Abstract
This study primarily focused on the anti-metastatic activity of doxorubicin (DOX) loaded in a pH-sensitive mixed polymeric micelle formed from two block polymers: poly(L-lactide) (PLLA) (Mn 3000)-b-poly(ethylene glycol) (PEG) (Mn 2000)-folate and poly(L-histidine) (PHis) (Mn 4700)-b-PEG (Mn 2000). Tumor formation and metastasis in mice were examined using a murine mammary carcinoma cell of 4T1 which is one of the most aggressive metastatic cancer cell lines. The efficacy was evaluated by tumor size, body weight change, survival rate, dorsal skin fold window chamber model, and histological observation of the lung, heart, liver and spleen after treatment with various DOX formulations. When the tumor reached 50–100 mm3 in size, the mice were treated by 4 times at a 3-day interval at a dose of 10 mg DOX/kg. The mixed micelle formulation resulted in retarded tumor growth, no weight loss, and no death for 4–5 weeks. In another set of the in vivo test for histological evaluation of the organs, the mice were similarly treated but the formulations were injected one day after 4T1 cell inoculation. The treatment by DOX loaded mixed micelle showed no apparent metastasis till 28 days. However, significant metastasis to the lung and heart was observed on Day 28 when the mice were treated with DOX carried by PBS, PLLA-b-PEG micelle and PHis-b-PEG micelle.
Keywords: breast cancer, 4T1 mammary carcinoma, folate, pH-sensitive micelle, poly(L-histidine-b-PEG), metastasis model
1. Introduction
Unique pH-sensitive nanotechnologies based on a mixed micelle composed of L-histidine copolymer-b-PEG and poly(L-lactic acid)-b-PEG-ligand have been proven to be effective for treating various sensitive and drug resistant xenograft solid tumors in mice. The pH sensitivity comes from the ionization of polyHis block as pH decreases. Receptor mediated active uptake by cancer cells, triggered release by physical disintegration of the micelles induced by endosomal pH, and endosomal escaping capability of ionized poly(L-histidine) were the major functionalities to achieve high cytosolic dosing in cancer cells while minimizing non-specific biodistribution. The detailed underlying mechanisms and results have been summarized in a recent review article [1]. Because the growth of human solid tumors engrafted in mice is mostly confined in the injected site, such xenograft models do not properly represent the metastatic nature of clinical tumors which is counted as a major cause of death from cancers [2–5].
4T1 breast cancer cell line was derived from a spontaneously arising BALB/c mammary tumor [6]. Its metastatic properties in syngeneic cancer models are well characterized and documented [5, 7]. The metastasis occurs via a hematogenous route to the liver, lungs, bone, and brain, closely resembling metastatic breast cancer in human patients [8]. The 4T1 cells grow aggressively and cause a uniformly lethal disease even after excision of a primary tumor [9, 10].
Despite significant efforts on anti-cancer drug delivery researches with nanoconstructs, a few systems have been tested for their efficacy on 4T1 metastatic models and demonstrated results with varying degrees of tumor growth inhibition. For example, a doxorubicin (DOX) loaded immunoliposome targeting MUC1 on 4T1-MUC1 tumor in mice showed some efficacy in early lesions but not in advanced lesions [11]. A similar efficacy in the early stage was confirmed by another immunoliposome equipped with 2C5 monoclonal antibody [12]. Folate decorated pH-sensitive micelles constructed by a block copolymer of poly(10-undecenoic acid) and the copolymer of N-isopropylacrylamide and N,N-diemthylacylamide demonstrated high accumulation of DOX in 4T1 tumor but in vivo antitumor efficacy was not reported [13]. Another micellar system with folic acid and pH-induced release mechanism (H40-poly(L-aspartate-doxorubicin)-b-PEG) was tested with 4T1 cell line and demonstrated high cytotoxicity against the cells. However, none of the tested systems reported the degree of prevention of metastasis in 4T1 models in animals.
This study investigated whether our pH-sensitive micelle system can inhibit or prevent the metastasis of 4T1 murine cells to major organs in mice and the efficacy was compared with control formulations. The results demonstrated that the pH-sensitive system decorated with folate was effective for suppressing 4T1 tumor growth and spreading of 4T1 cells in the organs examined.
2. Materials and Methods
2.1. Materials
4T1 mouse mammary tumor cell line was purchased from ATCC. The cells were cultured in RPMI 1640 medium, completed by adding 10% fetal bovine serum and 1% penicllin-streptomycin, at 37 °C in a humidified incubator in 5% CO2. Na2B4O7, NaN3, triethylamine (TEA), ethylenediamine tetraacetic acid (EDTA) and doxorubicin·HCl (DOX·HCl) were purchased from Sigma Chemical Co. (St. Louis, USA). Triethylamine, glutaraldehyde, methanol, chloroform, isopropyl alcohol, and dimethylsulfoxide (DMSO) were provided from J. T. Baker (Deventer, Netherlands). Penicillin-streptomycin, Tris–HCl (pH 8.4), fetal bovine serum (FBS), phosphate buffer solution (PBS) were purchased from Gibco Co. (Uxbridege, U.K). Cell counting kit-8 was purchased from Dojindo Molecular Technologies, Inc. (Gaithersburg, MD, USA). N,N'-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), folic acid, L-lactide, thionyl chloride, isopropylamine and 2-mercaptoethanol were obtained from Sigma. α-carboxy-ω-hydroxy PEG was prepared in house. Ketamine was from Abbott (IL, USA) and xylazine from Bayer (KS, USA).
