Abstract
We examined the effects of periodic access to a palatable, high sugar content food (candy) in 8 male baboons on the anorectic response to d-amphetamine, which increases dopamine, and dexfenfluramine, which increases serotonin. During candy access, up to 200 candies containing 75% of energy as sugar were available during the morning on Mondays, Wednesdays and Fridays; food pellets (19% of energy as sugar) were available in the afternoon and throughout the remaining days of the week. During candy access, baboons consumed a mean of 177 pieces of candy containing 696 kcal (2.91 MJ) in the morning compared to 44 food pellets and 150 kcal (0.63 MJ) in the morning on non-candy days. Food pellet intake was lower during candy access. Complete dose-response functions for the effects of the drugs on food pellet intake on days that candy was not available were determined before, during, and after the period of access to candy. Dexfenfluramine and amphetamine produced dose-dependent decreases in food pellet intake and increases in latency to eat food pellets before, during, and after candy access. During access to candy, the dose-response function for dexfenfluramine was shifted to the right indicating the development of tolerance, while that for amphetamine was shifted to the left indicating sensitization. Only the dose-response function for dexfenfluramine returned to baseline after candy access suggesting that the difference was specific to concurrent palatable food consumption. We hypothesize that tolerance to the effects of dexfenfluramine reflects a decrease in the satiating effect of serotonin release due to repeatedly eating large amounts of palatable food.
Keywords: Food Intake, Baboon, Sugar, Satiation, Dexfenfluramine, Amphetamine, Tolerance, Sensitization
Introduction
Behavioral similarities between excessive drug use, i.e., drug abuse and excessive food consumption, i.e., food abuse have long been noted [1], and a range of studies, mostly accomplished in laboratory rodents, highlight neurochemical and physiological similarities in drug abuse and overeating. For example, intermittent consumption of large amounts of a palatable food that increases dopamine release can produce similar changes in brain chemistry as the repeated use of a drug that also increases dopamine release [2]. Consumption of sucrose plus chow increased the place preference conditioned by amphetamine [3], suggesting that diet increased the rewarding efficacy of dopaminergic compounds. Further, in another study [4] when rats self-administered oral amphetamine and had access to granulated sucrose and rat chow, their amphetamine intake decreased compared to when they access to chow alone. One interpretation of these findings is that sucrose and amphetamine were functioning as economic substitutes for each other [5]; an effect we have observed in non-human primates [6, 7]. In several studies, propensity to drink sucrose solutions was correlated with larger locomotor responses to amphetamine [8], or propensity to acquire amphetamine self-administration [9]. Several studies have also demonstrated cross-sensitization between sugar and amphetamine [10, 11].
It is a bit surprising that little data exist on the effects of dietary manipulations on the response to serotonergic drugs. Furthermore, the data that do exist are contradictory. An early study [12] failed to see an effect of a high sucrose diet on the anorectic effects of the serotonin releaser dexfenfluramine in rats. Another research group, however, has observed an attenuated behavioral response, e.g., paw treading, of the direct serotonin 1-A receptor agonist 8-hydroxy-N.N-dipropyl-2-aminotetralin (8-OH-DPAT) following consumption of a diet high in sucrose in rats [13, 14], hypothesized to be related to serotonin receptor desensitization [15, 16].
Because the data obtained in rodents clearly shows that diet can affect response to drugs, but the direction of change varies across studies and drugs, in this study we compared the effect of a dietary manipulation on the anorectic effects of the serotonergic drug dexfenfluramine and the dopaminergic drug d-amphetamine in non-human primates.
A serotonergic and a dopaminergic drug were chosen based on suggestions that these 2 neurotransmitters affect different aspects of feeding behavior. An increase in serotonin, as caused by dexfenfluramine [17], has been hypothesized to be vital for the development of satiation [see 18 review]. An increase in dopamine, as caused by amphetamine [19], has been hypothesized to decrease hunger. If consumption of large amounts of a single macronutrient specifically affects hunger or satiation by altering the body’s response to food even in the absence of the macronutrient, it may in turn alter the response to pharmacological manipulations that affect hunger and satiation.
In order to investigate how altering macronutrient intake can alter the response to a drug it is important to have procedures that generate excessive intake of that nutrient. Rodents avidly consume sucrose and fat. If given access to fat [20] or sucrose [21] during a part of the day when rats normally eat little (light cycle), they will eat large amounts of fat or sucrose. The sugar or fat consumption increases total energy intake, alters macronutrient contribution to intake and disrupts the pattern of feeding [see review 22]. Rats given access to fat for 2 hr/day (3 hr before the dark cycle) 3 days per week eat massive amounts of fat during those 2 hrs [23, 24]. We have adapted these procedures to generate “binge” consumption of food high in fat or sugar in baboons [25, 26]. When free-feeding baboons are given access to a preferred candy food item in the morning 3 days a week they derive as much energy from that item in a single “meal” as they do from the standard diet the remainder of the day [26]. Thus, the procedures not only generated excessive intake of a sugar-based candy, but because the candy was only available intermittently, it modeled periodic over-consumption of treats or snack foods.
In the present study, a complete dose-response function for the effects of d-amphetamine, which increases dopamine levels, and dexfenfluramine, which increases serotonin levels on food pellet consumption was determined before, during and after an 8-week period of access to a high-sugar candy 3 days a week. We used a procedure that allowed baboons to earn 20 candies once every 15 minutes over 2 hours each morning (200 max). Baboons could then consume pellets during 4 possible afternoon meals, with number of meals and the size of each meal determined by each baboon. Although the baboons were not food deprived, other than during the dark cycle, the procedure limited the variability in the pattern of food intake. Limiting the rate of food intake by forcing breaks between eating the 20 candies has been shown to provide an eating baseline that was sensitive to the hypothesized effects of drugs on satiation [27].
