Abstract
Background
The peroxiredoxins (PRDXs) are emerging as regulators of antioxidant defense, apoptosis, and therapy resistance in cancer. Because their significance in prostate cancer (PCa) is unclear, we investigated their expression and clinical associations in PCa.
Methods
Transcript expression of PRDX1-6 in PCa was evaluated in cancer gene microarray datasets, whereas protein expression was evaluated by immunoblotting in prostate cell lines, and by immunohistochemistry (IHC) in prostate tissue microarrays (TMAs) containing tumor (n=80) and control (n=17) tissues. PRDX3 was also analyzed in TMAs containing PCa tissues from African-American and Caucasian patients (n=150 per group). PRDX expression was correlated with patients' clinicopathologic characteristics.
Results
Analysis of PRDX expression in cancer microarray datasets revealed consistent upregulation (tumor vs normal) of PRDX3 and 4. All PRDXs exhibited elevated protein expression in PCa cell lines, compared with non-tumor cells. IHC revealed significant overexpression of PRDX3 and 4 in PCa, associated with age, increased prostate specific antigen (PSA), tumor stage, or Gleason score. High PRDX3 staining was associated with early age and elevated Gleason score at time of radical prostatectomy in African-American but not in Caucasian patients with PCa. PSA recurrence free survival in patients with low PRDX3 tumor expression was significantly longer in Caucasians compared to African-Americans, but no difference was detected for high expression.
Conclusions
PRDXs exhibit differential expression in prostate tumors, with PRDX3 and 4 consistently upregulated. Their role in PCa development, and their potential as biological determinants of PCa health disparities and novel therapeutic targets, deserve further investigation.
Keywords: health disparities, oxidative stress, peroxiredoxins, prostate cancer, tissue microarrays
Introduction
Prostate cancer (PCa) is the most frequently diagnosed male cancer and the second leading cause of cancer death in men in the United States [1]. This growing public health challenge is aggravated by disparities in the incidence and mortality of PCa among African-American (AA) men, compared to other ethnic groups [2]. AA men present with PCa at a younger age compared to Caucasian (CC) men, and typically with more aggressive disease [3,4].
Emerging evidence suggests that chronic inflammation of the prostate, associated with an augmented state of cellular oxidative stress, contributes to PCa development by inducing molecular damage and DNA modifications [5,6]. This perturbation of the cellular redox homeostasis in the prostate microenvironment results in the upregulation of stress and redox proteins that protect cells against oxidative damage and apoptosis [6-10]. Evaluation of the expression and role of these proteins in PCa is required for defining molecular and cellular factors associated with prostate tumor aggressiveness and therapy resistance, developing more effective therapeutic interventions, identifying novel PCa biomarkers, and uncovering potential biological determinants of PCa health disparities.
The peroxiredoxin (PRDX) protein family consists of six anti-oxidant enzymes (PRDX1-6) that are emerging as key regulators of cellular anti-oxidant defense, and are being increasingly implicated in malignant transformation and therapy resistance [11-20]. The expression of individual PRDX is deregulated in various human cancers [11,12]. Overexpression of PRDX1-5 was observed in breast cancer [13,14], and that of PRDX1 and 6 in bladder cancer [15]. All the PRDXs, with the exception of PRDX4, were found overexpressed in malignant mesothelioma [16]. In lung carcinoma, PRDX1, 2, 4 and 6 were found upregulated in one study [17], while another study found overexpression of only PRDX1 and 3 [18]. Recent studies reported increased expression of PRDX2 and 3 in cervical cancer [19], and elevated PRDX2 and 6 in ovarian tumors [20].
In light of recent reports implicating PRDX in PCa [21-24], we hypothesized that these anti-oxidant enzymes are upregulated in PCa, and sought to evaluate their expression in prostate tumors using cancer gene microarray databases and tissue microarrays (TMAs). Our results showed differential expression of PRDX mRNA and protein in PCa, with consistent upregulation of PRDX3 and 4. The expression of these two proteins also correlated with various clinicopathologic parameters. We also observed clinical outcome differences related to PRDX3 expression between AA and CC patients with PCa. Our results underscore the PRDXs as potential disease markers, therapeutic targets, and biological determinants of health disparities in PCa.
Materials and Methods
Prostate Cell Lines
RWPE-1, RWPE-2, DU-145, PC3, LNCaP, MDA-PCa-2b, and 22RV1 cell lines were purchased from American Type Culture Collection (ATCC). BRF-41T and BRF-55T were purchased from AthenaES. PrEc and PrSc were obtained from Clonetics, Lonza. All cell lines were cultured as recommended by the suppliers and maintained in a humidified incubator with 5% CO2 at 37°C.
Antibodies
The following antibodies were used: monoclonal anti-β-actin (Sigma-Aldrich), and rabbit polyclonals anti-PRDX1 (1:2500 for IHC, 1:1000 for immunoblotting, Abcam), anti-PRDX2 (1:2500 for IHC, 1:10,000 for immunoblotting, Abcam), anti-PRDX3 (1:2500 for IHC, 1:5000 for immunoblotting, Abcam), anti-PRDX4 (1:2500 for IHC, 1:1000 for immunoblotting, Abcam), anti-PRDX5 (1:1000 for IHC, 1:1000 for immunoblotting, Santa Cruz Biotechnology), anti-PRDX6 (1:2500 for IHC, 1:3000 for immunoblotting, Abcam), and horseradish peroxidase (HRP)-labeled secondary IgG antibodies (Zymed).
Bioinformatics Analysis of Cancer Gene Microarray Databases
For comparison of PRDX mRNA expression between PCa and normal prostate tissues, we selected 13 datasets from the Oncomine database (Compendia Biosciences; Ann Arbor, MI; www.oncomine.org). These datasets, containing 800 gene microarrays of PCa and normal tissues, provide fold-change data for gene expression, with P values calculated by t-tests. We added an additional dataset created by the Prostate SPECS consortium (Strategic Partners for Evaluation of Cancer Signatures; www.pathology.uci.edu/faculty/mercola/UCISPECSHome.html), a NIH-funded multi-institution biomarker discovery program. This dataset comprised 85 microarrays (PCa, n=40; normal prostate, n=45), and is accessible through the GEO database (GSC17951). For this dataset, fold-change (tumor vs normal) and P values were calculated using the LIMMA statistical package from Bioconductor [25]. Thus a total of 14 datasets were used. Only six datasets had data for PRDX5; however, all datasets had data for PRDX1, 2, 3, 4, and 6.