2.2. Preparation of poly(L-lactide) (PLLA)-b-PEG-folate and poly(L-histidine) (PHis)-b-PEG
PLLA-b-PEG was synthesized by our routine method [14]. The hydroxyl group on PEG was used for the initiation of polymerization of L-lactide, while the carboxyl group was preserved for conjugation with folic acid. To prepare PLLA-b-PEG-folate, folic acid was reacted with 10 times ethylene diamine under the catalysis of DCC and NHS. After purification by precipitation in acetonitrile, the dark yellow powder was dried in vacuo. Ion-exchange column chromatography was used to separate non reacted folic acid and diaminated folic acid. PLLA-b-PEG was activated by DCC and NHS, and then coupled with diaminated folic acid. The raw product was purified by dialysis (MWCO 2000) to yield PLLA-b-PEG-folate.
For PHis-b-PEG synthesis, N-carboxy anhydride (NCA) was first synthesized by thionyl chloride treatment of Nα -CBZ-Nim -DNP-L-histidine. Then ring-opening polymerization of the NCA was initiated by isopropylamine. The purified product (poly(Nim-DNP-His) was further coupled with carboxylated PEG. After thiolysis deprotection using 2-mercaptoethanol, final product PHis (4.7k)-b-PEG(2k) was obtained.
2.3. Micelle fabrication and characterization
DOX˙HCl was dissolved in DMSO at room temperature with triethyleneamine at a 1:2 molar ratio of doxorubicin to triethylamine. The 1 ml of DOX solution (20mg/ml) was mixed with a 7 ml DMSO solution containing 40 mg of PLLA-b-PEG-folate (30 wt%) and PHis-b-PEG (70 wt%). The micelle was formed by dialysis against PBS. The final concentration of DOX in the micelle solution was 2 mg/ml as determined by high-performance liquid chromatography (HPLC). The particle size of the micelle (0.1 mg/ml) was measured by a Zetasizer. Photon correlation spectroscopy (PCS) was conducted with Zetasizer 3000 (Malvern instruments, USA) with He-Ne Laser beam at a wavelength of 633 nm at 25 °C on a fixed scattering angle of 90°. The mean particle sizes of the DOX mixed micelle, PLLA-PEG micelle and PHis-b-PEG micelle were 94 nm, 73nm and 152 nm, respectively.
2.4. Flow cytometry
4T1 cells were cultured in 75 mm2 cell culture flasks using RPMI 1640 media supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin. The culture medium was changed every other day. Incubator was maintained at 5.0% CO2 and 37 °C. The cells were seeded in a 6-well plate (1×106 cells/well) and incubated overnight and harvested by 0.2% (w/v) trypsin–0.1% (w/v) EDTA solution. 2 ml solution of each DOX loaded in PLLA-b-PEG micelle, PHis-b-PEG micelle and PHis-b-PEG/PLLA-b-PEG-folate mixed micelle was introduced to each well at a 20 µg DOX/ml concentration in medium and incubated for 20 min. The cells were trypsinized and washed 3 times with PBS solution and then fixed with 2.5% glutaradehyde. After filtering through a nylon mesh, cell fluorescence was measured by flow cytometry (FACSCAN, Becton Dickinson) described in detail by previous work [15].
2.5. Cell viability
The 4T1 cells (5×104 cells/ml) growing in flasks were harvested by treating with 0.2% (w/v) trypsin-0.1% (w/v) EDTA solution. The cells in RPMI 1640 completed medium (200 µL) were seeded in a 96-well plate (1×104 cells/well) and incubated for one day. The stock DOX solution, DOX loaded PLLA-b-PEG micelle, PHis-b-PEG micelle and PHis-b-PEG/PLLA-b-PEG-folate mixed micelle were diluted with completed RPMI 1640 medium to prepare the micelles with various DOX concentrations (from 10 to 0.0001 µg/ml) for dose-dependent cytotoxicity test. The pH of the culture medium was adjusted to pH 6.8 with 0.1 N HCl. After 72 h incubation, the cells were washed three times with PBS (pH 7.4). Medium containing 20 µl of Cell Counting Kit-8 solution (200 µl) was added to each well and the plate was incubated for an additional 4 h. The absorbance of each well was read with a microplate reader (Model 680 microplate reader, Bio-Rad) using a test wavelength of 450 nm [16].
2.6. Tumor growth
Five to six weeks old female nu/nu mice (BALB/c mice) were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were accommodated in autoclaved micro-isolator cages housed in appositive pressure containment rack and maintained under the guidelines of an approved protocol from University of Utah Institutional Animal Care and Use Committee. They were randomly assigned to experimental and control groups of ten animals each. The xenografts of murine carcinoma were developed by subcutaneously implanting 1×106 4T1 cells in the right rare flank from cell culture in the nude mice, as described previously [17]. When the tumor volume reach to around 50–100 mm3, animals were treated four times at a 3-day interval (Days 0, 3, 6, and 9) with DOX dissolved in PBS, DOX loaded in PLLA-b-PEG, loaded in PHis-b-PEG micelle, and loaded in PHis-b-PEG/PLLA-b-PEG-folate mixed micelle. Each formulation was injected intravenously via tail vein at a dose of 10 mg/kg through 25G5/8 needle. The tumor inhibition activity was assessed with tumor volume, which was calculated by the following equation: V = (w)2×(l)/2, where (w) and (l) are width and length of the tumor measured by a caliper. The body weight was measured simultaneously. The mice survival data was recorded.
