opiate negative urine samples (rs = .51, P = .003). Overall, 92% of monthly urines samples were opiate negative when participants were blocked by naltrexone, whereas 61% of the samples were opiate negative when participants were not blocked.
Detailed Analysis of Opiate and Cocaine Use
To understand why the increased naltrexone adherence in the Contingency group did not appear to affect opiate urinalysis results, we reviewed individual naltrexone adherence and urinalysis results (see Figures 4 for thrice-weekly urinalysis results and Figure S1 in Supporting Information for a figure showing the data for monthly urinalysis results). That review showed that while participants were blocked by naltrexone, they rarely provided urine samples that were positive for opiates alone, but more frequently provided samples that were positive for both opiates and cocaine. Although only 13 participants provided opiate positive urine samples while under depot naltrexone blockade (see Figure 4; Contingency Participants 18, 19, 21, 24, 25, 26, 27 and 28; Prescription Participants 7, 8, 12, 13 and 14), those participants provided 78 opiate positive urine samples and 88% of those samples (69 samples) were positive for both opiates and cocaine. Only 4 of the 13 participants (Participants 14, 24, 25 and 27) ever provided a sample that was positive for opiates and negative for cocaine while under naltrexone blockade.
Figure 4.
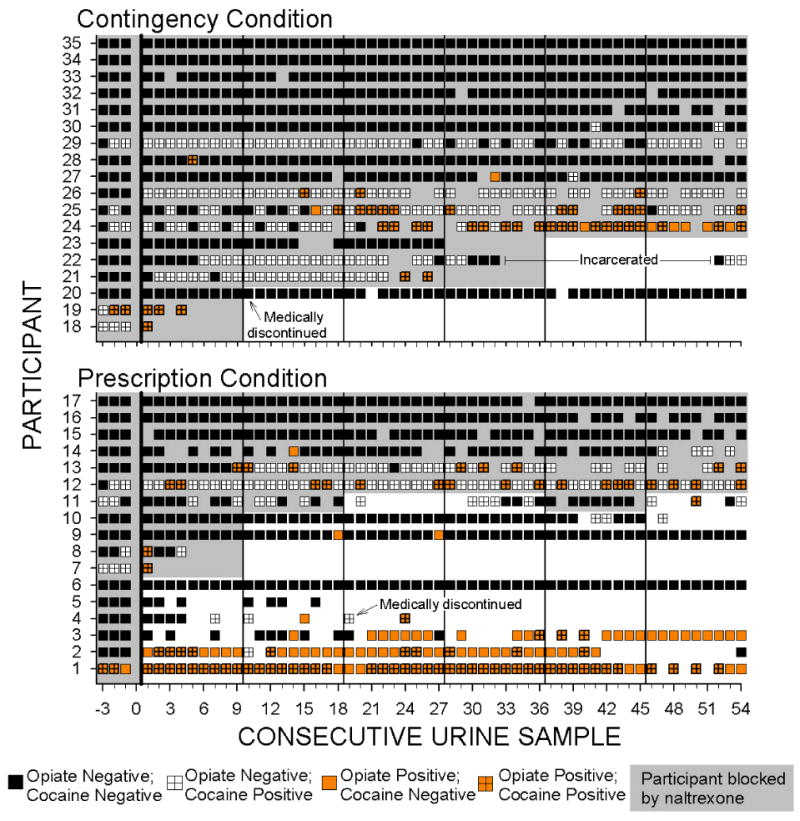
Naltrexone blockade and opiate and cocaine urinalysis results across consecutive trice weekly urine samples collected when participants attended the therapeutic workplace. Within each panel, rows of data represent the results for individual participants. Urinalysis results are based on samples collected three times per week, typically on Monday, Wednesday and Friday of each week. Samples prior to the left of the vertical black line at 0 on the horizontal axis were collected prior to random assignment while participants were taking oral naltrexone. Black squares indicate urine samples negative for both opiates and cocaine; orange squares indicate opiate positive urine samples; white squares with crosses indicate cocaine positive urine samples; orange squares with crosses indicate samples positive for both opiates and cocaine. Empty sections indicate missing samples. Shaded portions show when participants were blocked by naltrexone (i.e., the sample was collected within 3 weeks of the last naltrexone injection). Vertical lines after urine samples 0, 9, 18, 27, 36, and 45 indicate the time of scheduled injections. Within each panel, participants are arranged from top to bottom from those with the most to least naltrexone blockade, most to least opiate negative samples, and then most to least cocaine negative samples.
Exploratory GEE analyses (excluding missing samples) were conducted to determine whether opiate urinalysis results were associated with group (Contingency/Prescription), naltrexone blockade (samples collected within 3 weeks of the last injection; yes/no), or cocaine use (urine sample positive for cocaine; yes/no), or interactions of those variables. The GEE analyses provided preliminary evidence that opiate positive urine samples were associated with cocaine positive urine samples, both for monthly (Chi Square = 7.64, P = .006) and thrice weekly (Chi Square = 7.25, P = .007) urine samples, even when group and naltrexone blockade were controlled. (See Supporting Information for a table of the all results from these GEE analyses and for a figure that summarizes the data included in those analyses.)