Immunoblotting of PRDX
Procedures were carried out essentially as described previously [26]. Briefly, whole protein lysates from prostate cells were resolved in SDS-polyacrylamide gel electrophoresis (NuPAGE 4-12%, Invitrogen) and transferred to PVDF membranes (Millipore). Membranes were blocked with 5% dry milk solution in TBS-T buffer (20 mM Tris-HCl, pH 7.6, 140 mM NaCl, 0.1% Tween 20) for 1 h and probed with primary antibodies. After several washes with TBS-T, membranes were incubated with HRP-conjugated secondary antibodies for 30 minutes and then washed again with TBS-T. Protein bands were detected by enhanced chemiluminescence (Amersham).
Tissue Microarrays
Human PCa TMAs, commercially available from Imgenex Corp. (San Diego), were used for IHC analysis of PRDX. Two different Imgenex TMAs were used to increase the patient sample size: IMH-303, containing 40 PCa specimens and 9 matched adjacent normal tissues; and IMT-01291, containing 40 PCa specimens and 8 normal post-mortem control (hereafter referred to as ‘disease-free normal’) prostate tissues. Additionally, we acquired an ethnicity TMA, which at the time we conducted these studies was commercially available from the Cooperative for Prostate Cancer Tissue Resource, containing 150 AA and 150 CC PCa cases. This TMA was analyzed for PRDX3 expression. The manufacturers of these TMAs provided limited basic clinicopathologic information (age, tumor stage, PSA values, Gleason scores) corresponding to the tissue cores, with no patient identifiers. However, no information was available on neo-adjuvant treatment, surgical technique, year of surgery, institutions that collected the tissues, number of institutions, follow up routines, and tissue handling techniques. The limited patient follow up data associated with the Imgenex TMAs prevented any survival analysis. PSA recurrence free survival (PRFS, in months) follow-up information was provided for 61 CC and 46 AA PCa cases in the ethnicity TMA. These studies were approved by the Institutional Review Board.
IHC Analysis and Evaluation
TMAs were stained with a Biogenic i6000 autostainer (Biogenex Corporation) following the manufacturer's instructions and as described previously [26]. Briefly, paraffin embedded tissue sections in the TMA slides were deparaffinized and the slides were immersed in Citra-Plus antigen retrieval solution (Biogenex Corp.). Antigen retrieval was performed by microwaving the slides for 2 min at 100% power followed by 10 min at 20% power. Slides were then cooled in the antigen retrieval solution for 20 min. Endogenous peroxidase activity was quenched by treatment with 3% hydrogen peroxide in 10% methanol, and Power Block© universal blocking reagent (Biogenex Corp.) was used to block non-specific protein binding.
The TMA slides were incubated with primary antibodies, followed by 3 washes in PBS. Slides were then incubated with Multi-link© biotinylated secondary antibody (Biogenex Corp.) for 20 minutes, followed by incubation with streptavidin-coupled peroxidase supersensitive Label© (Biogenex Corp.) for 20 min. Immunostaining was detected by peroxidase activation of the 3-amino-9-ethycarbazole (AEC) chromagen (Romulin AEC-Biocare Medical). TMA were counterstained lightly with hematoxylin (Sigma) and mounted with permount (Fisher Scientific). For negative control the primary antibody was omitted and substituted with diluent only or non-specific rabbit IgG. Tissue sections were examined under an Olympus BX50 microscope, and images were acquired using a digital Spot RT3™ camera (Diagnostic Instruments). The TMA slides were scored blindly for PRDX immunoreactivity by a board certified pathologist. A 4-tier scoring system (0=negative, 1=weak, 2=moderate, 3=strong) was used to evaluate staining intensity. Tissue specimens that showed poor quality were excluded from the analyses.
Statistical analysis of IHC data and their relationship to patients' clinical outcomes was done using the SAS software package (version 9.01; SAS institute). For ease of statistical analysis, tissue specimens were grouped into two categories based on their scores. ‘High’ staining was determined as an intensity score of 3 while ‘<high’ (also referred to as low) staining had pooled scores of 0-2. Difference in expression levels of PRDXs between tumors and normal tissues were analyzed using Kruskal-Wallis's rank sum test. Associations between expression levels of PRDXs and clinicopathologic parameters were determined using Kendall's tau correlation analysis. Kaplan-Meier survival curves for PRFS were generated and the significance of association was tested by log-rank test. Probability values P<0.05 were considered statistically significant.
Results
Differential PRDX Protein Expression in PCa Cell Lines
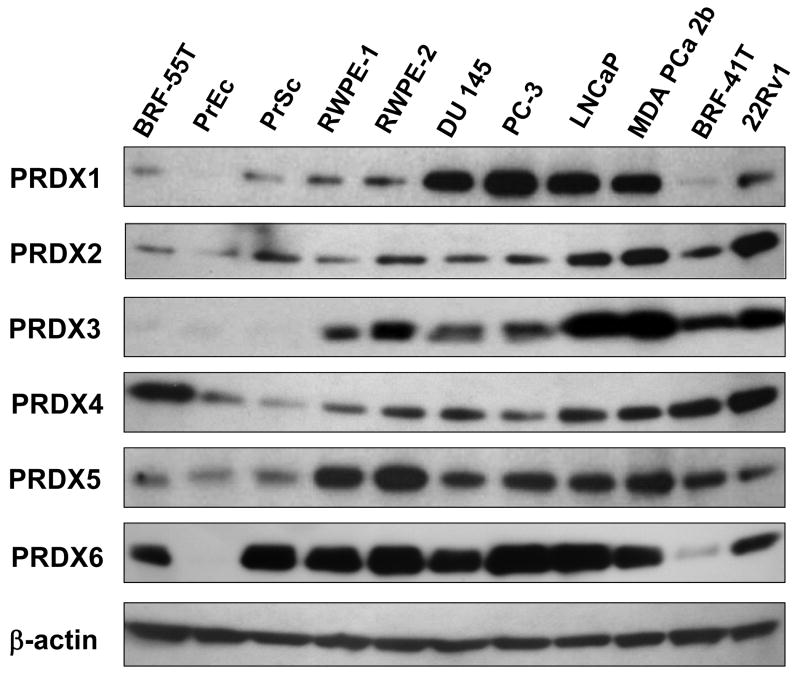
Immunoblotting analysis of PRDX protein expression in a panel of 11 prostate cell lines revealed that most PRDXs exhibited elevated expression in transformed or tumor-derived cell lines, compared to their relatively low levels in the primary normal cell lines PrEC and PrSC, and the benign prostatic hyperplasia (BPH) cell line BRF-55T (Fig. 1). Varying levels of PRDX protein expression were observed among the tumor and transformed cell lines. Differences in PRDX protein expression levels between tumor and non-tumor cell lines were most dramatic for PRDX1 and 3. These results prompted us to examine the transcript and protein levels of all six PRDXs in PCa tissues.