2.7. 4T1-GFP cell line
4T1 cell line was cultured with RPMI-1640 medium with 10% fetal bovine serum (GibcoBRL, USA) in a cell incubator at 37 °C. After growing to about 80% confluence, 4T1 cells were seeded in a 6-well culture plate at a density of 2 × 105 cells/well in 2 ml medium without antibiotics. After 24-hour culture, 4T1 cells in each well were transfected with 1 µg of pEGFP-N1 plasmids expressing green fluorescence protein by FuGENE 6 Transfection Reagent (Roche, Switzerland). After transfection for 48 hours, cells were cultured with fresh medium containing Kanamycin at a final concentration of 800 µg/ml for about 3 weeks until the formation of single cell clones. Subsequently, single cell clones were respectively picked up into a 24-well culture plate and cultured with media containing 800 µg/ml of Kanamycin. Finally, the positive 4T1-GFP cell clone, stably expressing green fluorescence protein, was identified by flow cytometry and observation under an inverted fluorescence microscope.
2.8. Dorsal skin fold window chamber
In order to visualize DOX loaded PHis-b-PEG/PLLA-b-PEG-folate mixed micelle inhibiting 4T1 cell proliferation in vivo, a titanium window chamber frame was implanted in the mice [18–22]. Mice were anesthetized with i.p. ketamine/xylazine at doses of 100 mg/kg and 10 mg/kg, respectively. About 1 cm diameter of circular skin was removed on the left side of the flap, leaving the skin and underlying fascia tissue on the right side intact. A pair of titanium windows was mounted with an alignment of the circular wound to the window, which was covered with a glass cover slip and a retaining ring after Green Fluorescent Protein transfected 4T1 (about 500 cells in 5 µL) was implanted onto the fascia. Following recovery from anesthesia and surgery, the animals were housed in an environmental chamber at 35 °C and 50 % humidity, with 12 hr light/12 hr dark cycle and access to rodent chow and water ad libitum. These conditions are necessary to maintain viability of the chamber and provide a high enough temperature to facilitate tumor growth. The fluorescence labeling 4T1 cells were observed under the intravital fluorescence microscopy [23, 24] using specially designed animal experiment Olympus microscope (Olympus BX51WI Microscope, Leeds Precision Instruments Inc., MN) [25, 26].
2.9. 4T1 metastasis examination
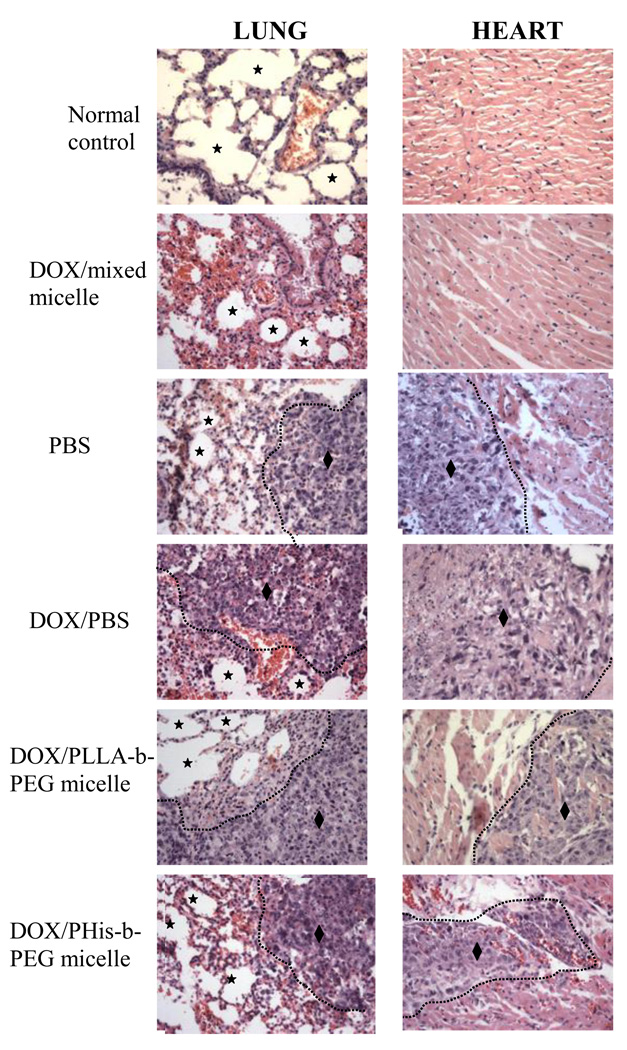
4T1 cells were cultured in completed RPMI 1640 media, at 37°C in a humidified incubator in 5% CO2. Cells were inoculated in the left flank area of female nude mice at a concentration of 1×106/mouse. Treatment started on the next day after cells inoculation in the body with the drug dose 10 mg/kg, totaling 4 treatments at a three-day interval (Days 1, 4, 7, and 10 post inoculation). Experimental groups include negative control (only PBS), DOX in PBS, DOX loaded PLLA-b-PEG, DOX loaded PHis-b-PEG and DOX loaded in PHis-b-PEG/PLLA-b-PEG-folate micelles. After four weeks, lung, liver, heart and kidney were excised and fixed in 10% formalin solution, followed by paraffin embedding for histological H & E staining. Representative picture of lungs and hearts are shown in Figure 6 with corresponding histological identification by H & E staining.
Figure 6.