The repeated administration of amphetamine to baboons results in the development of tolerance to its anorectic effects [28], i.e., the dose-response function determined during repeated administration was shifted to the right of the dose-response function determined before or after repeated administration. There was no evidence of cross-tolerance between dexfenfluramine and amphetamine [28]. Other studies have shown that rats who are tolerant to the effects of amphetamine on feeding behavior are cross-tolerant to the effects of other dopaminergic stimulant drugs [e.g., 29], indicating that drug exposure produced long-term changes in specific neurotransmitter function. We hypothesized that candy consumption 3 days a week would function as a drug does on central neurotransmitters altering brain response to other pharmacological manipulations, even in the absence of candy. Thus, we predicted that the dopaminergic drug, amphetamine, would produce smaller decreases in pellet intake when baboons were eating palatable food. In contrast, we predicted that the decrease in food intake caused by the serotonergic drug, dexfenfluramine, would not be affected by palatable food.
Method
Animals
Eight experimentally naïve adult male baboons (Papio cynocephalus anubis), initially weighing 17.5 to 23.1 (Mean = 19.9) kg were individually housed in custom-designed non-human primate cages (0.94 × 1.21 × 1.52 m high) at The New York State Psychiatric Institute. The room was illuminated with fluorescent lighting from 7:00 AM to 7:00 PM daily. In addition to food and candy earned during experimental sessions, two chewable vitamins, two pieces of fresh fruit, and a dog biscuit were also given daily. Water was available ad libitum from a spout located at the back of each cage. All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
Apparatus
A response panel holding, from bottom to top, a food hopper, 2 pull-type, “Lindsley” response levers spaced 0.30 m apart (Gerbrands, Arlington, MA), 4 stimulus lights (two above each lever), and 2 pellet dispensers (BRS-LVE model PDC-005, Beltsville, MD) was attached to the front of each cage. All schedule contingencies were programmed using Pascal on Macintosh (Cupertino, CA) computers located, along with the interface, in an adjacent room.
Brief Morning Candy and Food Pellet Sessions (Responding on Right Lever)
There were 10 brief sessions beginning each day at 9:00 AM at 15 min intervals (Table 1). The beginning of each session was signaled by illumination of the right light above the right lever. The first pull on the right lever started a 6 min timer. During that interval each time the right lever was pulled 10 times both lights above the right lever flashed 10 times. The first time the baboon pulled the lever 10 times after 6 min had elapsed the left light above the right lever was illuminated. The next time the right lever was pulled 10 times 10 food pellets (Table 1) were delivered accompanied by the flashing lights. When candy was available 10 to 20 pieces of original fruit-flavored Skittle® candy were delivered. Baboons had 15 min to respond during each brief session. Failure to complete the minimum number of lever presses in 15 min terminated that brief session. If the baboon earned his pellet or candy deliveries in less than 15 min, no lights would be illuminated until the start of the next brief session.
Table 1.
Weekly Schedule of Activities and the Experimental Day and Weekly Schedule of Candy and Pellet Availability during Brief Sessions
| Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|
| Pellets | Candy1,3 | Pellets2,3 | Candy1 | Pellets2 | Candy1 | Pellets |
| Experimental Day | ||||||
| 7:00 AM | Room Lights Turned On | |||||
| 7:00–8:30 AM | Morning Pellet Meal (Breakfast) Available | |||||
| 8:50 AM | Drug Injection | |||||
| 9:00–11:30 AM | 10 Brief Sessions for Pellets or Candy | |||||
| 11:30 AM–1:00 PM | Pellet Meal Available | |||||
| 1:30 PM–3:00 PM | Pellet Meal Available | |||||
| 3:30 PM–5:00 PM | Pellet Meal Available | |||||
| 5:30 PM–7:00 PM | Pellet Meal Available | |||||
| 7:00 PM | Rooms Lights Extinguished | |||||
Pellets were available before and after the period of candy availability
Dose-response functions were determined by giving active drug or placebo on Tuesdays and Thursdays. Control data were obtained after placebo administration on Tuesdays, Thursdays or Sundays
| Food Pellet | Candy | |
| Type | Grain-based | Skittle® candy |
| Details | Banana-flavored 1-g | 1 Jelly candy, 5 flavors |
| Manufacturer | Bio-Serv, Inc., Frenchtown, NJ | Mars Corp., Hackettstown, NJ] |
| Energy | 3.4 kcal (13.22 kJ) | 4.00 kcal (16.74 kJ) |
| Protein g (% Kcal) | 0.21 g (25%) | 0.002 g (0.2%) |
| Fat g (% Kcal) | 0.04 g (10%) | 0.044 g (9.8%) |
| Carbohydrate g (% Kcal) | 0.55 g (65%) | 0.910 g (90%) |
| Sugars g (% Kcal) | 0.16 g (19%) | 0.75 g (75%) |
| Other g (% Kcal) | 0.39 g (46%) | 0.15 g (15%) |
| Other (Ash, Vitamins) | 0.11 g | 0.01 g |
| Water | <10% | 3.7% |
Pulling the right lever 10 times resulted in the delivery of 10 food pellets [grain-based, banana-flavored one-g food pellets containing 3.4 kcal (13.22 kJ): 0.21 g protein, 0.4 g fat, 0.55 g carbohydrate (0.16 g sugars, 0.39 g other carbohydrates), 0.67 g minerals and vitamins, e.g., ash, 0.04 g fiber, < 10.0% moisture; caloric content derived from 25% protein, 10% fat, 19% sugar, 46% other carbohydrate; Bio-Serv, Inc., Frenchtown, NJ] accompanied by the lights above the lever flashing 10 times, or when candy was available the delivery of 10 to 20 pieces of original fruit-flavored Skittle® candy (4.00 kcal (16.74 kJ) : 0.002 g protein, 0.044 g fat, 0.91 g carbohydrate (0.75 g sugars, 0.15 g other carbohydrates), 0.01 g minerals and vitamins, 3.7% moisture; caloric content derived from 0.2% protein, 9.8% fat, 75% sugar, 15% other carbohydrate; Mars Corp., Hackettstown, NJ]
Pellet Meals (Responding on Left Lever)
Pellet meals were available once at 7:00 AM each morning and 4 times in the afternoon (Table 1). Pellet meal availability was signaled by the illumination of the right light over the left lever. If a baboon wanted to eat a meal of pellets it had to pull the left lever. The first pull on the left lever started a 30 min a timer. During that interval each time the left lever was pulled 10 times both lights above the left lever flashed 10 times. The first time the baboon pulled the lever 10 times after 30 min had elapsed, the left light above the left lever was illuminated. Each time the left lever was then pulled 10 times baboons received 1 food pellet accompanied by the flashing lights. There was a 10 s interval after each pellet delivery when responding was not counted. Pellet meals ended when the 90 min session terminated or when the baboon stopped responding for 10 min. As with the brief sessions, baboons had the option to not respond.