Fig. 1. Immunoblotting analysis of PRDX protein expression in a panel of human prostate cell lines.
The panel included non-tumor (BRF-55T, PrEc, PrSc), transformed normal (RWPE-1, RWPE-2), androgen-independent (DU-145, PC3), and androgen-responsive (LNCaP, MDA-PCa-2b, BRF-41T, and 22RV1) cell lines. Protein loading was assessed with antibody to β-actin.
Analysis of Cancer Gene Microarray Databases Reveals Consistent Upregulation of PRDX3 and 4 transcripts in PCa tissues
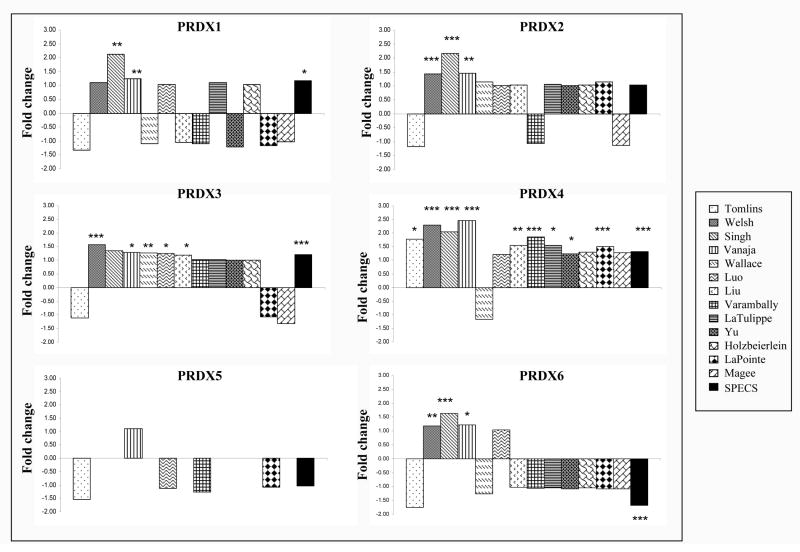
Initial evaluation of PRDX expression in PCa tissues was performed using cancer gene microarray datasets from the Oncomine and SPECS databases that compared PCa tissue to normal prostate tissue. PRDX3 and 4 were the most consistently upregulated (tumor vs normal) members of the PRDX family in the dataset collection, with significant upregulation of PRDX3 transcript in 6 of 14 datasets, and PRDX4 transcript in 10 of 14 datasets (Fig. 2). PRDX1, 2, and 6 were significantly upregulated only in 3 of 14 datasets, whereas no changes in PRDX5 transcript were detected in any dataset (only 6 datasets had data for PRDX5). The magnitude of the fold-increase observed for the individual PRDX was modest, with only PRDX4 showing more than 2-fold increase in multiple datasets. Since the PRDXs are relatively stable proteins with long half-lives, their mRNA levels might not correlate with protein levels [14].
Fig. 2. Transcript expression of PRDX1-6 in prostate cancer determined by analysis of cancer gene microarray databases.
All the datasets, except SPECS, were from the Oncomine database, and their names appear in the legend box at the right. Fold-changes and corresponding P values testing the difference in PRDX gene expression between PCa and normal prostate tissue (adjacent normal in most datasets) were provided by Oncomine. The P values for the SPECS database were calculated using the LIMMA statistical package [ ref. 25]. *P<0.05; **P<0.01, ***P<0.001.
IHC Analysis of Prostate TMAs Reveals Overexpression of PRDX3 and 4 in PCa
PRDX3 and 4, but not the other PRDXs, were previously reported as upregulated in a small number of prostate needle biopsy specimens using a proteomic approach [22]. However, to our knowledge there has not been a comprehensive IHC analysis of PRDX protein expression in a prostate TMA format. We performed IHC analyses of PRDX1-6 using two PCa TMAs containing a total of 80 prostate tumor tissues, 9 adjacent normal, and 8 disease-free normal prostate tissues. We were unable to score one disease-free normal prostate tissue for PRDX5 (n=7) and one tumor tissue sample each for PRDX2 (n=79) and PRDX3 (n=79) due to poor tissue quality. Clinicopathologic characteristics of the tissue specimens in the two TMAs are summarized in Table I The Gleason sum score and PSA levels were available for 40 and 35 tumor tissues, respectively. At the time of prostatectomy, the median age of the tumor tissue donors (n=80) was 67.5 years, and the median PSA level (n= 35) was 15.8 ng/ml (range 0.5-161 ng/ml), which was set as cut-off value for ease of statistical analysis.