Voucher Earnings and Attendance
Prescription participants earned about the same amount in vouchers (average of $28.61 per day; SD = $33.83) as Contingency participants (average of $34.76 per day; SD = $33.24; P = .33). Participants in the Contingency and Prescription groups attended the workplace (Table 2) and were retained in the therapeutic workplace at comparable rates (bottom panel of Figure 3; P = .96). Overall, 77% of participants were retained at 18 weeks. Five Contingency participants stopped attending the workplace and taking naltrexone injections (Figure 4), but all five stopped attending the workplace well before they were required to take the next scheduled injection and before their base pay would have been reset.
Comment
The extended-release depot formulation of naltrexone was developed to improve adherence to naltrexone. Yet participants in the Prescription group showed only modest adherence to depot naltrexone treatment. Although one-third of the participants accepted all of the depot injections, one-third of the participants refused all of the injections. Adherence was modest despite the fact that participants had free access to depot naltrexone at a facility located near the therapeutic workplace. Relative to the Prescription group, Contingency participants accepted a significantly greater percentage of depot naltrexone injections. All participants in the Contingency group accepted at least one injection, and two-thirds of the participants accepted all of the naltrexone injections. These data show that employment-based reinforcement was effective in maintaining adherence to depot naltrexone.
Retention in the therapeutic workplace did not differ between the groups. Approximately three-quarters of the participants in both groups were still attending the workplace at the end of the naltrexone treatment period. The high retention throughout the study is encouraging considering the participants' problematic work history. Overall, retention in the therapeutic workplace and the increased acceptance of injections in the Contingency group suggest that employment-based reinforcement can be an effective means of maintaining long-duration adherence to depot naltrexone treatment.
Over 80% of monthly urine samples were collected from both groups, which allowed for thorough evaluation of the effects of the intervention on drug use. Yet, there were no between-group differences in the percentages of opiate-negative urine samples. Because depot naltrexone blocks the effects of opioids (17), we expected increased opiate abstinence in the Contingency group considering the improved adherence. Although the groups did not differ in their rates of opiate-negative urine samples, there was a significant correlation between depot naltrexone adherence and opiate abstinence for the entire study sample, suggesting that depot naltrexone may have increased opiate abstinence.
Four factors might explain the failure to see effects of increased naltrexone adherence on opiate use: the small sample size, the overall low rates of opiate use in the population (some participants did not use opiates even when not protected by naltrexone), the fact that participants in both groups took some or all naltrexone injections, and the potentially deleterious effect of continued cocaine use on opiate use. The analyses showing that opiate positive urine samples were significantly associated with cocaine positive urine samples, independent of whether participants were blocked by naltrexone and group assignment, suggests that cocaine use may have compromised the effectiveness of naltrexone in reducing opiate use. However, given the low frequency of opiate positive urine samples when blocked by naltrexone and given the exploratory nature of these analyses, this conclusion should be considered speculative at this time, and in need of replication.
The relatively low adherence rate in participants who were simply offered free depot naltrexone injections suggests that the depot formulation alone, with its associated less-frequent dosing, is not sufficient to sustain good adherence in some individuals. In this study, employment-based reinforcement significantly improved adherence to depot naltrexone in opioid-dependent adults. The excellent retention in the therapeutic workplace suggests that employment-based reinforcement could be an effective means of promoting long-term adherence to depot naltrexone treatment. Overall, these data suggest that contingent access to workplaces could be used therapeutically to promote effective use of depot naltrexone and treat heroin addiction over extended periods of time.
Supplementary Material
Supporting Information: Additional Supporting Information may be found in the online version of this article:
Table S1. Analyses of opiate positive urinalysis resutls as a function of group, naltrexone protection and cocaine positive urinalysis results using GEE.
Figure S1. Naltrexone blockade and opiate and cocaine urinalysis results across consecutive months after random assignment.
Figure S2. The percentage of opiate positive urine samples as a function of the three different conditions under which the sample was submitted: whether or not the participant was blocked by naltrexone at the time the sample was collected (i.e., within three weeks of the last naltrexone injection), group assignment, and whether the sample was cocaine positive.
Acknowledgments
This research was supported by grants R01DA019497 and T32DA07209 from the National Institute on Drug Abuse (NIDA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The authors wish to thank Kylene Broadwater and Karly Diemer for their assistance with data management and study coordination, Jackie Hampton and Megan Duffy for participant recruitment and assessment, Mick Needham for supervising the workroom, Leticia Nanda for providing medical care to participants, and Paul Nuzzo for conducting statistical analyses.