Procedure
After baboons acclimated to the housing conditions, they were trained to respond for food pellets under the conditions described above i.e., 10 brief sessions in the morning and opportunities for a pellet meal through the day, except overnight when baboons rarely eat. Once responding for pellets was stable (no upward or downward trends in pellet intake) complete dose response functions were determined for d-amphetamine and dexfenfluramine, in that order, with dose range based on previous studies in baboons [e.g., 27]. Four doses of each drug plus placebo were tested with each dose-response function requiring about 3 weeks for determination: placebo was tested on 25% of the test days to control for conditioned responses. In order to further control for conditioned responses, a different dose order was used for each baboon during each of the 3 dose-response determinations. Furthermore, at each dose-response determination half of the baboons were tested with the smaller doses first and half were tested with the larger doses first. Dose order was systematically varied within and across baboons. Up to this point, baboons had never had Skittles candy. We then substituted Skittles candy for pellets during the morning brief sessions on Mondays, Wednesdays and Fridays. Baboons initially received 10 candies per completed brief session (100 possible per day). After 4 weeks, the number of candies delivered was increased to 15 per completed session (150 possible per day), and then 2 weeks later the number of candies delivered was increased to 20 per completed session (200 possible per day). The number of candies was increased until no baboon ate the maximal number of candies every day, i.e., 6 of the 8 baboons ate 200 candies on some days, but no baboon ate 200 candies every day.
Complete dose-response functions for the effects of drugs on consumption of pellets were then re-determined for dexfenfluramine and amphetamine in that order. Candy continued to be available on Mondays, Wednesdays and Fridays. Once the dose-response functions were determined during candy availability, candy was no longer available and food pellets were delivered following all completed brief morning sessions. In order to allow for any possible central effects of candy consumption to abate, baboons had access to only food pellets for 6 months after the last candy session. Then, the dose-response functions for the effects of drugs on consumption of pellets were re-determined for amphetamine and dexfenfluramine in that order.
Drugs
Dexfenfluramine HCl (0.12–1.0 mg/kg; Sigma Chemical Corp., St. Louis, MO, USA) was dissolved in sterile saline at a concentration of 30 mg/ml. D-Amphetamine HCl (0.06–0.5 mg/kg; Sigma Chemical Corp) was dissolved in sterile saline at a concentration of 20 mg/ml. Drug doses are expressed as total weight of the salt. Drugs were given intramuscularly (i.m.) in a thigh muscle (location varying among sessions) at 08:50 AM before brief sessions on Tuesdays and Thursdays. Dose order was counterbalanced. Although pharmacokinetic data on i.m. amphetamine and dexfenfluramine in the baboon are unavailable, previous studies in baboons indicate that the 2 drugs have a similar duration of action [e.g., 29, 30], and both drugs have a similar ½ life after oral administration in humans [31, 32].
Data Analysis
There were 3 principal outcome measures: 1) latency (min) to the first pellet of the afternoon meal sessions, 2) total number of candies or pellets earned during the 10 brief sessions, and 3) total number of pellets during the 4 afternoon pellet meal sessions. Latency to the first pellet meal was defined as the number of minutes between 11:30 AM, when the first afternoon pellet meal became available, and the first pellet delivery of the day. Because the first pellet acquisition component was completed prior to the first consumption component, the minimal latency was 30 min.
The first set of analyses assessed candy and pellet consumption and energy intake as a function of the amount of candy delivered during the each morning brief session using analyses of variance (ANOVA) with Candy condition (10, 15 and 20 candies per brief session) and Day (6 days) within each condition as within-group factors: food pellet intake and total caloric intake included a no candy condition (0, 10, 15, 20 candies per brief session). The second set of analyses assessed the response to drug administration as a function of candy availability. Because access to candy shifted baseline of pellets (see Figures 1 and 2), data for both drugs were converted to change from baseline and summarized using ANOVAs with Phase of the dose-response function (Before, During and After) relative to candy availability and Dose (4 doses) as within-group factors. Significant Phase × Dose effects were followed up with paired t-tests comparing the 3 Phases at each Dose, which were considered significantly different at p < 0.01. All other data were considered significantly different at p < 0.05, using Huynh–Feldt corrections where appropriate.
