Abstract
Purpose
This phase I study assessed the maximum tolerated dose (MTD), safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of SJG-136, a sequence-specific DNA cross-linking agent, in patients with advanced cancer.
Experimental Design
In Schedule A, seven patients received escalating doses of SJG-136 (6, 12, 24, and 48 µg/m2) daily for 5 of 21 days. Blood samples were collected for PK analysis on Days 1 and 5 of Cycle 1. In Schedule B, SJG-136 was given daily for 3 of 21 days (N=17; doses 20, 25, 30, and 35 µg/m2). Blood samples were collected on Days 1 and 3 of Cycles 1 and 2 for PK and PD analysis. Patients in Schedule B received dexamethasone and early diuretic care.
Results
Schedule A: dose-limiting toxicities included Grade 3 edema, dyspnea, fatigue, and delayed liver toxicity (Grade 3/4). PK analysis revealed dose-dependent increases in AUC and Cmax. Substantial changes in volume of distribution at steady-state (Vss) occurred after repeated dosing in some patients prior to the onset of edema. Schedule B: the same toxicities were manageable with steroid premedication and diuretic support. No significant myelosuppression occurred on either schedule. DNA interstrand cross-links correlated with systemic exposure of SJG-136 following the second dose in Cycle 1 and were still detectable immediately before Cycle 2.
Conclusions
The MTD of SJG-136 in this study was 30 µg/m2 administered on a daily × 3 basis with no myelosuppression effects. Coupled with supportive management, SJG-136 is now advancing to a Phase II trial in ovarian cancer.
Keywords: SJG-136, clinical trial, cancer, Phase I, pharmacokinetics, pharmacodynamics
INTRODUCTION
SJG-136 (NSC 694501) is a synthetic pyrrolobenzodiazepine dimer that exerts antitumor activity through DNA targeting (Figure 1, online).1, 2, 3 The two imine moieties of the molecule react with N-2 positions of the guanines on opposite DNA strands, forming sequence-specific cross-links with a preference for 5’ purine-GATC-pyrimidine sequences. In three-dimensional structures, SJG-136 binds in the minor groove of DNA causing minimal helix perturbation.3 The cytotoxic DNA interstrand cross-links form rapidly in cells and persist longer than those produced by other cross-linking agents.4,5 In a COMPARE analysis of 60,000 compounds in the National Cancer Institute anticancer drug screen, SJG-136 did not fit within clusters of any known DNA-binding agents, suggesting a distinct mechanism of action.5
Figure 1.
(online only). Chemical structure of SJG-136.
SJG-136 has demonstrated potent activity in a range of cell lines and tumor xenograft models.5, 6 Growth inhibition of sensitive cell lines (e.g. NCI-H522, HL-60, and Molt-4) was achieved at subnanomolar concentrations.5 SJG-136 was also cytotoxic in cell lines resistant to other DNA-interactive agents, including human ovarian cancer cell lines resistant to cisplatin.5 In xenograft models, DNA cross-links were detected in vivo in tumors at one hour after intravenous administration of SJG-136 and persisted over 24 hours.5 In athymic mice bearing various xenograft tumors, SJG-136 treatment caused significant growth delays ranging from 32 to 575% and tumor-free responses, particularly in glioma and melanoma and, to a smaller extent, in breast and ovarian carcinoma.6 In rodents, intravenous bolus administration of SJG-136 for five consecutive days conferred the best efficacy.6 In a recent phase I study of SJG-136 in patients with advanced solid tumors, the maximum tolerated dose (MTD) was 45 µg/m2 every 21 days on a three weekly schedule; however, significant toxicities, including vascular leak syndrome, liver toxicity and fatigue, were observed.7
Here we report findings from a Phase I clinical trial of SJG-136 in patients with advanced cancer designed to assess the MTD, safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of SJG-136.
PATIENTS AND METHODS
Patient Selection
Adult subjects were eligible to participate if they had histologically confirmed solid tumor malignancy that was metastatic or unresectable and for which standard curative or palliative measures did not exist or were no longer effective. Subjects must have recovered from acute adverse effects from prior therapies, have an ECOG performance status ≤ 1 and life expectancy over three months. Additional eligibility criteria included: (a) adequate bone marrow function (a white blood cell count ≥3.0×109/L, absolute neutrophil count ≥1.5×109/L, platelets ≥100×109/L); (b) adequate hepatic function (total bilirubin level within the upper limit of normal; and aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase ≤ 2.5 times the upper limit of normal); (c) adequate renal function (creatinine ≤1.5 mg/dL or calculated creatinine clearance ≥ 60 mL/min by Cockcroft-Gault method); (d) negative serum pregnancy test; and (e) a signed IRB approved consent form. Women of child-bearing potential and men had to use adequate barrier contraception.