The results of histopathologic examination of lung (left panel) and heart (right panel) were presented after treatment with various DOX loaded micelles to 4T1 tumor bearing mice. There was no 4T1 cancer metastasis in both lung and heart with treatment of DOX loaded in PHis-b-PEG/PLLA-b-PEG-folate mixed micelle. However, the features of tumor metastasis (♦) were found in negative group and treated groups by DOX in PBS, DOX in PLLA-b-PEG, and DOX in PHis-b-PEG. (★: Alveolar spaces of lung)
3. Results and Discussion
3.1. Cellular uptake at tumor extracellular pH
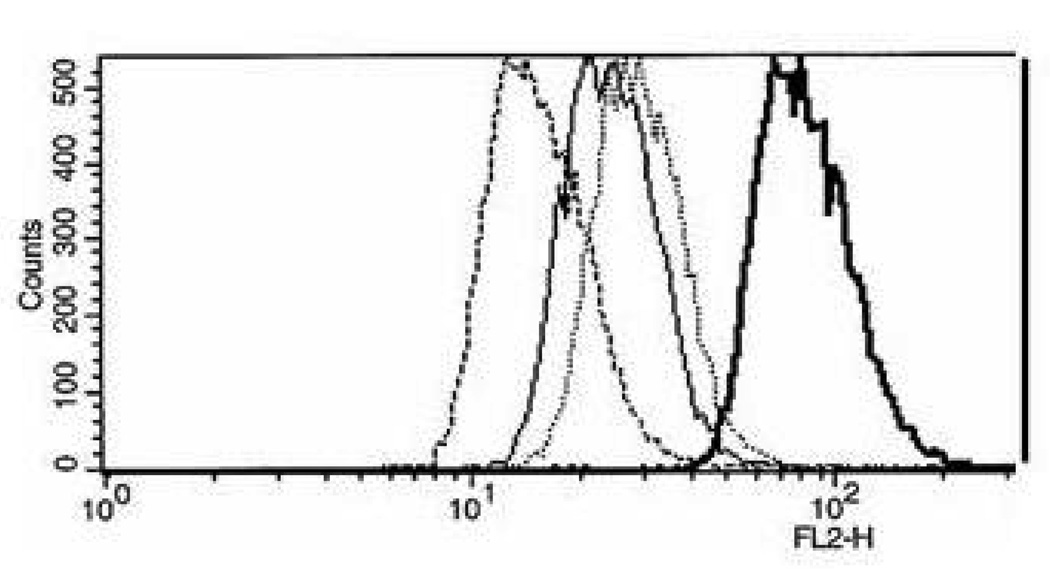
Fluorescence histograms of 4T1 cells incubated for 20 min with DOX carried by PLLA-b-PEG micelle, PHis-b-PEG micelle and PHis-b-PEG/PLLA-b-PEG-folate mixed micelle after being diluted with pH 6.8 complete medium to a concentration of 20 µg/ml DOX are presented in Figure 1. pH 6.8 was selected to mimic the slightly acidic environment of solid tumors [27]. All cell population-profile patterns were unimodal. The DOX fluorescence intensity of the cells incubated with DOX carried by PHis-b-PEG/PLLA-b-PEG-folate mixed micelle was higher than DOX by PHis-b-PEG micelle and PLLA-b-PEG micelle by approximately 4 and 8 times, respectively. Considering that PHis-b-PEG micelle was not stable below pH 7.2 [28] and DOX release rate from PHis-b-PEG micelle was significantly faster than that from PLLA-b-PEG micelle from our previous study [29], the result supports that 4T1 cells present folate receptors and receptor mediated uptake is faster than uptake of free DOX released from PHis-b-PEG micelle and fluid phase pinocytosis of PLLA-b-PEG micelle in a short time period (20 min).
Figure 1.
Fluorescence histograms of 4T1 cells incubated with DOX loaded in PLLA-b-PEG, PHis-b-PEG and PHis-b-PEG (70 wt%)/PLLA-b-PEG-folate (30 wt%) mixed micelles. The DOX concentration in the incubation media was maintained constant at 20 µg/ml. Cells incubated in pH 6.8 medium with PHis-b-PEG/PLLA-b-PEG-folate mixed micelles (bold lines), PHis-b-PEG (dotted line), PLLA-b-PEG (regular line) and control plain medium without DOX (bold dotted line). All cells were incubated for 20 min after adding micelles.