Figure 1.
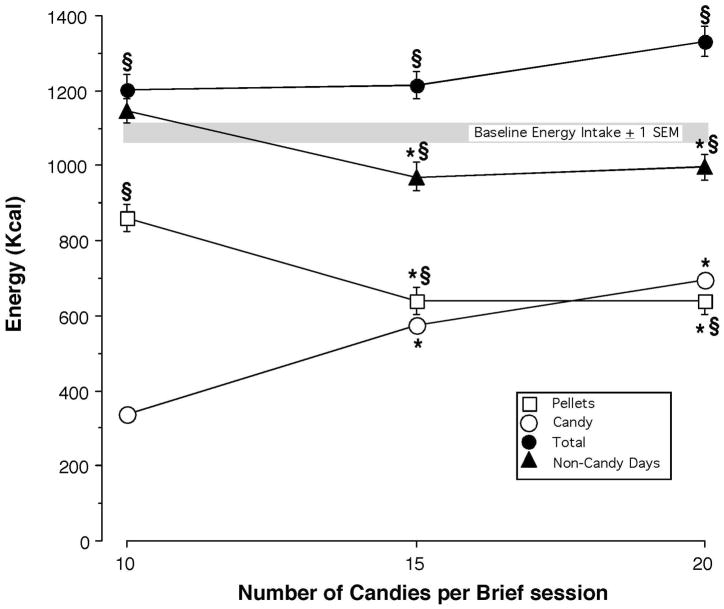
Mean energy intake derived from candy, pellets (afternoon pellets + 7:00 AM meal) and total (candy + pellets) on days that candy was available as a function of number of candies delivered each brief session. Mean total energy intake on days that candy was not available as a function of number of candies delivered each brief session is also shown. The gray bar represents mean total energy intake under baseline conditions prior to candy availability. Error bars represent ± 1 standard error of the mean (SEM); n = 8. Error bars for energy derived from candy fit within the symbols showing mean intake. An * indicates a significant difference (P < 0.05) between the 15 or 20 candy condition and the 10 candy condition. An § indicates a significant difference (P < 0.05) between a candy condition and the baseline prior to candy availability
Figure 2.
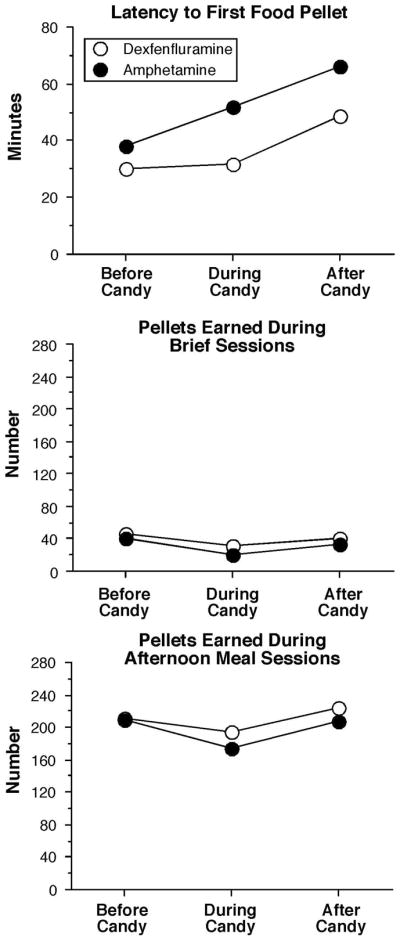
Mean baseline measures of eating behavior before, during and after a period of access to candy as a function of drug (n = 8). Error bars omitted for clarity.
Results
Effect of Candy Access on Food Intake
Figure 1 compares energy intake derived from food pellets and candy as the number of candies available during each brief morning sessions was increased from 10 to 20. Prior to candy access baboons consumed 1089 ± 29 Kcal (4.56 MJ; Mean ± SEM) each day based on the total number of pellets (320 ± 9) consumed during 7:00 AM “breakfast” meal session, the morning brief sessions and the afternoon meal sessions. Baboons earned 1) 84 ± 3 (out of 100 candies) when 10 candies were available; 2) 144 ± 1 (out of 150) when 15 candies were available and 3) 174 ± 5 (out of 200) when 20 candies were available during each brief session. Energy derived from candy increased, F (2, 14) = 59.1, p < 0.0001, as candy intake increased, i.e., when 20 candies were available, baboons consumed an average of 697 kcal (2.91 MJ) during the brief candy sessions with 75% of the energy derived from sugar. As candy consumption increased energy derived from pellets decreased, F (2, 14) = 25.2, p < 0.0001, such that when 15 or 20 candies were available a similar amount of calories were derived from candy in the morning as from pellets over the remainder of the day. Energy derived from pellets when candy was available was less than consumed prior to candy availability under all 3 candy amounts, F (3, 21) = 28.4, p < 0.0001.
In spite of the decrease in pellet consumption, total daily energy intake (candy + pellets) was increased by 100–200 Kcal when candy was available, F (3, 21) = 5.8, p < 0.009. Of note, when 15 or 20 candies were available during brief sessions on Mondays, Wednesdays and Fridays there was a decrease in total energy intake, i.e., number of food pellets consumed, on the days that candy was not available, F (3, 21) = 4.5, p < 0.04, with total energy intake about 100 Kcal less than observed prior to candy availability. Thus candy consumption altered feeding behavior on the days that candy was not available For example, when 20 candies were available baboons increased their energy intake by about 200 Kcal 3 days a week (+ 600 Kcal) and decreased their energy intake by about 100 Kcal 4 days a week (−400 Kcal) such that weekly energy intake increased by about 200 Kcal.