Excluded were subjects with uncontrolled illness, including ongoing or active infection, HIV, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situations that would limit compliance with study requirements. Patients were prohibited from study if they had chemotherapy or radiotherapy within four weeks (six weeks for nitrosoureas or mitomycin C) before entering the study or those with residual adverse events due to agents administered more than four weeks earlier. Patients were not allowed to receive any other investigational agent while on SJG-136.
Trial Design, Dose Escalation, and Modification
Schedule A: SJG-136 was given on a daily × 5 schedule every 21 days. An accelerated titration design was used to minimize potential exposure to low drug levels.8 Starting dose of SJG-136 was 6 µg/m2/day. One patient per cohort was enrolled. In the absence of a Grade 2 or higher toxicity, cohorts were expanded to three subjects at that level. Subsequent cohorts received double doses (12, 24, and 48 µg/m2/day).
Schedule B: SJG-136 was administered on a shortened daily × 3 dosing schedule every three weeks with steroid premedication (dexamethasone, 8 mg/day, Day −1 through Day 3) and early diuretic treatment with spironolactone. Spironolactone was started at 25 mg daily if weight increased by 2 lbs (1kg) or more. The dose has been titrated upwards in 25 mg increments every 2 days until stabilization. The starting dose of SJG-136 was 20 µg/m2/day, representing the equivalent of well tolerated 12 mcg/m2/day dose given for five consecutive days on Schedule A. Three patients per dose level were enrolled and the dose escalation was more conservative (20, 25, 30 and 35 µg/m2/day). Patients were monitored for toxicities during later cycles of treatment until the MTD and DLT were defined.
Drug Formulation and Administration
SJG-136 (NSC 694501, lot LJ1-122-1) was obtained under contract with the NCI Pharmaceutical Management Branch (Bethesda, MD). SJG-136 was supplied in solution (10 µg/ml, each ml containing 47.1 mg of dimethylacetamide). The drug was infused intravenously over 20 min, followed by 20 ml of 5% dextrose solution.
Patient Evaluation
Dose-limiting toxicity (DLT), assessed using the National Cancer Institute Common Toxicity Criteria (CTC version 3.0), was defined as suspected drug toxicities during Cycle 1 of therapy, including: Grade 4 neutropenia lasting for over 5 days; Grade 3–4 neutropenia associated with a fever ≥ 38.5°C; documented infection associated with Grade 3–4 neutropenia; Grade 4 thrombocytopenia; Grade 3 thrombocytopenia with bleeding; Grade 3–4 non-hematologic toxicity (excluding nausea, vomiting, or diarrhea that was not treated with appropriate supportive measures); inability to initiate Cycle 2 within 2 weeks of scheduled treatment due to slow recovery from drug-induced toxicities.
Tumor response was assessed using RECIST criteria, measuring changes in only the largest diameter of the lesions.9 Measurements were taken by conventional techniques (CT, MRI, x-ray).
Pharmacokinetic (PK) Analysis
Serial blood samples were collected before the start of infusion, during the infusion at 10 minutes and at the end of infusion at 20 min, followed by 22, 25, 30, 50, 65, 80, 110, 130, and 260 minutes, after SJG-136 administration on Days 1 and 5 of Cycle 1 for Schedule A, and on Days 1 and 3 of Cycles 1 and 2 for Schedule B. The samples were centrifuged immediately after collection at 5,000 × g for 3 min at 4°C. The plasma was separated and stored at −80 °C pending analysis.
SJG-136 plasma concentration levels were determined using liquid chromatography-tandem mass spectrometry analysis.10 Noncompartmental PK analyses were performed using the WinNonlin software package, version 4.1 (Pharsight Corp., Palo Alto, CA). The area under the plasma concentration-time curve (AUC) was estimated by combining the AUC from time 0 to 4 h (AUC0-4h, calculated using the linear trapezoidal method) and the remaining AUC from 4 h to infinity (AUC4h-∞, calculated by extrapolation of the log-linear terminal phase). Total clearance (CL) and volume of distribution at steady state (Vss) were calculated as follows: CL = Dose/AUC; Vss = Mean Residence Time (MRT) × CL.
Pharmacodynamic (PD) Analysis: Determination of DNA interstrand cross-linking
Peripheral blood mononuclear cells (PBMC) were collected from the patients enrolled on Schedule B prior to the first and third doses of SJG-136 during Cycles 1 and 2. The formation of DNA interstrand cross-links was measured using a modification of the Single Cell Gel Electrophoresis (Comet) assay as described previously.5,7,11 DNA cross-linking in PBMC samples following SJG-136 dosing was expressed as the percentage decrease in tail moment (%DNA in tail × tail length) compared to the baseline value obtained before the first dose in Cycle 1. The greater the % decrease in tail moment, the greater the level of DNA interstrand cross-linking.
