Abstract
A series of microPET imaging studies were conducted in anesthetized rhesus monkeys using the dopamine D3-selective partial agonist, [18F]5. There was variable uptake in regions of brain known to express a high density of D3 receptors under baseline conditions. Pretreatment with lorazepam (1 mg/kg, i.v. 30 min) to reduce endogenous dopamine activity prior to tracer injection resulted in a dramatic increase in uptake in the caudate, putamen, and thalamus, and an increase in the binding potential (BP) values, a measure of D3 receptor binding in vivo. These data indicate that there is a high level of competition between [18F]5 and endogenous dopamine for D3 receptors in vivo.
Keywords: D3 receptors, Positron Emission Tomography, endogenous dopamine
INTRODUCTION
Alterations in the dopaminergic pathways are thought to be involved in the etiology of a variety of neurological and neuropsychiatric disorders, including Parkinson’s Disease, dystonia and schizophrenia (Jardemark et al., 2002; Kapur and Mamo, 2003; Karimi et al., in press; Korczyn, 2003; Lee et al., 1978; Luedtke and Mach, 2003; Missale et al., 1998; Nieoullon, 2002; Perlmutter et al., 1997). In addition, activation of the dopaminergic pathways may mediate the reinforcing effects of pyschostimulants, including cocaine and amphetamines (Nader et al., 1999; Uhl et al., 1998; Volkow et al., 2002).
Molecular genetic studies have defined two types of dopamine receptors, the D1-like (D1 and D5 receptor subtypes) and the D2-like (D2, D3 and D4 receptor subtypes), based upon structural and pharmacological similarities. D1-like receptors are structurally similar and positively linked to the activation of adenylyl cyclase via coupling to the Galpha(S)/Galpha(olf) class of G proteins (Herve et al., 2001). Stimulation of the D2-like receptors results in the coupling with the Gi/Go class of G proteins, leading to the inhibition of adenylyl cyclase activity (Sealfon and Olanow, 2000; Sibley and Monsma, 1992; Vallone et al., 2000).
There is approximately 78% amino acid homology in the transmembrane spanning (TMS) regions of the D2 and D3 receptors that comprise the ligand binding site of these receptors (Sokoloff et al., 1990). Despite the similarities in the structure of the D2 and D3 receptors, the D2 and D3 receptors differ in their a) neuroanatomical localization, b) levels of receptor expression, c) efficacy in response to agonist stimulation, and d) regulation and desensitization(Joyce, 2001; Luedtke and Mach, 2003; Mach et al., 2004). Because of the high localization of D3 receptors in limbic structures of the brain and the possibility that abnormalities in striatal D3 receptors contribute to the pathophysiology of dystonia, there has been a concerted effort to develop D3-selective radiotracers for imaging the expression of this receptor in a variety of CNS disorders.
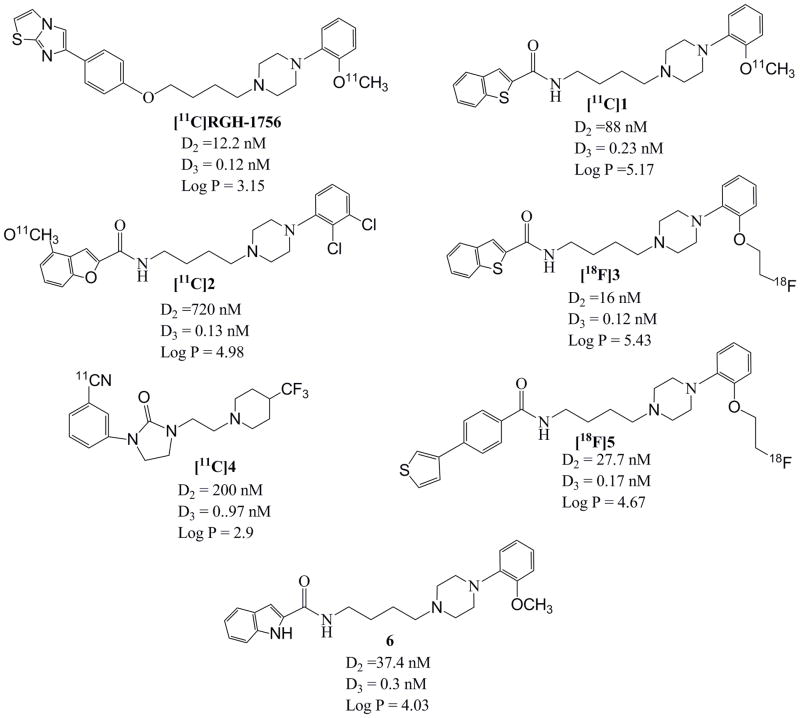
Previous efforts to image the D3 receptor have focused on the preparation of radiolabeled analogs of the conformationally-flexible benzamide analogs including, RGH-1756 (Langer et al., 2000; Sovago et al., 2005), the benzothiophene-2-carboxamide analog 1 (Kuhnast et al., 2006), the benzofuran-2-carboxamide analog 2 (Turolla et al., 2005), and [18F]3, which is a 2-fluoroethoxy analog of FAUC 356 (Hocke et al., 2008). However, the low uptake of these compounds in regions of the brain known to express D3 receptors was attributed to the high lipophilicity of these analogs, which could cause low uptake in the CNS and high level of nonspecific binding for the amount of the radiotracer that does cross the blood-brain barrier. Recently, a 11C-labeled imidazolidinone analog, [11C]4, was prepared and evaluated in vivo. This compound has a subnanomolar affinity for D3 receptors and a 100-fold selectivity for D3 versus D2 receptors. The log P value of this compound is 2.9, which means that it should readily cross the blood-brain barrier(Waterhouse, 2003). Unfortunately, in vivo imaging in rhesus monkeys revealed no specific binding to D3 receptors (Bennacef et al., 2009).