During the 5 months before access to candy baboons gained on average 1.9 kg, with a mean weight of 21.8 ± 1.9 kg (± SD). During the 6 months of access to candy baboons gained a similar amount of weight: on average 1.8 kg, with a mean weight of 23.6 ± 2.7 kg. Baboons maintained a stable rate of growth during candy access in spite of consumption of larger amounts of sugar.
Baseline Feeding Behavior
Figure 2 compares baseline pellet intake measures before, during and after candy availability, i.e., measures at the time each drug was tested. The data show how feeding behavior varied slightly across times that the drugs were tested and that pellet intake was affected by access to candy. Latency to the first pellet of the afternoon meal sessions increased as the study progressed from about 40 min to 60 min. The number of food pellets earned during brief sessions and the afternoon meals decreased when candy was available and returned to baseline after candy availability. Throughout the study, baboons received pellets during 3 of the 4 possible meal sessions and consumed 10–40 pellets during the breakfast meal session, regardless of candy or drug condition.
Effects of Amphetamine and Dexfenfluramine on Pellet Intake
The effects of dexfenfluramine on pellet intake are shown in the left set of panels and the effects of amphetamine are shown in the right set of panels of Figure 3. Dexfenfluramine produced dose-dependent increases in the latency to the first pellet of the afternoon sessions, F (3, 21) = 13.0, p < 0.0003 (Top Panel), decreases in the number of pellets earned during brief sessions, F (3, 21) = 18.1, p < 0.0001 (Middle Panel), and afternoon meal sessions, F (3, 21) = 32.8, p < 0.0001 (Bottom Panel), when averaged across all three dose-response functions. The dose-response function for many of the effects of dexfenfluramine was shifted to the right during candy availability such that larger doses were needed to produce effects seen with smaller doses before and after candy access. These changes in response to dexfenfluramine occurred on days when the baboons were not eating candy.
Figure 3.
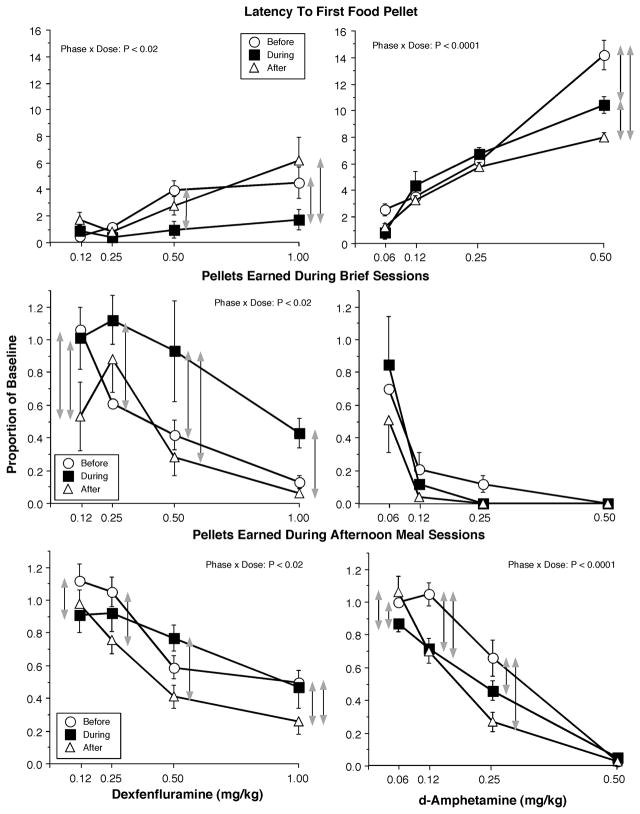
Mean latency to the first pellet of an afternoon meal, mean number of food pellets earned during the 10 brief sessions and mean number of food pellets earned during the afternoon meals for each drug, graphed as a proportion of baseline, as a function of dose. Data were obtained before, during and after a period of access to candy (n = 8). Error bars represent ± 1 standard error of the mean (SEM). Gray arrows indicate significant differences (P < 0.05) between dose-response functions at each dose based on the Post-hoc analyses.
There were significant Phase × Dose interactions for the effects of dexfenfluramine on the number pellets earned both during brief, F (6, 42) = 3.0, p < 0.02, and afternoon meal, F (6, 42) = 3.2, p < 0.02, sessions and latency to the first pellet of the afternoon, F (6, 42) = 4.1, p < 0.02. For example, 0.50 mg/kg dexfenfluramine decreased the number of pellets earned during brief sessions by 50% before and after access to candy, but was without effect during candy availability, F (2, 14) = 10.6, p < 0.002. A similar change was observed for latency to the first pellet, F (2, 14) = 10.0, p < 0.004. By contrast, dexfenfluramine produced larger decreases in pellet consumption during meals after candy access, F (2, 14) = 4.4, p < 0.05. These analyses confirm that tolerance developed to the effects of dexfenfluramine during access to candy, but the response to dexfenfluramine returned towards baseline, i.e., before access dose-response function, after candy access suggesting that the change in effect was related to candy access, not time alone.
Amphetamine produced dose-dependent increases in the latency to the first pellet of the afternoon sessions, F (3, 21) = 161.6, p < 0.0001, and decreases in the number of pellets earned during brief sessions, F (3, 21) = 14.5, p < 0.006, and afternoon meal sessions, F (3, 21) = 103.7, p < 0.0001. In contrast to dexfenfluramine, amphetamine produced significantly smaller decreases in afternoon pellet intake, F (2, 14) = 5.6, p < 0.02, and smaller effects on latency to the first afternoon pellet, F (2, 14) = 10.7, p < 0.001, during and after access to candy. Significant Phase x Dose effects indicated that the lesser effects on pellet intake was significant only for the smaller doses, F (6, 42) = 10.0, p < 0.0001, while the lesser effect on latency was significant only for the largest dose, F (6, 42) = 9.2, p < 0.0001.