Table 1. Association of PRDX expression in Imgenex prostate tissue microarrays with patients' clinical characteristics.
| Characteristic | Number of patients | PRDX IHC staining (high vs low), P* | |||||
|---|---|---|---|---|---|---|---|
| PRDX1 | PRDX2 | PRDX3 | PRDX4 | PRDX5 | PRDX6 | ||
| Age (years) | 0.3942 | 0.1536 | 0.1395 | 0.0214 | 0.2989 | 0.0680 | |
| ≤ 67.5 | 40 | ||||||
| > 67.5 | 40 | ||||||
| Tumor stage | 0.0203 | 0.3341 | 0.0061 | 0.0283 | 0.7650 | 0.0179 | |
| pT1 | 0 | ||||||
| PT2 | 43 | ||||||
| pT3 | 33 | ||||||
| pT4 | 4 | ||||||
| Gleason score | 0.5145 | 0.0061 | 0.1520 | 0.0085 | 0.0094 | 0.0504 | |
| 5 | 0 | ||||||
| 6 | 2 | ||||||
| 7 | 15 | ||||||
| 8 | 6 | ||||||
| 9 | 15 | ||||||
| 10 | 2 | ||||||
| PSA (ng/ml) | 0.777 | 0.777 | 0.0134 | 0.2341 | 0.6203 | 0.0564 | |
| ≤ 15.8 | 18 | ||||||
| >15.8 | 17 | ||||||
P values testing the association of immunohistochemical PRDX expression (high vs low comparison) in PCa tissues with patients' characteristics were calculated using Kendall's tau b correlation analysis. At time of prostatectomy, the median age of tissue donors (n=80) was 67.5 years and the median PSA level (n=35) was 15.8 ng/ml (range 0.5-161 ng/ml).
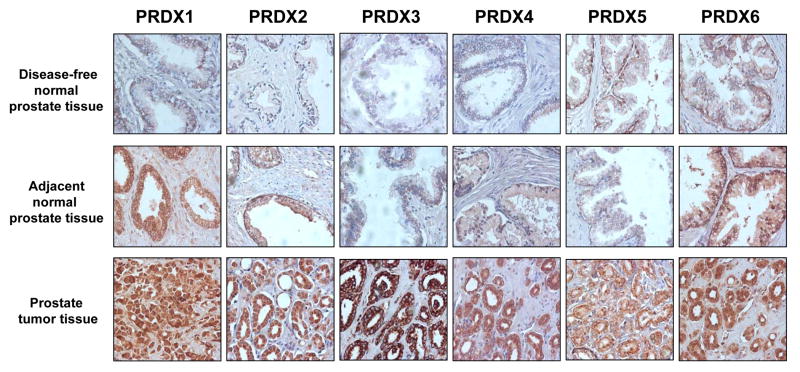
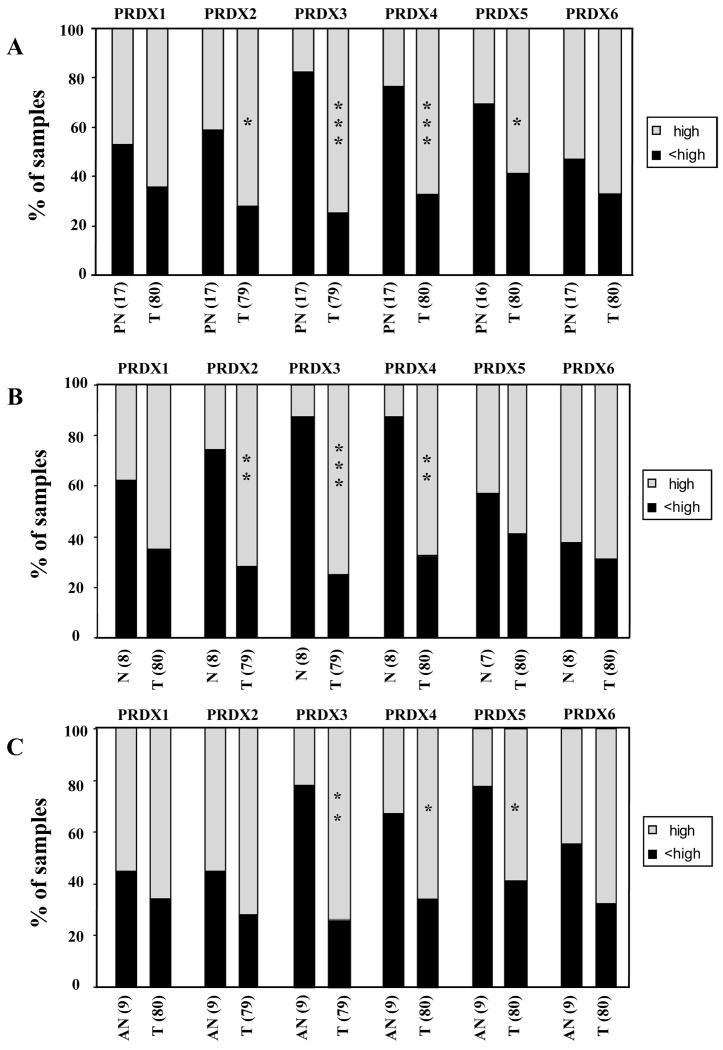
Fig. 3 shows representative tissue cores immunostained with specific antibodies against the different PRDXs. Analysis of PRDX expression in prostate tumors compared to normal control tissues (i.e, pool of disease-free normal and adjacent normal) showed robust overexpression of PRDX3 and 4 in the tumors (P<0.001) (Fig. 4A). PRDX2 levels were also significantly elevated in tumor tissues but only when compared to disease-free normal tissues (P<0.01) (Fig. 4B), whereas PRDX5 was significantly elevated only when compared to adjacent normal tissues (P<0.05) (Fig. 4C). Our analysis showed that over 60% of PCa tissues had high expression of all the PRDXs (Fig. 4A). However, 38-63% of disease-free normal prostate tissues had elevated expression of PRDX1, 5, and 6 (Fig. 4B), whereas 45-56% of adjacent normal prostate tissues exhibited high levels of PRDX1, 2, and 6 (Fig. 4C). By contrast, only 12% of disease-free normal tissues and 22-33% of adjacent normal tissues displayed high expression of PRDX3 and 4 (Fig. 4B,C).
Fig. 3. Immunohistochemical analysis of PRDX proteins in prostate tumor tissues.
Representative examples of immunohistochemical staining of PRDX in prostate tissue microarrays (TMAs) are shown. TMAs were stained for the individual PRDX using specific antibodies, as indicated in Materials and Methods.