In this study, we report the synthesis of [18F]N-[4-[4-(2-(2-fluoroethoxy)phenyl)piperazine-1-yl]butyl]-4-(3-thienyl)benzamide, [18F]5, a radiolabeled D3 partial agonist that is capable of imaging the dopamine D3 receptor in vivo with positron emission tomography. We also report that there is a high level of competition between [18F]5 and endogenous dopamine for D3 receptors. However, D3 dopamine receptors can be efficiently and reproducibly imaged following the administration of the benzodiazepine agonist, lorazepam, which transiently reduces synaptic dopamine levels (Dewey et al., 1992; Nader et al., 2006).
MATERIALS AND METHODS
Radiosynthesis
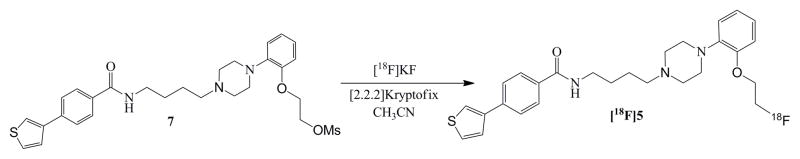
The synthesis of the mesylate precursor, 7, and unlabeled 5 (HPLC standard) will be published separately. [18F]5 was prepared via displacement of the corresponding mesylate precursor with [18F]potassium fluoride/Kryptofix 2.2.2 in DMSO at 80°C for 10 min.. The final product was purified by reversed-phase HPLC (C-18 column; 52% methanol: 48% ammonium formate buffer) in 50% yield and a specific activity of ~1,500 Ci/mmol.
Receptor Binding Assays
In vitro binding assays were conducted using the assay conditions described by Chu et al. in 2005. The radioligand used in the binding assay was [125I]IABN, which has a high affinity for dopamine D2, D3 and D4 receptors (Luedtke et al., 2000).
Intrinsic Activity Assay
The intrinsic activity at dopamine D3 receptors was determined using the cAMP assay conditions as described in 2005 by Chu et al. In this assay, quinpirole was used as a reference full agonist at both D2 and D3 dopamine receptors.
PET Data acquisition
MicroPET imaging studies were conducted on a Focus 220 microPET scanner (Siemens Medical Systems, Knoxville, TN, USA). Male rhesus monkeys (8 – 12 kg) were initially anesthetized with ketamine (10–15 mg/kg) and injected with glycopyrrolate (0.013 – 0.017 mg/kg) to reduce saliva secretions. PET tracers were administered ~ 90 minutes after ketamine injection. Subjects were intubated and placed on the scanner bed with a circulating warm water blanket and blankets. A water-soluble ophthalmic ointment was placed in the eyes, and the head was positioned in the center of the field using gauze rolls taped in place. Anesthesia was maintained with isoflurane (1.0 – 1.75 % in 1.5 L/min oxygen flow). Respiration rate, pulse, oxygen saturation, body temperature, and inspired/exhaled gasses were monitored throughout the study. Radiotracers and fluids were administered using a catheter placed percutaneously in a peripheral vein. For the metabolism studies, a catheter was placed percutaneously in the contralateral femoral artery to permit the collection of arterial blood samples and for the determination of the blood time-activity-curve using a continuous flow detection system (Hutchins et al., 1986). In each microPET scanning session, the head was positioned supine with the brain in the center of the field of view. A 10-minute transmission scan was performed to check positioning; once confirmed, a 45 minute transmission scan was obtained for attenuation correction. Subsequently, a 120-minute dynamic emission scan was acquired after administration of ~5 mCi of [18F]5 via the venous catheter. For the lorazepam studies, animals were given an intravenous dose of the drug (1 mg/kg in saline) approximately 30 min prior to injection of the radiotracer. Blocking studies were also conducted in lorazepam-treated animals by administering compound 6 (1 mg/kg, i.v.), a structurally-related benzamide analog (Figure 1), 5 min prior to tracer injection.