RESULTS
Characteristics of Patients
In Schedule A, seven patients were enrolled at doses of 6 – 48 µg/m2/day. In Schedule B, 17 patients were enrolled at doses of 20 – 35 µg/m2/day. The patient characteristics are listed in Table 1.
Table 1.
Patient Demographic and Disease Characteristics.
| Characteristics | Schedule A (Daily×5 schedule) |
Schedule B (Daily×3 schedule) |
||
|---|---|---|---|---|
| Number of patients enrolled | 7 | 17* | ||
| Age (Years) | ||||
| Median | 46 | 59 | ||
| Range | 23–75 | 40–84 | ||
| Gender | ||||
| Male | 5 | 9 | ||
| Female | 2 | 8 | ||
| Race | ||||
| Caucasian | 6 | 15 | ||
| African American | 1 | 2 | ||
| ECOG | ||||
| PS=0 | 2 | 4 | ||
| PS=1 | 5 | 13 | ||
| Number of prior chemotherapy regimens | ||||
| Median | 3 | 3 | ||
| Range | 1–5 | 1–6 | ||
| Primary tumor site | Colon | 3 | Colon | 5 |
| Anaplastic thyroid | 1 | Bladder | 2 | |
| Melanoma | 1 | Head & neck | 2 | |
| Osteosarcoma | 1 | NSCLC | 1 | |
| Prostate | 1 | SCLC | 1 | |
| Ovarian | 1 | |||
| Paraganglioma | 1 | |||
| Gastric | 1 | |||
| Leiomyosarcoma | 1 | |||
| Osteosarcoma | 1 | |||
| Unknown primary | 1 | |||
Of the 17 patients enrolled on the daily × 3 schedule, 14 patients were evaluable and three were taken off the study at or prior to completion of cycle 1.
Toxicity
Tables 2 (online only) and 3 list suspected drug-related adverse events including NCI-CTC grades on Schedule A and B, respectively. Initially, SJG-136 was administered daily for 5 days every 21 days with dose escalation of 6, 12, 24 and 48 µg/m2/day (Schedule A). DLTs during Cycle 1 included soft tissue edema, fatigue and dyspnea. Similar toxicities occurred during Cycle 2 in two patients treated at the 24 µg/m2/day dose level. These episodes were preceded by a precipitous drop in serum albumin without proteinuria. Most symptoms resolved over 2–3 weeks following discontinuation of SJG-136 and aggressive diuresis with spironolactone. Other non-dose-limiting toxicities included delayed increases in transaminases (Grade 2–4), alkaline phosphatase (Grade 2) and myalgias (Grade 2). No significant myelosuppression was observed. Although MTD was not achieved as defined in the protocol, the development of intolerable fluid retention necessitated a change in the study regimen to Schedule B as it was difficult to give repeated cycles of therapy.
Table 2.
Number of patients experiencing toxicity during Cycle 1 only (Schedule A) or Cycles 1 and 2 (Schedule B).
| No. of patients with toxicity (Grade 1 and 2 / 3 and 4) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| SJG 136 Dose Level |
Schedule A (Daily × 5) |
Schedule B (Daily × 3) |
|||||||
| Toxicity | µg/m2/day (No. patients) |
6 (n=1) |
12 (n=1) |
24 (n=3) |
48 (n=2) |
20 (n=4)a |
25 (n=4)b |
30 (n=6) |
35 (n=2) |
| Edema | n/o | n/o | 3/0 | 1/1c | 1/0 | 1/0 | 4/1d | 2/0 | |
| Weight gain | n/o | n/o | n/o | 0/1c | n/o | n/o | 1/0 | 1/0 | |
| Fatigue | n/o | 1/0 | 3/0 | 2/0 | 3/0 | 2/0 | 4/0 | 0/1d | |
| Anorexia | n/o | 1/0 | 1/0 | 2/0 | 1/0 | 1/0 | 2/0 | 1/0 | |
| Dyspnea | n/o | n/o | 1/0 | 0/1c | n/o | n/o | n/o | 1/1d | |
| Myalgias | n/o | 1/0 | n/o | n/o | n/o | n/o | n/o | n/o | |
| Liver transaminases (ALT/AST) | n/o | 1/0 | 2/0 | 1/0 | 0/1 | 1/0 | n/o | 0/1d | |
| Alkaline phosphatase | n/o | n/o | 1/0 | n/o | 1/0 | n/o | n/o | 0/1d | |
| Bilirubin | n/o | 1/0 | n/o | 0/1c | n/o | n/o | n/o | n/o | |
| Hypoalbuminemia | n/o | n/o | 1/0 | 2/0 | 1/0 | n/o | 1/0 | 1/0 | |
| Hypophosphatemia | n/o | n/o | n/o | n/o | 1/0 | n/o | 1/1d | n/o | |
| Anemia | n/o | n/o | n/o | n/o | 1/0 | 1/0 | n/o | 1/0 | |
| Leukopenia | n/o | n/o | n/o | n/o | n/o | n/o | n/o | n/o | |
| Thrombocytopenia | n/o | n/o | n/o | n/o | n/o | n/o | n/o | n/o | |
1 patient signed the consent but never received the treatment
1 patient not evaluable due to disease progression after cycle 1
DLT on Schedule A
DLT on Schedule B
n/o, no toxicity observed; DLT, dose limiting toxicity
Table 3.