Fig. 4. Differential expression of PRDX proteins in prostate tumor tissues.
Tissue microarrays were stained for the individual PRDX using specific antibodies, and the individual cores were blindly scored using the following scale: 0=no staining, 1=low staining, 2=moderate staining, 3=strong staining. Scored tissues were divided in two groups: high staining (score 3, light bars), and <high (low) staining (scores 0-2, dark bars). The percentage of specimens in the two staining categories was plotted for prostate tumor tissues (T, n=80) compared to (A) pooled normal prostate tissues (PN, n=17), (B) disease-free normal prostate tissues (N, n=8), and (C) adjacent normal prostate tissues (AN, n=9). *P<0.05; **P<0.01, ***P<0.001. P values were determined with Kruskal-Wallis test.
Correlation between clinicopathologic characteristics and PRDX protein expression revealed that overexpression of PRDX3 and 4 in prostate tumors was significantly associated with increase in tumor stage (P<0.05) (Table I). Although the expression of PRDX1 and 6 was not significantly different between tumor and normal tissues (Fig. 4), elevated levels of these two proteins in the tumors were also significantly associated with increase in tumor stage (Table I). Elevated expression of PRDX2, 4, and 5 in the tumor tissues was associated with increase in Gleason sum score (P<0.05) (Table I), whereas elevated PRDX3 expression was associated with increase in PSA levels (P<0.05) (Table I). Enhanced PRDX4 expression was also associated with increase in age at prostatectomy (Table I). The limited patient follow-up data associated with the Imgenex TMAs precluded correlating PRDX expression with survival outcomes in Kaplan-Meier analyses.
Ethnic Differences in the Association of PRDX3 Expression in Prostate Tumors with Clinical Outcomes
The elevated levels of PRDX3 in the AA-derived cell line MDA-PCa-2b (Fig. 1), its robust overexpression in prostate tumors compared to normal tissues, and its association with increase in tumor stage and PSA, prompted us to analyze its expression in ethnicity PCa TMA containing 150 cases from AA PCa patients and 150 cases from CC PCa patients. The clinicopathologic characteristics of the patients in this TMA are summarized in Table II. Seven tissues from the AA group and 14 tissues from the CC group were not scored due to poor tissue quality. Although there were no significant differences in the percent of PRDX3 immunopositive prostate tissues when comparing AA (133 out of 143, 93%) with CC (131 out of 136, 96.3%) tissues, we observed that the proportion of tumor tissues with high PRDX3 expression was higher in the CC group (62 out of 136, 45.6%) than in the AA group (37 out of 143, 25.9%). However, high PRDX3 expression correlated with early age at prostatectomy (P<0.05) and elevated Gleason sum score (P<0.05) in the AA group but not in the CC group (P=0.4590 and P=0.3394, respectively) (Table II, Kendall's tau test). Since most tumor specimens in this TMA were in the pT2 stage, it was not possible to correlate PRDX3 expression with increase in tumor stage.
Table II. Association of PRDX3 expression in ethnicity prostate cancer microarrays with patients' clinical characteristics.
| Characteristics | Number of Patients | PRDX3 IHC staining (high vs low), P* | ||
|---|---|---|---|---|
| African-American | Caucasian | African-American | Caucasian | |
| Age (years) | 0.0051 | 0.4590 | ||
| ≤ 62 | 97 | 68 | ||
| > 62 | 46 | 68 | ||
| Gleason score | 0.0292 | 0.3394 | ||
| 5 | 4 | 2 | ||
| 6 | 43 | 42 | ||
| 7 | 82 | 81 | ||
| 8 | 8 | 5 | ||
| 9 | 6 | 6 | ||
P values testing the association of immunohistochemical PRDX3 expression (high vs low comparison) in PCa tissues with patients' clinical characteristics were calculated using Kendall's tau b correlation analysis.
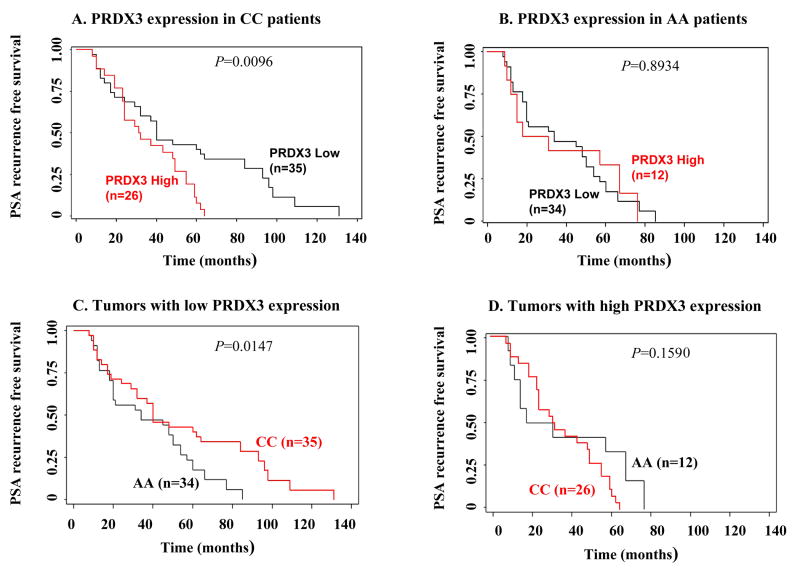
We also evaluated by Kaplan-Meier analysis the association between PRDX3 prostate tumor expression and PSA recurrence free survival (PRFS) in AA and CC patients for which disease progression data was available. In the CC group, low (<high) expression of PRDX3 was associated with longer PRFS (P<0.05, log rank test) (Fig. 5A). By contrast, there was no significant difference in PRFS between AA patients with low or high PRDX3 expression (P=0.8934) (Fig. 5B). When the CC and AA groups were compared with each other, the PRFS in patients with low (<high) PRDX3 tumor expression was significantly longer in the CC group than in the AA group (P<0.05) (Fig. 5C). No significant difference in PRFS was detected when AA and CC patients with high PRDX3 expression were compared (Fig. 5D) (P=0.1590).