Figure 1.
Structures of D3-selective ligands reported in the literature.
Time Activity Curves and Metabolite analysis
Time activity curves for the initial 5 min post injection were determined using the continuous flow detection system attached to a percutaneous arterial catheter. Arterial blood samples for metabolite analysis were taken in a heparinized syringe from the same catheter at 5, 15, 30 and 60 min post injection. The 5 min sample was taken immediately after the pump for the detector was turned off; the arterial line was flushed prior to collection of subsequent samples. Additional blood samples were taken at 45, 90 and 120 min for the TAC (Figure 4C). Metabolite analysis was performed using a solid-phase extraction technique previously used for similar studies (Mach et al., 1997). A 1 mL aliquot of whole blood was centrifuged to separate plasma from packed red cells. Each fraction was counted, a 400 μL aliquot of plasma was removed, counted and deproteinated with 6 ml of 2: 1 methanol: 0.4 M perchloric acid mixture. The supernatant was diluted in 4 mL water and applied to an activated C-18 light Sep-Pak. The cartridge was neutralized with 2 mL 1N NaOH, then rinsed twice with water, and extracted with two portions of methanol (2 mL, 1 mL). All samples were counted in a Ludlum well counter. The methanol extracts were combined, concentrated in vacuo and rediluted to 150 μL of methanol for injection onto the HPLC. HPLC analysis was performed using a reversed-phase Phenomenex analytical column (Prodigy 250*4.6) with a mobile phase of methanol: 0.1 M ammonium formate buffer, pH 4.5 60:40. The flow rate was 0.8 ml/min, 0.5 min/fraction and 36 fractions were collected and counted or each sample. The location of the parent UV peak was determined by injection of cold standard. The purity and stability of the injectate were confirmed by analysis of an in vitro control (reserved injectate added to a pre-scan blood sample and processed as described above after all the ex vivo samples were completed). This control also confirmed the stability of the radiotracer under the conditions used to process the blood for metabolite analysis. The percentages of unchanged parent compound and its metabolites were determined by decay correcting the counts and dividing the amount of recovered activity in all samples and multiplying by 100. (Table 2) Only a single peak for the parent compound was observed in the in vitro control and > 95% of the activity was recovered.
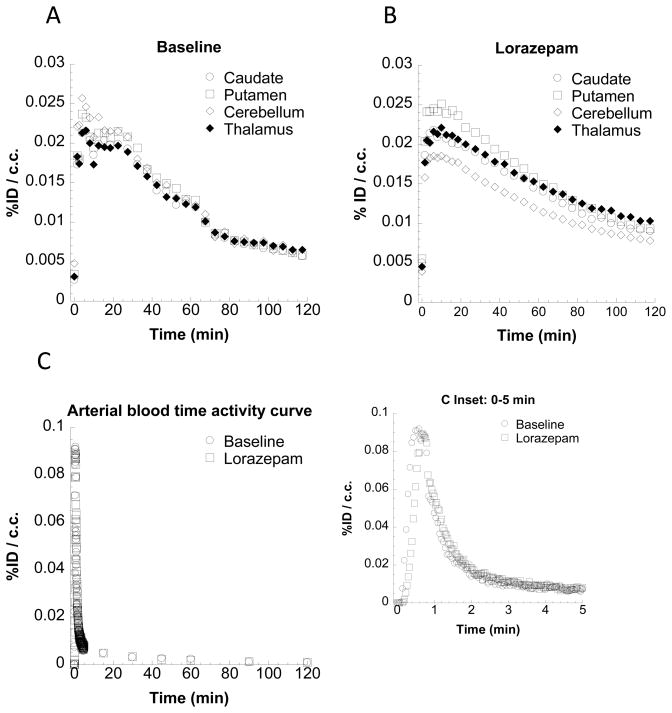
Figure 4.
Blood and Tissue time-activity curves (TACs) of [18F]5 in from microPET imaging studies.. A and B show regional brain TACs from baseline and lorazepam studies. The uptake of [18F]5 in the representative monkey brain regions (caudate, putamen, cerebellum, and thalamus) reached peak accumulation in the caudate and putamen 5 min post-i.v. injection. Lorazepam (1 mg/kg/i.v.) treatment increased [18F]5 uptake in the caudate, putamen and thalamus. C shows that lorazepam treatment did not change the arterial blood TAC compared to baseline data. The inset graph shows that there was also no change during the initial 5 minutes.