3.2. Cell cytotoxicity
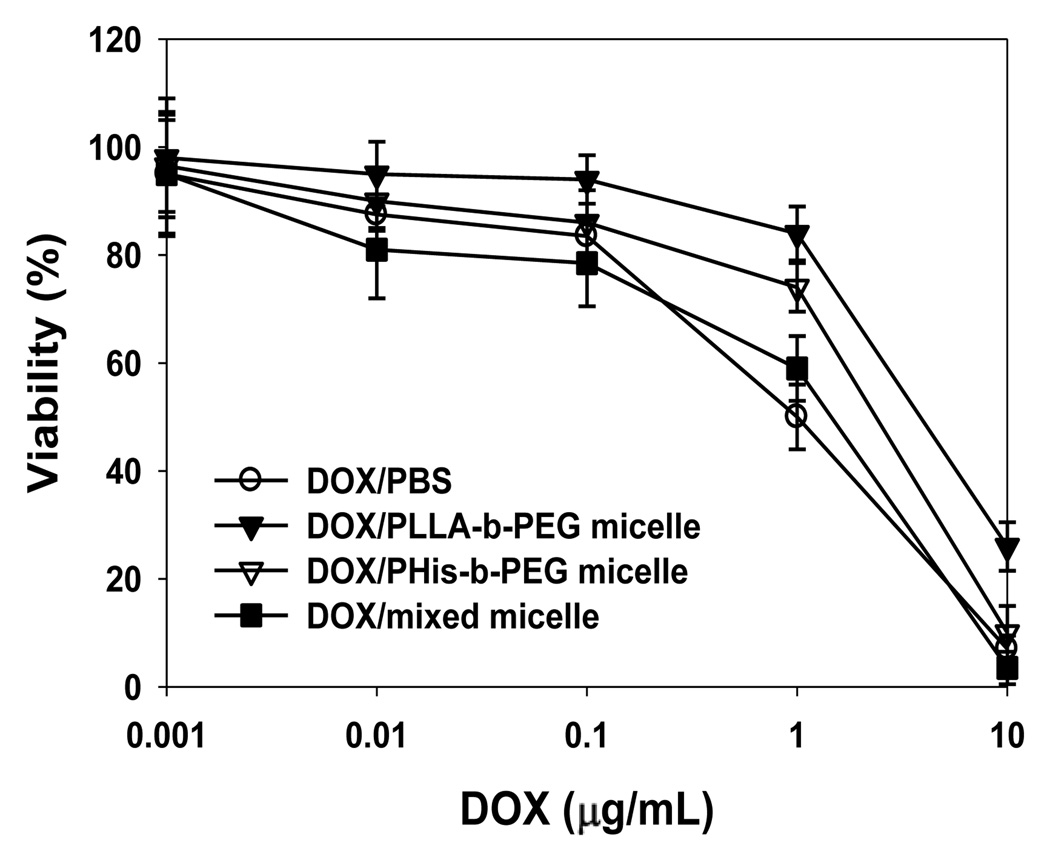
Cell cytotoxicity of DOX in PBS and DOX carried by various micelles against 4T1 cells was studied. The IC50 of DOX, a concentration at which 50% cells are killed, was 1.3, 1.4, 2.5 and 6.7 µg/ml for DOX in PBS, PHis-b-PEG/PLLA-b-PEG-folate, PHis-b-PEG and PLLA-b-PEG micelles, respectively (Fig. 2). The cytotoxicity of DOX carried by the mixed micelle was equivalent to free DOX and higher than that of DOX carried by PLLA-b-PEG and PHis-b-PEG micelles. This is attributed to higher cellular uptake and triggered release of DOX by endosomal pH. The cytotoxicity of DOX loaded in PHis-b-PEG was greater than DOX loaded in PLLA-b-PEG micelle because drug release rate from pH-sensitive PHis-b-PEG micelle at pH 6.8 is higher than pH-insensitive PLLA-b-PEG micelle. It is worth noting that no cytotoxicity was observed when 4T1 cells were treated with blank micelles. The micelles are developed to overcome resistance in Dox treatment. However, in this article, we are using 4T1 cells, not 4T1 resistant cells. So theoretically there shouldn’t be a difference between DOX in PBS and PHis-b-PEG/PLLA-b-PEG-folate groups, and there isn’t seemed to be a difference in the result. For PHis-b-PEG group, the slightly higher IC50 value is probably due to the dual effects of no targeting moiety and less stability due to lack of PLLA-b-PEG in micelle fabrication, but the pH sensitivity of the micelle is preserved and as a result the IC50 value is lower than that of PLLA-b-PEG group. The highest IC50 value is observed in PLLA-b-PEG group, since this group has neither targeting moiety nor triggered release mechanism.
Figure 2.
The viability of murine breast cancer 4T1 cells was studied after incubation with various micelles in pH 6.8 complete RPMI 1640 media, free DOX (a), DOX-loaded in PLLA-b-PEG micelle (b), DOX loaded in PHis-b-PEG(c) and DOX loaded in PHis-b-PEG/PLLA-b-PEG-folate micelles (d) at 37 °C for 72 h.