Discussion
Baboons, who were naïve to the hard-cased gel candy (Skittles), readily consumed nearly the maximal amount of candy available during brief morning sessions 3 days a week. Furthermore, the number of candies consumed and the energy intake on the mornings candy was available was 4–5 times greater than that observed when only pellets were available in the morning, indicating a substantial increase in morning eating. Increasing the number of candies available increased candy intake and decreased intake of food pellets during the rest of the day, such that baboons increased total daily caloric intake on candy days by only 15%. There was a reduction of caloric intake on the remaining 4 days of the week so that total weekly caloric intake increased by less than 5%. Finally, the baboons did not show an accelerated growth rate during candy access. However, contribution of sugar to total energy intake increased to about 50% when candy was available; sugars accounted for less than 20% of total energy intake on days when only food pellets were available. Limiting access to candy to 3 days a week resulted in large intake of candy on those days with near complete energy compensation, i.e., a reduction in pellet intake, later in the day and on non-candy days. These results replicate findings using a similar feeding schedule with foods high in fat or carbohydrate in another omnivore, the laboratory rat [e.g., 23, 24].
Consumption of a diet high in sugars increased some of the anorectic effects of amphetamine, i.e., a shift to the left in the dose-response function. Yet, it is difficult to call this shift sensitization due to concurrent sugar intake because the effect persisted after candy access. In contrast to expectations, consumption of a diet high in sugars did produce a shift to the right for the dose-response function of dexfenfluramine, i.e., tolerance developed to the effects of the serotonergic drug, dexfenfluramine. Consumption of large amounts of candy 3 mornings a week altered baboons’ response to dexfenfluramine even when candy was not available that day.
It has been suggested that consumption of highly-palatable preferred foods that are high in fat and sugar content produce similar effects in the central dopamine system as produced by drugs of abuse [e.g., 33]. For example, when rodents respond for drugs of abuse there is increased dopamine release in the nucleus accumbens, as measured using microdialysis [34, see 35 for a review]. Similarly, when rodents respond for food pellets or sugar there is increased dopamine release in the nucleus accumbens [e.g., 36, 37, 38]; increases that are larger in the presence of food deprivation [39]. Because the drugs were given peripherally in the present study it is not possible to differentiate central from peripheral drug action. However, based on the putative repeated release of dopamine during consumption of highly palatable foods, it was reasonable to hypothesize that tolerance would develop to the anorectic effects of amphetamine.
Data obtained in laboratory rodents make a compelling argument for the modulation of the central dopaminergic system by the consumption of highly palatable foods. The present results in non-human primates were obtained using feeding schedules that required operant responding, compared to the free-access to food, procedures used with laboratory rodents. The maintenance diets and preferred nutrients also differed from those used with laboratory rodents. The large number of procedural differences may account for the unexpected outcome. The decreases in pellet consumption induced by amphetamine replicate earlier studies in non-human primates and rodents without access to candy [e.g., 29, 40, 41], but not the earlier studies in rodents with access to palatable foods. The present design relied on within animal comparisons obtained over a long time span with shifting baselines, while the studies in rodents have relied on between group designs, with groups varying by diet most often using a single type of sugar compared to the combination of sugars in Skittles candy. Species and/or procedural factors, of which there are many, must account for the failure to see significant changes in the behavioral effects of amphetamine as a consequence of sugar ingestion, as robustly reported in rodents.
Obtaining a dose-response function 6 months after access to candy was important to confirm that changes in responsivity to drug were transient and tied to the diet. Although pharmacological dose-response functions were obtained, the type and amount of high sugar diet available was not varied, such that different results might have been obtained with varying levels of sugar consumption. Furthermore, in the rat studies the total amount of palatable food was determined by the individual rat, not by the design, as was accomplished here. An alteration in the response to amphetamine may have occurred if baboons could have eaten even more candy.
A greater locomotor response to a single dose of amphetamine tested 9 days after a period of high sugar consumption has been reported in rats [42]. The present data provide partial support for an increase in sensitivity to amphetamine as a consequence of excessive sugar consumption. Unfortunately, the dose-response for the effects of amphetamine on afternoon pellet consumption obtained after the period of candy access was also shifted to the left of the dose-response function determined before and during candy access, suggesting an increase in sensitivity to the effects of amphetamine with repeated intermittent exposure to amphetamine. The original assumption of this study was that excessive consumption of sugar, perhaps by releasing endogenous dopamine, would result in tolerance to the behavioral effects of amphetamine. Clearly, this was not the case.
The data clearly show that tolerance developed to the effects of dexfenfluramine on pellets earned during the morning brief pellet sessions and the latency to the first pellet of the afternoon meal session. This tolerance, as shown by a shift to the right of the dose-response functions, was only evident during access to candy suggesting that the tolerance was related to increased sugar consumption or volume of morning food intake on the days that drug was not given. As with the results for amphetamine, a between groups design would be necessary to rule out other experiential factors that might account for the shifts in sensitivity. This finding differs from one previous study in rats [12], but is similar to other studies that reported a decreased response to a serotonin receptor agonist in rats who had access to a high sugar diet for 3 to 5 weeks [13, 14]. While it is difficult to compare our findings with these studies due to differences in duration of exposure, amount and type of sugar intake, other dietary factors and species, in combination the findings suggest that excessive sugar consumption or meal size can induce tolerance to the effects of serotonergic drugs.