Table 2.
Percent parent compound in arterial blood samples
| Time (min) | % Parent Compound | |
|---|---|---|
| Baseline | Lorazepam* | |
| 5 | 27.9 ± 4.5 | 33.6 ± 3.3 |
| 15 | 15.8 ± 1.7 | 16.7 ± 4.6 |
| 30 | 12.7 ± 4.7 | 11.4 ± 1.4 |
| 60 | 7.9 ± 3.3 | 4.2 ± 2.8 |
30 min pre-treatment, 1 mg/kg/i.v.
Image processing and analysis
Acquired list mode data were histogrammed into a 3D set of sinograms and binned to the following time frames: 3 × 1 min, 4 × 2 min, 3 × 3 min and 20 × 5 min. Sinogram data were corrected for attenuation and scatter. Maximum a posteriori (MAP) reconstructions were done with 18 iterations and a beta value of 0. A 1.5 mm Gaussian filter was applied to smooth each MAP reconstructed image. These images were then co-registered with MRI images to identify the regions of interest with AnalyzeDirect software (AnalyzeDirect, Inc., Overland Park, KS). 3D regions of interest were manually drawn through all planes of co-registered MRI images for the caudate, putamen and cerebellum. The regions of interest were then overlaid on all reconstructed PET images to obtain time–activity curves. Activity measures were standardized to dose of radioactivity injected to yield %ID/c.c. (Figure 4A and B.).
RESULTS
In vitro binding studies indicate that 5 has a high affinity for D3 receptors (0.17 nM) and ~160-fold selectivity for D3 versus D2 receptors (Table 1). Functional assays demonstrated that 5 partially inhibited forskolin-dependent adenylyl cyclase activity relative to quinpirole (~ 35% maximal response) in CHO cells transfected with hD3 receptor (Table 1), indicating that it is a partial agonist at D3 receptors. These data are similar to that previously reported by our group for a diverse panel of N-phenyl piperazine analogs structurally similar to 5 (Chu et al., 2005).
Table 1.
| D2long | D3 | D4 | D2:D3 Ratio | %IA D2 | %IA D3 |
|---|---|---|---|---|---|
| 27.7 ± 5.4 nM | 0.17 ± 0.01 nM | 246 ± 13.3 nM | 163 | 29.3 ± 7.3 | 34.5 ± 1.7 |
Ki values (nM) were determined using human receptors expressed in HEK cells with 125I-IABN. The Ki values represent the mean values for n > 3 determinations.
Percent intrinsic activity (%IA) at human D2 or D3 receptors was determined using a forskolin-dependent adenylyl cyclase whole cell assay at a concentration of test compound >10 × Ki value. The mean values (n > 3) were normalized to values obtained using the full agonist quinpirole.
The synthesis of [18F]5 was achieved in approximately 60 min in an overall radiochemical yield of 50% from starting [18F]fluoride. The specific activity of the final compound was >1,500 mCi/μmol (end of synthesis), which is suitable for microPET imaging studies (Figure 2).
Figure 2.
Radiosynthesis of [18F]5.
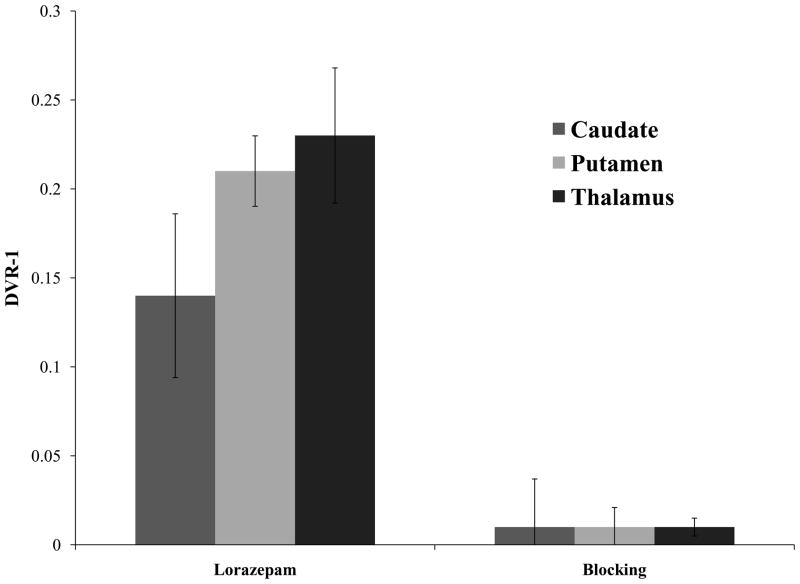