3.3. Inhibition of 4T1 tumor growth
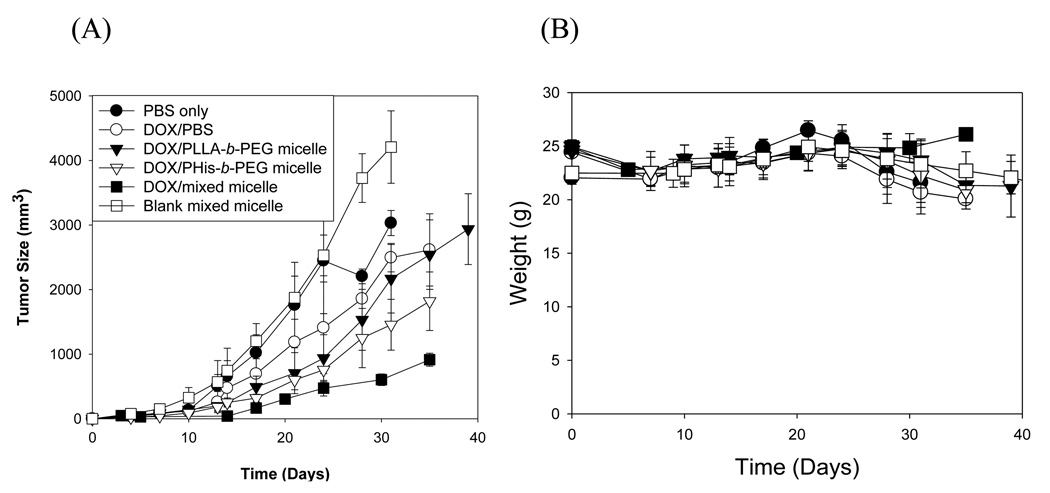
In negative control groups (PBS and blank Phis-b-PEG/PLLA-b-PEG-folate micelle), an exponential growth of tumor and a drop in body weight at the later stage were observed. The body weight loss despite an increase in tumor volume suggests physical weakness owing to the disease without any particular toxicity of the carrier. All groups treated with DOX showed a decrease in tumor growth rate but in varying degree. In DOX/PBS group, initial treatment retarded the growth of tumor. However, the growth rate became the fastest in the later stage among the treated groups with the body weight loss also the greatest, reflecting DOX toxicity. The group treated with DOX/PLLA-b-PEG micelle had a slower growth rate of tumor than DOX/PBS group. This finding is consistent with what was reported in previous articles [30]. The encapsulation of DOX in the core of the micelle reduces the random exposure of DOX to normal tissues. The passive accumulation of the micelles to the tumor sites due to EPR (enhanced permeability and retention) effect [30] increased efficacy. The DOX/PHis-b-PEG micelle, which may serve as a positive control (without folate) of our test micelle, although its composition is not identical), is more effective than the DOX/PLLA-b-PEG micelle. This effect is relevant to triggered release of DOX by extracellular pH. With a rapid cellular uptake, DOX/PHis-b-PEG/PLLA-b-PEG-folate formulation was the most effective for suppressing the tumor growth rate and it is the only group demonstrating no weight loss for entire observation period.
3.4. Animal survival test
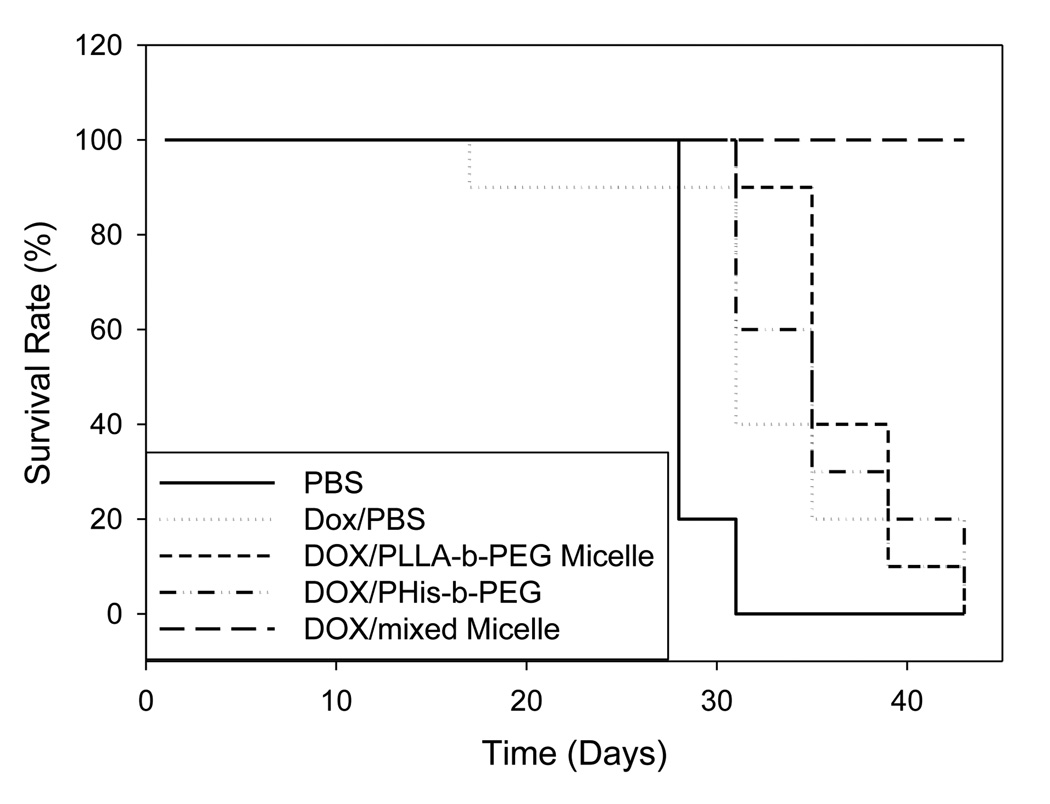
Survival curves are shown in Figure 4. The mice in negative control group (PBS only) were all moribund at the 31st day after inoculation of 4T1 cells. The treated groups with DOX in PBS, PHis-b-PEG and PLLA-b-PEG prolonged the survival time but all remained moribund at the 43rd day after inoculation. All mice in the group treated with DOX in PHis-b-PEG/PLLA-b-PEG-folate mixed micelles survived for the entire duration of the 43 day experiment. In a separate preliminary study, we observed that the mice in the test group had a survival rate of 9/10 for 70 days (data not presented).
Figure 4.
Survival curves for 4T1 tumor bearing mice. Mice were aged until moribund for up to 45 days after treatment with DOX in PBS, DOX loaded in PLLA-b-PEG, DOX loaded in PHis-b-PEG and DOX loaded in PHis-b-PEG/PLLA-b-PEG-folate micelles on 4T1 breast cancer cells subcutaneously transplanted in nu/nu mice. Each formulation was intravenously administered four times at a three-day interval at a dose of 10 mg/kg.