Release of serotonin has been hypothesized to play a role in satiation [see 18 review], and perhaps a parallel mechanism was in play here such that increased sugar intake during each brief session acted like a mini-meal causing serotonin release and ultimately a decrease in sensitivity to serotonin, i.e., tolerance. This possibility has implications for understanding the genesis of obesity. Repeated eating past the point of “fullness” or satiation, partially signaled by increasing serotonin levels, or eating in the face of fullness signals, may ultimately lead to a decrease in sensitivity to the neurochemical signals that indicate that it is time to stop eating. The decreased sensitivity would lead to an increase in meal size and a further decrease in sensitivity to satiety signals, including those induced by drug action. However, any central effects of candy access on serotonin did not disrupt the ability of baboons to maintain a stable, slow growth pattern appropriate for young adult male baboons [43, 44], and did not disrupt consumption of the regular diet. It would be interesting to determine if eating behavior during the rest of the day and on non-candy days would indeed be altered during candy access if a wider variety of palatable foods had been available.
Summary
Consumption of a large number of candies high in sugar content 3 days a week decreased the sensitivity of baboons to the anorectic effects of dexfenfluramine. In contrast baboons became more sensitive to the effects of amphetamine as the study progressed, irrespective of candy consumption. Because 1) dexfenfluramine decreases food intake predominantly by increasing serotonin [45], and 2) increased serotonin levels play a role in signaling satiation [e.g., 46, 47], we hypothesize that tolerance to the effects of dexfenfluramine is a proxy measure for a decrease in the satiety signal produced by serotonin release during meals of highly palatable food. This hypothesis has implications for developing novel approaches to treating obesity, such as the development of agents to reverse serotonergic desensitization. The results of the current study clearly show that altering the sugar content of the diet, by providing occasional access to large amounts of palatable food, reduces the responsivity to serotonergic agents without affecting the response to dopaminergic agents in baboons.
Acknowledgments
This research was supported by DA-04130 from The National Institute on Drug Abuse, and approved by the New York State Psychiatric Institute Animal Care and Use Committee. The assistance of Jean Willi, Angel Ramirez, Daniel Peralta, Malgorzata Zawodna and Drs. Suzette Evans, Mohamed Osman and Moshe Shalev is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 3.Vitale MA, Chen D, Kanarek RB. Chronic access to a sucrose solution enhances the development of conditioned place preferences for fentanyl and amphetamine in male Long-Evans rats. Pharmacol Biochem Behav. 2003;74(3):529–39. doi: 10.1016/s0091-3057(02)01034-1. [DOI] [PubMed] [Google Scholar]
- 4.Kanarek RB, Marks-Kaufman R. Dietary modulation of oral amphetamine intake in rats. Physiol Behav. 1988;44:501–505. doi: 10.1016/0031-9384(88)90312-5. [DOI] [PubMed] [Google Scholar]
- 5.Bickel WK, Green L, Vuchinich RE. Behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration. J Exp Anal Behav. 1995;64(3):257–262. doi: 10.1901/jeab.1995.64-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foltin RW. Food and amphetamine self-administration by baboons: Effects of alternatives. J Exp Anal Behav. 1997;68:47–66. doi: 10.1901/jeab.1997.68-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foltin RW. Food and cocaine self-administration by baboons: effects of alternatives. J Exp Anal Behav. 1999;72(2):215–34. doi: 10.1901/jeab.1999.72-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sills TL, Vaccarino FJ. Individual differences in sugar intake predict the locomotor response to acute and repeated amphetamine administration. Psychopharmacology. 1994;116(1):1–8. doi: 10.1007/BF02244864. [DOI] [PubMed] [Google Scholar]
- 9.DeSousa NJ, Bush DE, Vaccarino FJ. Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology (Berl) 2000;148(1):52–8. doi: 10.1007/s002130050024. [DOI] [PubMed] [Google Scholar]
- 10.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122(1):17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 11.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- 12.Yeomans M, Clifton P. Exposure to sweetened solutions enhances the anorectic effect of naloxone but not d-fenfluramine. Physiol Behav. 1997;62(2):255–262. doi: 10.1016/s0031-9384(97)00112-1. [DOI] [PubMed] [Google Scholar]
- 13.Inam QU, Haleem MA, Haleem DJ. Effects of long term consumption of sugar as part of meal on serotonin 1-a receptor dependent responses. Pak J Pharm Sci. 2006;19(2):94–8. [PubMed] [Google Scholar]
- 14.Jabeen B, Haleem DJ. Desensitization of pre and post synaptic 5-HT-1A receptor responses following long term consumption of sugar rich diet: implications for sugar-induced obesity. Pak J Pharm Sci. 2008;21(4):327–32. [PubMed] [Google Scholar]
- 15.Inam QU, Jabeen B, Haleem MA, Haleem DJ. Long-term consumption of sugar-rich diet decreases the effectiveness of somatodendritic serotonin-1A receptors. Nutr Neurosci. 2008;11(6):277–82. doi: 10.1179/147683008X344183. [DOI] [PubMed] [Google Scholar]
- 16.Inam QU, Haleem MA, Haleem DJ. Attenuation of somatodendritic responses to 8-hydroxy-2-di-npropylamino tetralin following long-term dietary sugar consumption in rats. J Coll Physicians Surg Pak. 2009;19(7):401–5. [PubMed] [Google Scholar]