MicroPET studies were conducted in rhesus monkeys under 1% isoflurane anesthesia (n = 4). These initial microPET imaging studies yielded variable results (Figure 3), with one study demonstrating a high uptake in the caudate and putamen (monkey 4), but the other studies (monkeys 1–3) indicating no specific uptake of the radiotracer in regions of brain known to express D3 receptors (Xu et al., 2009; Xu et al., 2010). To determine if endogenous dopamine may be responsible for the variability of the PET results, a second series of studies were conducted in which the animal received an intravenous dose of lorazepam (1.0 mg/kg) 30 min prior to the injection of the radiotracer. Lorazepam was previously shown to increase striatal [11C]raclopride binding (Dewey et al., 1992), and we previously used this paradigm in our evaluation of the effect of endogenous dopamine to interfere with the binding of the D2/3 radiotracer, [18F]FCP, in PET studies in rhesus monkeys (Nader et al., 2006). The results of this study indicate that pretreatment with lorazepam eliminates the between-subject variability of the uptake of [18F]5 in regions of the brain known to express D3 receptors (Figure 3). There was also a notable increase in [18F]5 in the thalamus, a region of brain known to express D3 versus D2 receptors (Rabiner et al., 2009). The tissue-time activity curves in the lorazepam-treated animals relative to the baseline study, demonstrated a dramatic increase in D3 receptor availability as measured by the Logan DVR-1 analyses (Figure 4) (Logan, 2000). Lorazepam had no effect on the blood curve (Figure 4) or metabolism (Table 2) of [18F]5. This indicates that the increases seen in the brain were not the result of a change in the input function of the parent compound. Blocking studies with compound 6, a ligand having a high affinity and selectivity for D3 versus D2 receptors (Figure 1), are consistent with [18F]5 labeling D3 versus D2 receptors in vivo (Figure 5).
Figure 3.
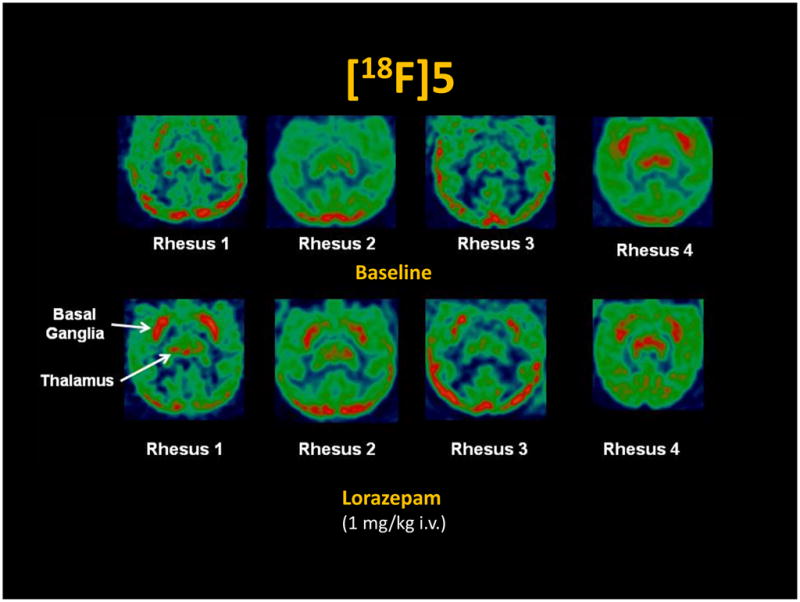
MicroPET imaging studies of [18F]5 in rhesus monkeys.
Figure 5.
Binding potential (BP) analysis in caudate, putamen and thalamus of microPET scans from baseline lorazepam treatment and blocking studies (under lorazepam). Blocking with Compound 6 (1 mg/kg/i.v.) significantly decreased [18F]5 binding potential in striatal regions: caudate (p < 0.05), putamen (p < 0.001) and extra-striatal regions: thalamus (p < 0.005).
DISCUSSION
The development of PET radiotracers having a high selectivity for dopamine D3 versus D2 receptors has been an active area of research in recent years. Although a number of D3 selective radiotracers, labeled with either carbon-11 (t = 20.4 min) or fluorine-18 (t = 110 min), have been reported over the past decade, none have proven to be useful for imaging this receptor in vivo. Consequently, studies aimed at imaging the D3 receptor must resort to using nonselective, high D2/D3 affinity radiotracers such as the radiolabeled antagonists [11C]raclopride (Volkow et al., 2008; Yokoi et al., 2002), [11C]fallypride or [18F]fallypride (Buchsbaum et al., 2006; Narendran et al., 2009; Narendran et al., 2004) and [11C]FLB457 (Cselenyi et al., 2006; Vandehey et al., 2010), and full agonists at D2 and D3 receptors, [11C](+)-PHNO (Ginovart et al., 2006; Narendran et al., 2006), and [11C]NPA (Hwang et al., 2000; Narendran et al., 2004). Although methods have been developed to separate the “D3 signal” from the “D2 signal” (Rabiner et al., 2009), radiotracers capable of imaging the D3 receptor without interference from the labeling of D2 receptors are clearly needed for in vivo studies with PET.