3.5. Cells growing in window chamber
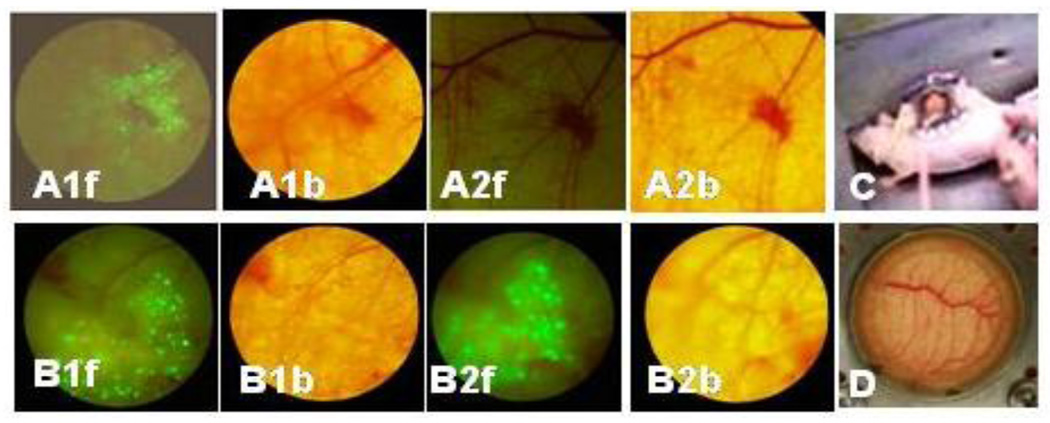
Green Fluorescent Protein (GFP) transfected 4T1 cells were inoculated into a mouse window chamber as shown in Figure 5. The growth of cells was directly visualized in the window chamber by observing green fluorescence using fluorescence microscopy. The fluorescence was observed on the first day after implantation of GFP transfected 4T1 cells in the fascia (as observed in fluorescent images in Fig. 5 A1f and B1f) indicating the rapid growth of cells. In one mouse (n=1), the DOX/PHis-b-PEG/PLLA-b-PEG-folate mixed micelle solution was injected intravenously. The other mouse received no treatment. After three days of treatment, almost no green fluorescence was observed (Fig. 5 A2f) for the treated mouse, while an increase in green fluorescence in mouse received no treatment (Fig. 5 B2f) was apparent. This observation directly supports the efficacy of DOX/mixed micelle in tumor suppression (Fig. 3) and prolonged survival time (Fig. 4).
Figure 5.
Mouse dorsal skin fold window chamber made of two symmetrical titanium frames (C and D). Green Fluorescent Protein labeled 4T1 murine breast cancer cells were inoculated into mouse window chamber (A and B). After implanting 4T1 breast cancer cells, Cells were growing in the window chamber observed by fluorescence microscope at first day (A1f and B1f; on the bright filed A1b and B1b). Green 4T1 images at the third day after i.v. injection of PHis-b-PEG/PLLA-b-PEG-folate mixed micelle were shown (A2f; on the bright field A2b) and without treatment (B2f, on the bright field B2b), respectively.
Figure 3.
Effects of the DOX in PBS, DOX loaded in PLLA-b-PEG micelle, DOX loaded in PHis-b-PEG and DOX loaded in PHis-b-PEG/PLLA-b-PEG-folate micelle on the growth of 4T1 breast cancer cells subcutaneously transplanted in nu/nu mice (female, n = 10). Each micelle was intravenously administered four times at a three-day interval at a dose of 10 mg/kg (A). Body weight changes of 4T1 tumor bearing nu/nu mice (female, n = 10) treated with micelles (B).
3.6. Histological examination of 4T1 metastasis
Metastatic tumors are usually very common during the later stages of cancer. Metastatis usually occurs via the blood or/and the lymphatic routes. 4T1 breast cancer cells spontaneously produces highly metastasis tumor that can spread into the lung, liver, lymph node and brain while the primary tumor still growing in situ [31,5]. After treatment of mice with DOX loaded in folate conjugated pH sensitive mixed micelles, lung, heart, liver, and spleen of the animals were analyzed. They showed a significant decrease in tumor metastasis. Histological analysis of primary tumors showed that these effects were due to the ability of mixed micelle to recognize 4T1 cancer cells and releasing DOX inside the cells hence blocking cell proliferation as well as the routes of cell spreading and thus preventing metastasis.
The infiltration of tumor cells into the heart was observed in many cases in the experiment as shown in Figure 6. It might be that the metastasized tumor in lung grew and invaded into the heart. However, there was no sign of tumor metastasis in the lung and heart after treatment with the mixed micelle when compared to images without any treatment (Figure 6). In the liver and kidney, a few cases showed foci of tumor metastasis. The summary of 4T1 metastasis experiment is listed in Table 1.
Table 1.