Fig. 5. Kaplan-Meier curves of PSA recurrence-free survival (PRFS) in relationship to PRDX3 expression in ethnicity prostate cancer tissue microarrays.
Low (<high, scores 0-2) and high (score 3) PRDX3 expression was correlated with PRFS in (A) Caucasian (CC) and (B) African-American (AA) patients. PRFS was compared between AA and CC patients expressing low (<high) (C) and high (D) PRDX3 in prostate tumors. P values were calculated using the log-rank test.
As indicated above, although we observed that high PRDX3 expression significantly correlated with elevated Gleason sum score in the total AA group (Table II), comparison of low and high PRDX3 expression with PRFS in AA patients showed no significant difference (Fig. 5B). By contrast, while elevated PRDX3 expression did not correlate with Gleason sum score in the total CC group (Table II), low PRDX3 expression was significantly associated with longer PRFS and in CC patients (Fig. 5A). These results were intriguing given the documented correlation between the severity of Gleason scores and PSA recurrence [27,28]. This prompted us to perform additional statistical tests to evaluate the association between Gleason scores and PRFS in both ethnic groups.
Since PRFS information was available for only 46 out of 143 AA and 61 out of 136 CC patients, we first evaluated in each patient sub-group the correlation between Gleason sum scores and PRFS. We found that elevated Gleason sum scores correlated with shorter PRFS in the AA sub-group (n=46, P<0.05, Kendall's tau test) but not in the CC sub-group (n=61, P=0.8869, Kendall's tau test). To further explore this finding we separated the Gleason score 7 in both ethnic groups into grades 3+4 and 4+3, which have been shown to be significantly associated with different clinical courses, with 4+3 tumors linked to more aggressive disease and biochemical recurrence [28]. We then evaluated the PRFS of patients with Gleason grades 3+4 (AA, n=21; CC, n=25) and 4+3 (AA, n=7; CC, n=6), within each ethnic sub-group and between both sub-groups. There was a moderate, albeit not significant, association between Gleason grade 4+3 and shorter PRFS within each sub-group (AA, P=0.0888; CC, P=0.0778; log rank test, Kaplan-Meier curves not shown). However, when the AA sub-group was compared with the CC sub-group with respect to the PRFS of grade 4+3 tumors, we observed that AA patients had a significantly shorter PRFS than CC patients (P<0.05, log rank test, Kaplan-Meier curves not shown). No significant differences were found between AA and CC patients when the PRFS curves of 3+4 tumors were compared (P=0.6545, log rank test, Kaplan-Meier curves not shown). We did not observe a significant difference between PRDX3 expression (low or high) and Gleason grades 4+3 or 3+4 within either ethnic group. It should be noted, however, that the sample size with Gleason grade 4+3 was very small, which may have negatively influenced statistical significance.
Discussion
The role of PRDXs in PCa is unknown, although growing evidence suggests their involvement in PCa progression and oxidative stress resistance. For instance, PRDX1 interacts with androgen receptor (AR) in PCa cell lines, and enhances AR-mediated transactivation of target genes [23,24]. PRDX1 and 5 are regulated in PC3 cells by ETS transcription factors, and their silencing sensitizes cells to oxidative stress-mediated death [29]. PRDX6 is downregulated in mice lacking the homeobox gene Nkx3.1, resulting in increased oxidative damage during prostate carcinogenesis [30]. Antisense-mediated suppression of PRDX1-4 suggested their non-redundant role against oxidative stress and chemotherapy in PCa cells [31].
The present study showed consistent upregulation of PRDX3 and 4 in PCa, inferred by analysis of transcript expression in cancer gene microarray databases, and analysis of protein expression by immunoblotting in a panel of prostate cell lines, and immunohistochemistry in TMAs. While PRDX1 was expressed at high levels in the DU145, PC3, LNCaP, and MDA-PCa-2b cell lines compared to the normal cell lines, its expression in PCa tissues was not significantly elevated when compared to normal tissues. This discrepancy suggests that the cell culture microenvironment might provide factors that maintain an elevated expression of PRDX1 in PCa cell lines. PRDX1 is elevated in some cancers [14-16], and has been implicated in PCa [23,24]; however, some studies point to this protein as a tumor suppressor [32].
We observed that a relatively high percentage of disease-free normal and adjacent normal prostate tissues displayed high levels of PRDX1, 2, 5, and 6. By contrast, a relatively small percentage of disease-free normal tissues displayed high expression of PRDX3 and 4, although the percentage of adjacent normal tissues expressing high levels of these two proteins was higher. While it is likely that some PRDXs may be constitutively expressed at elevated levels in non-cancer tissues, it cannot be ruled out that field cancerization may account for their relatively elevated levels in adjacent normal tissues. Recently, Nonn et al. [33] proposed that field cancerization exists in the prostate, and that “morphologically normal” tissue adjacent to PCa tissue exhibits molecular abnormalities such as increased expression of cancer-associated genes or proteins, changes in gene methylation patterns, increased oxidative DNA damage and angiogenesis, and TMPRSS2-ERG-fusions.
A previous study by Lin et al [22] revealed by immunoblotting analysis that PRDX3 and 4, but not 1 and 2, are upregulated in PCa. These authors based their conclusions on analysis of nine prostate needle biopsy specimens from PCa patients and fourteen specimens from BPH patients. Cha et al. [14] examined by qRT-PCR array the mRNA expression of PRDX1-6 in several human tissues and cancers, and observed that PRDX3 and 4 transcripts were expressed at relatively low levels, compared to the other PRDXs, in the normal tissues, including three prostate tissues. However, in cancer tissues, including twelve PCa specimens, PRDX3 and 4 transcripts appeared to be induced approximately to the same levels of most of other PRDXs [14]. Although these previous studies did not examine the relationship between the expression of PRDX3 and 4 and clinicopathologic parameters, they support our conclusion that these two proteins are the most consistently upregulated members of the PRDX family in PCa.