In the current study, we present evidence demonstrating that [18F]5 is a potential radiotracer for imaging D3 receptors in vivo with PET. [18F]5 has a subnanomolar affinity for D3 receptors and a 160-fold higher affinity for D3 compared to D2 dopamine receptors. An important observation in the current study is the ability of endogenous dopamine to compete with D3 receptors in vivo to the extent that it is necessary to decrease synaptic levels of dopamine by pretreatment with lorazepam to image D3 receptors in the anesthetized rhesus monkey. This observation is consistent with previous reports regarding the high in vivo occupancy of D3 receptors by endogenous dopamine, including: 1) the binding of [125I]iodosuplride to D3 receptors in autoradiography studies requires either extensive washing of tissue slices to remove endogenous dopamine, or the in vivo depletion of monoamines with tetrabenazine prior to euthanasia and tissue preparation (Schotte et al., 1996; Schotte et al., 1992) and, 2) endogenous dopamine protects the D3 receptor from alkylation by 1-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) and the spiperone analog, N-(p-isothiocyanatophenethyl) spiperone (NIPS), in vivo whereas no such protection was observed at D2 receptors (Levant, 1995; Zhang et al., 1999). Finally, several in vitro binding studies have revealed that dopamine has a higher affinity for cloned D3 versus D2 receptors (reviewed in Levant, 1997), and supports a greater occupancy of D3 receptors by endogenous dopamine in vivo.
An unresolved question from the current study relates to the ability to image D3 receptors in vivo without the need to deplete the dopaminergic synapse of endogenous neurotransmitter. It must be stressed that the current study was conducted under conditions of isoflurane anesthesia. Previous studies by Votaw and colleagues have shown that isoflurane increases the trafficking of the dopamine transporter (DAT) from the plasma membrane to the cell interior (Votaw et al., 2003; Votaw et al., 2004), which may contribute to the increase in extracellular dopamine concentrations measured in microdialysis studies (Adachi et al., 2005; Tsukada et al., 1999). Potentiation of GABAergic neurotransmission by pretreatment with lorazepam decreases activity of dopaminergic neurons (Dewey et al., 1992). Consequently, lorazepam should minimize the effect of isoflurane-induced DAT internalization and prevent increased synaptic dopamine levels, thereby leading to an increase in the availability of D3 receptors for labeling with [18F]5. It is unlikely that all D3 receptors in the CNS are occupied by endogenous dopamine. If this were the case, it would impair imaging D3 receptors using the nonselective D2/D3 radiotracers such as [18F]fallypride and [11C](+)-PHNO. It is important to note that both [18F]fallypride (a D2/D3 antagonist) and [11C](+)-PHNO (a D2/D3 full agonist) are capable of imaging extrastriatal D3 receptors in isoflurane-anesthetized nonhuman primate brain (Christian et al., 2000; Rabiner et al., 2009). In fact, one study reported that isoflurane anesthesia increased the binding of [11C](+)-PHNO to striatal D2/D3 receptors in Sprague-Dawley rats relative to awake animals (McCormick et al., 2006). Further studies are clearly needed, in either awake rhesus monkeys or in translational imaging studies in human subjects, to determine the ability of [18F]5 to image D3 receptors under conditions where physiological levels synaptic dopamine are present.
In summary, the results of the current study indicate that it is possible to image dopamine D3 receptors in vivo with PET with [18F]5 even though this has not been demonstrated with structurally-related radiotracers. It should be noted that all of the radiotracers shown in Figure 1 have a similar high (i.e., subnanomolar) affinity for D3 receptors and high selectivity for D3 versus D2 receptors (>100-fold). The key step taken in the current study was the use of lorazepam to reduce synaptic dopamine levels under conditions of isoflurane anesthesia, which should minimize competition between endogenous dopamine and [18F]5 for D3 receptors and increase D3 receptor availability. Additional studies are ongoing to determine if this effect is observed with other, high affinity D3-selective radiotracers developed by our group.
Acknowledgments
The authors would like to thank the staff of the Nonhuman Primate microPET facility for their assistance and technical expertise. This research was funded by NIH grants DA16181, NS048056, and NS058714.