4T1 metastasis in mice treated with various doxorubicin formulations. Organs including lung, liver, heart, spleen and kidney were excised and fixed in 10% formalin solution, followed by paraffin embedding for histological H & E staining after terminatingthe experiment. The data were presented by the number of metastasis cases/the number of 4T1 cancer animals.
| Organ | Mixed micelle |
Negative control (PBS) |
DOX in PBS |
DOX in PLLA-b-PEG micelle |
DOX in PHis-b-PEG micelle |
|---|---|---|---|---|---|
| Lung | 0/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| Heart | 0/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| Liver | 0/5 | 1/5 | 0/5 | 1/5 | 1/5 |
| Kidney | 0/5 | 3/5 | 1/5 | 1/5 | 1/5 |
| Spleen | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
4. Conclusion
DOX loaded folate-pH sensitive micelles have the ability to suppress cancer cell proliferation and retard tumor growth and cancer cell metastasis. Folic acid acting as a targeting moiety combined with pH sensitive core phase carrier can recognize the cancer cells and trigger the drug release at tumor sites thus effectively killing them. The results from these studies provide evidence for the role of DOX loaded folate conjugated pH sensitive mixed micelle as therapeutic agents for inhibiting breast cancer growth and metastasis. The novel pH sensitive system of folate conjugated mixed micelle loaded with DOX is a promising new treatment for cancer metastasis.
Acknowledgement
This work was supported by NIH CA101850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee ES, Gao ZG, Bae YH. Recent progress in tumor pH targeting nanotechnology. J. Controlled Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart IR. "Seed and soil" revisited: Mechanisms of site-specific metastasis. Cancer Metastasis Rev. 1982;1:5–16. doi: 10.1007/BF00049477. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986;5:29–49. doi: 10.1007/BF00049529. [DOI] [PubMed] [Google Scholar]
- 4.Fodstad O, Aamdal S, McMenamin M, Nesland JM, Pini A. A new experimental metastasis model in athymic nude mice, the human malignant melanoma LOX. Int. J. Cancer. 1988;41:442–449. doi: 10.1002/ijc.2910410322. [DOI] [PubMed] [Google Scholar]
- 5.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 6.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2:331–334. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morecki S, Yacovlev E, Diab A, Slavin S. Allogeneic cell therapy for a murine mammary carcinoma. Cancer Res. 1998;58:3891–3895. [PubMed] [Google Scholar]
- 9.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60:2710–2715. [PubMed] [Google Scholar]
- 10.Moase EH, Qi W, Ishida T, Gabos Z, Longenecker BM, Zimmermann GL, Ding L, Krantz M, Allen TM. Anti-MUC-1 immunoliposomal doxorubicin in the treatment of murine models of metastatic breast cancer. Biochim. Biophys. Acta. 2001;1510:43–55. doi: 10.1016/s0005-2736(00)00334-5. [DOI] [PubMed] [Google Scholar]
- 11.ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin. Cancer Res. 2009;15:1973–1980. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu SQ, Wiradharma N, Gao SJ, Tong YW, Yang YY. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomaterials. 2007;28:1423–1433. doi: 10.1016/j.biomaterials.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Na K, Lee KH, Lee DH, Bae YH. Biodegradable thermo-sensitive nanoparticles from poly(L-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur. J. Pharm. Sci. 2006;27:115–122. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Han SK, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation. Colloids Surf. A: Physicochem. Eng. Asp. 2003;214:49–59. [Google Scholar]
- 15.Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J. Drug Target. 2005;13:391–397. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J. Controlled Release. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Wu NZ, Klitzman B, Rosner G, Needham D, Dewhirst MW. Measurement of material extravasation in microvascular networks using fluorescence video-microscopy. Microvasc. Res. 1993;46:231–253. doi: 10.1006/mvre.1993.1049. [DOI] [PubMed] [Google Scholar]
- 18.Wu NZ, Braun RD, Gaber MH, Lin GM, Ong ET, Shan S, Papahadjopoulos D, Dewhirst MW. Simultaneous measurement of liposome extravasation and content release in tumors. Microcirculation. 1997;4:83–101. doi: 10.3109/10739689709148320. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenbeld HC, Yuan F, Michel CC, Jain RK. Perfusion of single tumor microvessels: application to vascular permeability measurement. Microcirculation. 1996;3:349–357. doi: 10.3109/10739689609148307. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs SK, Monskey WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, Yuan F, Jain RK. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 22.Tozer GM, Prise VE, Wilson J, Cemazar M, Shan S, Dewhirst MW, Barber PR, Vojnovic B, Chaplin DJ. Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: intravital microscopy and measurement of vascular permeability. Cancer Res. 2001;61:6413–6422. [PubMed] [Google Scholar]
- 23.Dewhirst MW, Shan S, Cao Y, Moeller B, Yuan F, Li CY. Intravital fluorescence facilitates measurement of multiple physiologic functions and gene expression in tumors of live animals. Dis. Markers. 2002;18:293–311. doi: 10.1155/2002/820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang G, Jiang Y, Shen Y, Nam KH, Lee D, Gao Z. DNA loaded carrier preferential extravasation from tumor blood vessel. Int. J. Pharm. 2009;369:155–161. doi: 10.1016/j.ijpharm.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Lee ES, Gao ZG, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J. Controlled Release. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong P, Kleemann HW, Tannock IF. Cytostatic potential of novel agents that inhibit the regulation of intracellular pH. Br. J. Cancer. 2002;87:238–245. doi: 10.1038/sj.bjc.6600424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J. Controlled Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Gao ZG, Lee ES, Bae YH. In vivo evaluation of doxorubicin-loaded polymeric micelles targeting folate receptors and early endosomal pH in drug-resistant ovarian cancer. Mol. Pharm. 2009;6:1353–1362. doi: 10.1021/mp900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. Polyethylene glycol-diacyllipid micelles demonstrate increased accumulation in subcutaneous tumors in mice. Pharm. Res. 2002;19:1424–1429. doi: 10.1023/a:1020488012264. [DOI] [PubMed] [Google Scholar]
- 31.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]