Although our study was limited by the incomplete clinical and follow-up patient data associated with the commercial TMAs, it is nevertheless, to our knowledge, the first comprehensive study on the expression and clinical associations of PRDX in PCa. Our results revealed that the upregulation of PRDX3 and 4 was significantly associated with increase in tumor stage, PSA, or Gleason score, suggesting a possible role in PCa progression. These clinical associations need to be confirmed using more comprehensive TMAs or individual PCa tissue blocks from a large patient cohort, with complete clinical and follow-up patient data.
It is not clear why PRDX3 and 4 are the most upregulated PRDXs in PCa. PRDX3 has been localized to the mitochondria [34], whereas PRDX4 is present in the endoplasmic reticulum (ER), lysosomes, and in proximity to the mitochondria [15,35]. It is possible that PCa cells, which exhibit high metabolism leading to increased mitochondrial ROS [36], upregulate these two proteins as part of their anti-oxidant defense response, in order to protect organelles from oxidative damage and prevent cell death [37,38].
Using an ethnicity PCa TMA we were able to detect some significant differences in clinical outcomes between CC and AA patients in relationship to PRDX3 tumor expression status. For instance, high expression of PRDX3 in prostate tumors was associated with younger age and Gleason score at the time of radical prostatectomy in AA but not in CC patients, whereas low expression was associated with a significantly longer PRFS in CC patients. PRDX3 expression did not appear to influence PRFS in AA patients since there were no significant differences in PRFS relative to low or high PRDX expression in this patient group. In general, the AA group had a much shorter PRFS than the CC group. These results suggested that low PRDX3 expression was a predictor of a more favorable outcome in the CC PCa patients but not in the AA patients, which in general appeared to have a more aggressive disease. It should be taken into consideration, however, that our study was limited by the scarcity of follow-up data on PRFS for both ethnic groups, particularly for the AA patients, which could have influenced the statistical significance of the results.
We also found that elevated Gleason scores were significantly associated with shorter PRFS in the AA group but not in the CC group. These results were somewhat surprising because the severity of Gleason scores typically correlate with prostate cancer progression and biochemical recurrence [27,28]. A previous study by Sakr et al [28] that correlated the major Gleason grade proportion (3+4 versus 4+3) to clinicopathologic parameters in a PCa patient population of 228 AA and 304 CC demonstrated that a Gleason score 4+3 was significantly associated with more advanced disease, higher PSA levels, higher incidence of PSA recurrence, and particularly, with a higher proportion of AA patients. These investigators also found that a 3+4 PCa correlated with longer PRFS in CC patients but not in AA patients [28]. In our study we found that AA patients with a Gleason grade 4+3 had a shorter PRFS compared to CC patients, which would be consistent with the notion that the AA patients had a more aggressive disease. We were, however, unable to demonstrate a significant association between elevated PRDX3 expression and Gleason grade 4+3 in either patient group. It should be emphasized that most of the Gleason grades associated with the commercial ethnicity TMAs used in our study were 3+3 or 3+4, with very few patient samples exhibiting a 4+3 PCa. This small sample size may have precluded a more statistically robust analysis of the relationship between Gleason grades, PRDX3 expression, and PRFS, within each ethnic group and between the two groups.
The significance of these intriguing observations is unclear and warrants confirmation with larger patient cohorts from different ethnic groups associated with complete clinicopathologic information. It is possible that the CC group in the ethnicity TMA included more men in which PCa was detected at earlier tumor stage compared to the AA group, which could explain the more favorable disease outcome in CC patients with low PRDX3 expression. It could be also speculated that differential expression, activity, function, or secretion of PRDX3 or other pro-survival proteins in prostate tumors from different ethnic populations might reflect the influence of gene-environment interactions that could contribute to ethnic differences in tumor aggressiveness, as recently implicated in studies on the IGF-II gene in breast cancer [39]. Our observations could be also extended to other genes that were previously found upregulated by gene expression profiling in PCa tumors from AA patients compared to CC patients [40].
In summary, our data indicates a consistent upregulation of PRDX3 and 4 in PCa, linked to specific clinical outcomes. Further analysis of the expression, activity levels, and functions of these PRDXs in ethnically diverse prostate tumor tissues and cellular models, and their modulation by environmental stressors, would be essential for defining the potential role of these antioxidant proteins as novel biomarkers and therapeutic targets. Such studies could also provide new insights into the role of PRDXs as potential biological determinants contributing to PCa health disparities.
Acknowledgments
We are indebted to Dr. Melanie Mediavilla-Varela (Moffitt Cancer Center, Tampa, FL) for her valuable technical assistance during the earlier stages of this work. We thank Dr. Dan Mercola (UC Irvine) for facilitating access to the SPECS database. We also thank Dr. Nathan Wall (LLU) for critically reading this manuscript and providing valuable suggestions. This work was supported by the following research grants: NIH-NCMHD 5P20MD001632, NIH-NIGMS 5R25GM60507, NIH-NCI UO1CA114810-03, and CDMRP-PCP W81XWH-04-1-0087.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jones BA, Liu WL, Araujo AB, Kasl SV, Silvera SN, Soler-Vila H, Curnen MG, Dubrow R. Explaining the race difference in prostate cancer stage at diagnosis. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2825–2834. doi: 10.1158/1055-9965.EPI-08-0203. [DOI] [PubMed] [Google Scholar]