- 17.Blundell JE, Latham CJ, Leshem MB. Differences between the anorexic actions of amphetamine and fenfluramine - possible effects on hunger and satiety. J Pharm Pharmacol. 1976;28:471–477. doi: 10.1111/j.2042-7158.1976.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 18.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs : effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67(1):27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Blundell JE, Latham CJ, Moniz E, McArthur RA, Rogers PJ. Structural analysis of the actions of amphetamine and fenfluramine on food intake and feeding behaviour in animals and in man. Curr Med Res Opin. 1979;6:34–54. [Google Scholar]
- 20.Panksepp J, Krost K. Modification of diurnal feeding patterns by palatability. Physiol Behav. 1975;15(6):673–7. doi: 10.1016/0031-9384(75)90118-3. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch E, Walsh M. Effect of limited access to sucrose on overeating and patterns of feeding. Physiol Behav. 1982;29(1):129–34. doi: 10.1016/0031-9384(82)90376-6. [DOI] [PubMed] [Google Scholar]
- 22.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82(1):123–30. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65(3):545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 24.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28(4):436–45. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Bisaga A, Danysz W, Foltin RW. Antagonism of glutamatergic NMDA and mGluR5 receptors decreases consumption of food in baboon model of binge-eating disorder. Eur Neuropsychopharmacol. 2008;18:794–802. doi: 10.1016/j.euroneuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foltin RW. “Tasting and wasting” behavior in non-human primates: Aberrant behavior or normal behavior in “times of plenty”. Physiol Behav. 2006;89(4):587–597. doi: 10.1016/j.physbeh.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Foltin RW, Danysz W, Bisaga A. A novel procedure for assessing the effects of drugs on satiation in baboons: effects of memantine and dexfenfluramine. Psychopharmacology (Berl) 2008;199(4):583–92. doi: 10.1007/s00213-008-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foltin RW. Differential development of tolerance to the effects of d-amphetamine and fenfluramine on food intake in baboons. J Pharmacol Exp Ther. 1990;252:960–969. [PubMed] [Google Scholar]
- 29.Woolverton WL, Kandel D, Schuster CR. Tolerance and cross-tolerance to cocaine and d-amphetamine. J Pharmacol Exp Ther. 1978;205:525–535. [PubMed] [Google Scholar]
- 30.Foltin RW, Fischman MW. Food intake in baboons: effects of d-amphetamine and fenfluramine. Pharmacol Biochem Behav. 1988;31:585–592. doi: 10.1016/0091-3057(88)90234-1. [DOI] [PubMed] [Google Scholar]
- 31.de la Torre R, Farre M, Navarro M, Pacifici R, Zuccaro P, Pichini S. Clinical pharmacokinetics of amfetamine and related substances: monitoring in conventional and non-conventional matrices. Clin Pharmacokinet. 2004;43(3):157–185. doi: 10.2165/00003088-200443030-00002. [DOI] [PubMed] [Google Scholar]
- 32.Richards RP, Gordon BH, Ings RM, Campbell DB, King LJ. The measurement of d-fenfluramine and its metabolite, d-norfenfluramine in plasma and urine with an application of the method to pharmacokinetic studies. Xenobiotica. 1989;19(5):547–553. doi: 10.3109/00498258909042294. [DOI] [PubMed] [Google Scholar]
- 33.Avena NM, Rada P, Hoebel BG. Sugar and Fat Bingeing Have Notable Differences in Addictive-like Behavior. J Nutr. 2009;139(3):623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47(Suppl 1):190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Wise RA. Drug self-administration viewed as ingestive behaviour. Appetite. 1997;28(1):1–5. doi: 10.1006/appe.1996.0059. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 37.Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumantal lever pressing for food but not free food consumption. Pharmacol Biochem Behav. 1994;49(1):25. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 38.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 39.Radhakishun FS, van Ree JM, Westerink BH. Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett. 1988;85(3):351–6. doi: 10.1016/0304-3940(88)90591-5. [DOI] [PubMed] [Google Scholar]
- 40.Leibowitz SF. Catecholaminergic mechanisms of the lateral hypothalamus: their role in the mediation of amphetamine anorexia. Brain Res. 1975;98(3):529–45. doi: 10.1016/0006-8993(75)90371-6. [DOI] [PubMed] [Google Scholar]
- 41.Sills TL, Baird JP, Vaccarino FJ. Individual Differences in the Feeding Effects of Amphetamine - Role of Nucleus Accumbens Dopamine and Circadian Factors. Psychopharmacology. 1993;112(2–3):211–218. doi: 10.1007/BF02244913. [DOI] [PubMed] [Google Scholar]
- 42.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122(1):17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 43.Mahaney M, Leland M, Williamsblangero S, Marinez Y. Cross-Sectional Growth Standards for Captive Baboons. 2. Organ Weight by Body Weight. J Med Primatol. 1993 SEP–OCT;22(7–8):415–427. [PubMed] [Google Scholar]
- 44.Strum SC. Weight and age in wild olive baboons. Am J Primatol. 1991;25:219–237. doi: 10.1002/ajp.1350250403. [DOI] [PubMed] [Google Scholar]
- 45.Heal DJ, Cheetham SC, Prow MR, Martin KF, Buckett WR. A comparison of the effects on central 5-HT function of sibutramine hydrochloride and other weight-modifying agents. Br J Pharmacol. 1998;125(2):301–8. doi: 10.1038/sj.bjp.0702067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clifton PG. Meal patterning in rodents: psychopharmacological and neuroanatomical studies. Neurosci Biobehav Rev. 2000;24(2):213–22. doi: 10.1016/s0149-7634(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 47.Kirkham TC, Blundell JE. Effect of naloxone and naltrexone on the development of satiation measured in the runway: comparisons with d-amphetamine and d-fenfluramine. Pharmacol Biochem Behav. 1986;25:123–128. doi: 10.1016/0091-3057(86)90241-8. [DOI] [PubMed] [Google Scholar]