Footnotes
Disclosures: In recent years, Dr. Bigelow has received consulting payments from Abbott Laboratories, Acura Pharmaceuticals, Takeda Pharmaceuticals, and Teva Pharmaceuticals, and through his university has received research support from Titan Pharmaceuticals and Pain Therapeutics, Inc.
Conflict of Interest: The other authors report no conflicts of interest or financial disclosures.
References
- 1.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 2.Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58:503–8. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- 3.Vaillant GE. A 20-year follow-up of New York narcotic addicts. Arch Gen Psychiatry. 1973;29:237–41. doi: 10.1001/archpsyc.1973.04200020065009. [DOI] [PubMed] [Google Scholar]
- 4.Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD ALIVE Study. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort,1988-2000: description and determinants. Am J Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- 5.Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93:73–92. [PubMed] [Google Scholar]
- 6.Magura S, Rosenblum A. Leaving methadone treatment: lessons learned, lessons forgotten, lessons ignored. Mt Sinai J Med. 2001;68:62–74. [PubMed] [Google Scholar]
- 7.National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA. 1998;280:1936–43. [PubMed] [Google Scholar]
- 8.Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303–10. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 9.Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. Effects in man. Arch Gen Psychiatry. 1973;28:784–91. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- 10.Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- 11.Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology (Berl) 1999;145:162–74. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- 12.Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279:524–38. [PubMed] [Google Scholar]
- 13.Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, et al. Management of relapse in naltrexone maintenance for heroin dependence. Drug Alcohol Depend. 2007;91:289–92. doi: 10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: novel treatment for opioid dependence. Expert Opin Investig Drugs. 2007;16:1285–94. doi: 10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]
- 15.Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess. 2007;11:iii, iv, 1–85. doi: 10.3310/hta11060. [DOI] [PubMed] [Google Scholar]
- 16.Modesto-Lowe V, Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp Clin Psychopharmacol. 2002;10:213–27. doi: 10.1037//1064-1297.10.3.213. [DOI] [PubMed] [Google Scholar]
- 17.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl) 2002;159:351–60. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rounsaville BJ. Can psychotherapy rescue naltrexone treatment of opioid addiction? NIDA Res Monogr. 1995;150:37–52. [PubMed] [Google Scholar]
- 20.Grabowski J, O'Brien CP, Greenstein R, Ternes J, Long M, Steinberg-Donato S. Effects of contingent payment on compliance with a naltrexone regimen. Am J Drug Alcohol Abuse. 1979;6:355–65. doi: 10.3109/00952997909001724. [DOI] [PubMed] [Google Scholar]
- 21.Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–35. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 22.Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- 23.Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58:755–61. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman K. Exploring the limits and utility of operant conditioning in the treatment of drug addiction. The Behavior Analyst. 2004;27:209. doi: 10.1007/BF03393181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Exp Clin Psychopharmacol. 2001;9:14–23. doi: 10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- 26.Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: three-year abstinence outcomes. Exp Clin Psychopharmacol. 2002;10:228–40. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- 27.Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, et al. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. J Appl Behav Anal. 2007;40:387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donlin WD, Knealing TW, Needham M, Wong CJ, Silverman K. Attendance rates in a workplace predict subsequent outcome of employment-based reinforcement of cocaine abstinence in methadone patients. J Appl Behav Anal. 2008;41:499–516. doi: 10.1901/jaba.2008.41-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: a randomized controlled trial. Addiction. 2009;104:1530–8. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Association American, Psychiatric Association. Task Force on DSM-IV Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 31.Knealing TW, Roebuck MC, Wong CJ, Silverman K. Economic cost of the therapeutic workplace intervention added to methadone maintenance. J Subst Abuse Treat. 2008;34:326–32. doi: 10.1016/j.jsat.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–23. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, et al. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res Hum Retroviruses. 1994;10(Suppl 2):S281–3. [PubMed] [Google Scholar]
- 34.Compton WM, Cottler LB, Dorsey KB, Spitznagel EL, Mager DE. Comparing assessments of DSM-IV substance dependence disorders using CIDI-SAM and SCAN. Drug Alcohol Depend. 1996;41:179–87. doi: 10.1016/0376-8716(96)01249-5. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson GS. WRAT-3: Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]
- 36.Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J Consult Clin Psychol. 2004;72:839–54. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- 37.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 38.Singer JD. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. J Educ Behav Stat. 1998;24:323–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information: Additional Supporting Information may be found in the online version of this article:
Table S1. Analyses of opiate positive urinalysis resutls as a function of group, naltrexone protection and cocaine positive urinalysis results using GEE.
Figure S1. Naltrexone blockade and opiate and cocaine urinalysis results across consecutive months after random assignment.
Figure S2. The percentage of opiate positive urine samples as a function of the three different conditions under which the sample was submitted: whether or not the participant was blocked by naltrexone at the time the sample was collected (i.e., within three weeks of the last naltrexone injection), group assignment, and whether the sample was cocaine positive.