- 3.Karami S, Young HA, Henson DE. Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31(1):29–34. doi: 10.1016/j.cdp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93(5):388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 5.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67(22):10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 8.So A, Hadaschik B, Sowery R, Gleave M. The role of stress proteins in prostate cancer. Curr Genomics. 2007;8(4):252–261. doi: 10.2174/138920207781386951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68(6):1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 10.Mediavilla-Varela M, Pacheco FJ, Almaguel F, Perez J, Sahakian E, Daniels TR, Leoh LS, Padilla A, Wall NR, Lilly MB, De Leon M, Casiano CA. Docetaxel-induced prostate cancer cell death involves concomitant activation of caspase and lysosomal pathways and is attenuated by the stress oncoprotein LEDGF/p75. Molecular Cancer. 2009;8:68. doi: 10.1186/1476-4598-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B, Wang Y, Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009;286(2):154–160. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7(5-6):768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- 13.Karihtala P, Mäntyniemi A, Kang SW, Kinnula VL, Soini Y. Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003;9(9):3418–3424. [PubMed] [Google Scholar]
- 14.Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan C, Cha EJ, Lee HL, Han KH, Lee KM, Kim WJ. Enhanced expression of peroxiredoxin I and VI correlates with development, recurrence and progression of human bladder cancer. J Urol. 2006;175(4):1512–1516. doi: 10.1016/S0022-5347(05)00659-2. [DOI] [PubMed] [Google Scholar]
- 16.Kinnula VL, Lehtonen S, Sormunen R, Kaarteenaho-Wiik R, Kang SW, Rhee SG, Soini Y. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002;196(3):316–323. doi: 10.1002/path.1042. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Kim YS, Lee HL, Shim JY, Lee KS, Oh YJ, Shin SS, Choi YH, Park KJ, Park RW, Hwang SC. Expression of peroxiredoxin and thioredoxin in human lung cancer and paired normal lung. Respirology. 2006;11(3):269–275. doi: 10.1111/j.1440-1843.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 18.Lehtonen ST, Svensk AM, Soini Y, Pääkkö P, Hirvikoski P, Kang SW, Säily M, Kinnula VL. Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer. 2004;111(4):514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Yu M, Han S, Oh I, Choi YJ, Kim S, Yoon K, Jung M, Choe W. Expression of human peroxiredoxin isoforms in response to cervical carcinogenesis. Oncol Rep. 2009;21(6):1391–1396. [PubMed] [Google Scholar]
- 20.Pylväs M, Puistola U, Kauppila S, Soini Y, Karihtala P. Oxidative stress-induced antioxidant enzyme expression is an early phenomenon in ovarian carcinogenesis. Eur J Cancer. 2010;46(9):1661–1667. doi: 10.1016/j.ejca.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard C, Mecham B, Dumpit R, Coleman I, Bhattacharjee M, Chen Q, Sikes RA, Nelson PS. Conserved gene expression programs integrate mammalian prostate development and tumorigenesis. Cancer Res. 2009;69(5):1739–1747. doi: 10.1158/0008-5472.CAN-07-6817. [DOI] [PubMed] [Google Scholar]
- 22.Lin JF, Xu J, Tian HY, Gao X, Chen QX, Gu Q, Xu GJ, Song JD, Zhao FK. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121(12):2596–2605. doi: 10.1002/ijc.23016. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Yu X, Ip C, Mohler JL, Bogner PN, Park YM. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res. 2007;67(19):9294–9303. doi: 10.1158/0008-5472.CAN-07-0651. [DOI] [PubMed] [Google Scholar]
- 24.Chhipa RR, Lee KS, Onate S, Wu Y, Ip C. Prx1 enhances androgen receptor function in prostate cancer cells by increasing receptor affinity to dihydrotestosterone. Mol Cancer Res. 2009;7(9):1543–1552. doi: 10.1158/1541-7786.MCR-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- 26.Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb MJ, Sheets SM, Wu X, Casiano CA. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005;62(1):14–26. doi: 10.1002/pros.20112. [DOI] [PubMed] [Google Scholar]
- 27.Wittschieber D, Köllerman J, Schlomm T, Sauter G, Erbersdobler Nuclear garding versus gleason grading in small samples containing prostate cancer: a tissue microarray study. Pathol Oncol Res. 2010 doi: 10.1007/s12253-010-9270-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Sakr WA, Tefilli MV, Grignon DJ, Banerjee M, Dey J, Gheiler EL, Tiguert R, Powell IJ, Wood DP. Gleason score 7 prostate cancer: a heterogeneous entity? Correlation with pathologic parameters and disease-free survival. Urology. 2000 Nov 1;56(5):730–4. doi: 10.1016/s0090-4295(00)00791-3. [DOI] [PubMed] [Google Scholar]
- 29.Shiota M, Izumi H, Miyamoto N, Onitsuka T, Kashiwagi E, Kidani A, Hirano G, Takahashi M, Ono M, Kuwano M, Naito S, Sasaguri Y, Kohno K. Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high-mobility group protein B1. Cancer Sci. 2008;99(10):1950–1959. doi: 10.1111/j.1349-7006.2008.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang X, DeWeese TL, Nelson WG, Abate-Shen C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005;65(15):6773–6779. doi: 10.1158/0008-5472.CAN-05-1948. [DOI] [PubMed] [Google Scholar]
- 31.Shen C, Nathan C. Nonredundant antioxidant defense by multiple two-cysteine peroxiredoxins in human prostate cancer cells. Mol Med. 2002;8(2):95–102. [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28(10):1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009;69(13):1470–1479. doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279(40):41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 35.Giguère P, Turcotte ME, Hamelin E, Parent A, Brisson J, Laroche G, Labrecque P, Dupuis G, Parent JL. Peroxiredoxin-4 interacts with and regulates the thromboxane A(2) receptor. FEBS Lett. 2007;581(20):3863–3868. doi: 10.1016/j.febslet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Chowdhury SK, Raha S, Tarnopolsky MA, Singh G. Increased expression of mitochondrial glycerophosphate dehydrogenase and antioxidant enzymes in prostate cancer cell lines/cancer. Free Radic Res. 2007;41(10):1116–1124. doi: 10.1080/10715760701579314. [DOI] [PubMed] [Google Scholar]
- 37.De Simoni S, Goemaere J, Knoops B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+ Neurosci Lett. 2008;433(3):219–224. doi: 10.1016/j.neulet.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 38.Wang HQ, Du ZX, Liu BQ, Gao YY, Meng X, Guan Y, Zhang HY. TNF-related apoptosis-inducing ligand suppresses PRDX4 expression. FEBS Lett. 2009;583(9):1511–1515. doi: 10.1016/j.febslet.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Kalla-Singh S, Tan QW, Brito C, De León M, Garberoglio C, De León D. Differential insulin-like growth factor II (IGF-II) expression: a potential role for breast cancer survival disparity. Growth Horm IGF Res. 2010;20(2):162–170. doi: 10.1016/j.ghir.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, Stephens RM, Caporaso NE, Loffredo CA, Ambs S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68(3):927–936. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]