References
- Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T. Isoflurane anesthesia induces biphasic effect on dopamine release in the rat striatum. Brain Res Bull. 2005;67(3):176–181. doi: 10.1016/j.brainresbull.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Bennacef I, Salinas CA, Bonasera TA, Gunn RN, Audrain H, Jakobsen S, Nabulsi N, Weinzimmer D, Carson RE, Huang Y, Holmes I, Micheli F, Heidbreder C, Gentile G, Rossi T, Laruelle M. Dopamine D3 receptor antagonists: the quest for a potentially selective PET ligand. Part 3: Radiosynthesis and in vivo studies. Bioorg Med Chem Lett. 2009;19(17):5056–5059. doi: 10.1016/j.bmcl.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J, Kemether E, Oakes TR, Mukherjee J. D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophr Res. 2006;85(1–3):232–244. doi: 10.1016/j.schres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Christian BT, Narayanan TK, Shi B, Mukherjee J. Quantitation of striatal and extrastriatal D2 dopamine receptors using PET imaging of [18F]fallypride in nonhuman primates. Synapse. 2000;38(1):71–79. doi: 10.1002/1098-2396(200010)38:1<71::AID-SYN8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorg Med Chem. 2005;13(1):77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Cselenyi Z, Olsson H, Halldin C, Gulyas B, Farde L. A comparison of recent parametric neuroreceptor mapping approaches based on measurements with the high affinity PET radioligands [11C]FLB 457 and [11C]WAY 100635. Neuroimage. 2006;32(4):1690–1708. doi: 10.1016/j.neuroimage.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12(10):3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97(4):1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Herve D, Le Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, Jaber M, Studler JM, Girault JA. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J Neurosci. 2001;21(12):4390–4399. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocke C, Prante O, Salama I, Hubner H, Lober S, Kuwert T, Gmeiner P. 18F-Labeled FAUC 346 and BP 897 derivatives as subtype-selective potential PET radioligands for the dopamine D3 receptor. Chem Med Chem. 2008;3(5):788–793. doi: 10.1002/cmdc.200700327. [DOI] [PubMed] [Google Scholar]
- Hutchins GD, Hichwa RD, Koeppe RA. A Continuous Flow Input Function Detector for H215 O Blood Flow Studies in Positron Emission Tomography. Nuclear Science, IEEE Transactions. 1986;33(1):546–549. [Google Scholar]
- Hwang DR, Kegeles LS, Laruelle M. (−)-N-[11C]propyl-norapomorphine: a positron-labeled dopamine agonist for PET imaging of D2 receptors. Nucl Med Biol. 2000;27(6):533–539. doi: 10.1016/s0969-8051(00)00144-x. [DOI] [PubMed] [Google Scholar]
- Jardemark K, Wadenberg ML, Grillner P, Svensson TH. Dopamine D3 and D4 receptor antagonists in the treatment of schizophrenia. Curr Opin Investig Drugs. 2002;3(1):101–105. [PubMed] [Google Scholar]
- Joyce JN. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol Ther. 2001;90(2–3):231–259. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Karimi M, Moerlein SM, Videen TO, Luedtke RR, Taylor M, Mach RH, Perlmutter JS. Decreased striatal dopamine receptor binding in primary focal dystonia: a D2 or D3 defect? Movement Disorders. doi: 10.1002/mds.23401. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczyn AD. Dopaminergic drugs in development for Parkinson’s disease. Adv Neurol. 2003;91:267–271. [PubMed] [Google Scholar]
- Kuhnast B, Valette H, Besret L, Demphel S, Coulon C, Ottaviani M, Guillermier M, Bottlaender M, Dolle F. Synthesis and radiolabeling of N-[4-[4-(2-[11C]methoxyphenyl)piperazin-1-yl]butyl]benzo[b]thiophene-2-carboxamide -- a potential radiotracer for D3 receptor imaging with PET. Nucl Med Biol. 2006;33(6):785–795. doi: 10.1016/j.nucmedbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Langer O, Gulyás B, Sandell J, Laszlovszky I, Kiss B, Domány G, Ács T, Farde L, Halldin C. Radiochemical labelling of the dopamine D3 receptor ligand RGH-1756. Journal of Labelled Compounds and Radiopharmaceuticals. 2000;43(11):1069–1074. [Google Scholar]
- Lee T, Seeman P, Rajput A, Farley IJ, Hornykiewicz O. Receptor basis for dopaminergic supersensitivity in Parkinson’s disease. Nature. 1978;273(5657):59–61. doi: 10.1038/273059a0. [DOI] [PubMed] [Google Scholar]
- Levant B. Differential sensitivity of [3H]7-OH-DPAT-labeled binding sites in rat brain to inactivation by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline. Brain Res. 1995;698(1–2):146–154. doi: 10.1016/0006-8993(95)00879-u. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharm Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27(7):661–670. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Freeman RA, Boundy VA, Martin MW, Huang Y, Mach RH. Characterization of 125I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38(4):438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Curr Pharm Des. 2003;9(8):643–671. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- Mach RH, Huang Y, Freeman RA, Wu L, Vangveravong S, Luedtke RR. Conformationally-flexible benzamide analogues as dopamine D3 and σ2 receptor ligands. Bioorg Med Chem Lett. 2004;14(1):195–202. doi: 10.1016/j.bmcl.2003.09.083. [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Gage HD, Morton TE. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav. 1997;57(3):477–486. doi: 10.1016/s0091-3057(96)00449-2. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Ginovart N, Vasdev N, Seeman P, Kapur S, Wilson AA. Isoflurane increases both the specific binding ratio and sensitivity to amphetamine challenge of [11C]-(+)-PHNO. NeuroImage. 2006;31(Supplement 2):T33–T33. [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Nader MA, Green KL, Luedtke RR, Mach RH. The effects of benzamide analogues on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1999;147(2):143–152. doi: 10.1007/s002130051154. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, Vora S, Litschge M, Kendro S, Cooper TB, Mathis CA, Laruelle M. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63(6):447–461. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi-Dargham A, Laruelle M. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52(3):188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60(7):485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, Moerlein SM. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17(2):843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63(9):782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen P, Bonaventure P, Leysen J. Endogenous dopamine limits the binding of antipsychotic drugs to D3 receptors in the rat brain: a quantitative autoradiographic study. The Histochemical Journal. 1996;28(11):791–799. doi: 10.1007/BF02272152. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Leysen JE. Autoradiographic evidence for the occlusion of rat brain dopamine D3 receptors in vivo. Eur J Pharmacol. 1992;218(2–3):373–375. doi: 10.1016/0014-2999(92)90196-b. [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Olanow CW. Dopamine receptors: from structure to behavior. Trends Neurosci. 2000;23(10 Suppl):S34–40. doi: 10.1016/s1471-1931(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sovago J, Farde L, Halldin C, Schukin E, Schou M, Laszlovszky I, Kiss B, Gulyas B. Lack of effect of reserpine-induced dopamine depletion on the binding of the dopamine-D3 selective radioligand, [11C]RGH-1756. Brain Res Bull. 2005;67(3):219–224. doi: 10.1016/j.brainresbull.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N, Nakanishi S. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res. 1999;849(1–2):85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- Turolla EA, Matarrese M, Belloli S, Moresco RM, Simonelli P, Todde S, Fazio F, Magni F, Kienle MG, Leopoldo M, Berardi F, Colabufo NA, Lacivita E, Perrone R. 11C-labeling of n-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl]arylcarboxamide derivatives and evaluation as potential radioligands for PET imaging of dopamine D3 receptors. J Med Chem. 2005;48(22):7018–7023. doi: 10.1021/jm050171k. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Vandenbergh DJ, Rodriguez LA, Miner L, Takahashi N. Dopaminergic genes and substance abuse. Adv Pharmacol. 1998;42:1024–1032. doi: 10.1016/s1054-3589(08)60922-9. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24(1):125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Vandehey NT, Moirano JM, Converse AK, Holden JE, Mukherjee J, Murali D, Nickles RJ, Davidson RJ, Schneider ML, Christian BT. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30(5):994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13(5–6):355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, Ma J, Pradhan K, Tomasi D, Thanos PK, Ferre S, Jayne M. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28(34):8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw J, Byas-Smith M, Hua J, Voll R, Martarello L, Levey AI, Bowman FD, Goodman M. Interaction of isoflurane with the dopamine transporter. Anesthesiology. 2003;98(2):404–411. doi: 10.1097/00000542-200302000-00021. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Byas-Smith MG, Voll R, Halkar R, Goodman MM. Isoflurane alters the amount of dopamine transporter expressed on the plasma membrane in humans. Anesthesiology. 2004;101(5):1128–1135. doi: 10.1097/00000542-200411000-00012. [DOI] [PubMed] [Google Scholar]
- Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5(6):376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [3H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl]benzamide, a selective radioligand for dopamine D3 receptors. I. In vitro characterization. Synapse. 2009;63(9):717–728. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [3H]4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl) butyl)benzamide: a selective radioligand for dopamine D3 receptors. II. Quantitative analysis of dopamine D3 and D2 receptor density ratio in the caudate-putamen. Synapse. 2010;64(6):449–459. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248–259. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- Zhang K, Weiss NT, Tarazi FI, Kula NS, Baldessarini RJ. Effects of alkylating agents on dopamine D3 receptors in rat brain: selective protection by dopamine. Brain Res. 1999;847(1):32–37. doi: 10.1016/s0006-8993(99)02024-7. [DOI] [PubMed] [Google Scholar]