Number of patients experiencing toxicity during Cycle 2–4 (Schedule A) or Cycle 3–6 (Schedule B).
| No. of patients with toxicity (Grade 1 and 2 / 3 and 4) |
|||||||
|---|---|---|---|---|---|---|---|
| SJG 136 Dose Level |
Schedule A (Daily × 5)a |
Schedule B (Daily × 3) |
|||||
| Toxicity | µg/m2/day (No. patients) |
12 (n=1) |
24 (n=2) |
20 (n=1) |
25 (n=1) |
30 (n=2) |
35 (n=1) |
| Edema | 1/0 | 1/2 | 1/0 | n/o | 2/0 | 1/0 | |
| Weight gain | n/o | 4/0 | n/o | n/o | 2/0 | 1/0 | |
| Fatigue | 1/0 | 0/3 | 1/0 | n/o | 1/0 | 1/0 | |
| Anorexia | 1/0 | 2/0 | n/o | n/o | 1/0 | 1/0 | |
| Dyspnea | 1/0 | 1/2 | n/o | n/o | 2/0 | 0/1 | |
| Myalgias | 1/0 | 3/0 | n/o | n/o | 1/0 | n/o | |
| Liver transaminases (ALT/AST) | 1/0 | 1/2b | 0/1 | 1/0 | 1/0 | n/o | |
| Alk.Phosphatase | n/o | 1/1b | 1/0 | n/o | 1/0 | n/o | |
| Bilirubin | 1/0 | n/o | n/o | n/o | n/o | n/o | |
| Hypoalbuminemia | 2/0 | 2/0 | 1/0/ | n/o | 1/0 | n/o | |
| Hypophosphatemia | n/o | n/o | n/o | n/o | n/o | n/o | |
| Anemia | 1/0 | n/o | n/o | 1/0 | n/o | n/o | |
| Leukopenia | 1/0 | n/o | n/o | n/o | n/o | n/o | |
| Thrombocytopenia | 1/0 | n/o | n/o | n/o | n/o | n/o | |
no patients on dose level 6 or 48 µg/m2/day received more than one cycle
one patient experienced delayed onset of grade 3 and 4 elevation of ALT, AST and alkaline phosphatase, which started 60 days after the last dose and lasted for 3 months
n/o, no toxicity observed
On Schedule A, only three patients were evaluable for toxicities in Cycles 2 through 4. Patients receiving SJG-136 at the doses of 6 and 48 µg/m2/day did not get more than 1 cycle. In one patient on 24 µg/m2/day, the onset of Grade 3 and 4 elevation of AST, ALT and alkaline phosphatase was delayed 60 days after the last dose and lasted 3 months.
Due to observed toxicities, a more conservative approach to dose was adopted (20, 25, 30 and 35 µg/m2/day for 3 days every 21 days; Schedule B), along with steroid premedication and early diuretic treatment. DLTs during Cycles 1 and 2 included edema, fatigue, dyspnea, hypophosphatemia, and elevated ALT and alkaline phosphatase. Two patients at the dose level 35 µg/m2/day experienced DLTs within first cycle of therapy, making further dose escalation unsafe. The delayed Grade 4 liver toxicity did not recur, possibly due to dexamethasone pretreatment, and edema was manageable with spironolactone. Again, no significant myelosuppression was observed. Five patients were evaluable for toxicity in subsequent cycles. Dyspnea was the only Grade 3 toxicity experienced during Cycles 3 through 6.
Pharmacokinetic Analysis
For Schedule A, the PK parameters following the intravenous administration of SJG-136 on Days 1 and 5 during Cycle 1 were obtained for seven patients (Table 4, online). The plasma concentrations of SJG-136 at all dose levels were above the limit of quantification (0.056 ng/ml) with no signs of accumulation after multiple dosing. Considerable interpatient variation occurred. Following the first dose (C1D1), a dose-dependent increase in the systemic exposure to SJG-136 was observed, as represented by Cmax and AUC, although the systemic exposure at 48 µg/m2/day appears to be lower than expected (Figure 2, open symbols and Table 4). At the 6 and 12 µg/m2/day dose levels, the PK parameters were comparable following the first and fifth doses of SJG-136 (C1D1 vs C1D5). However, for patients treated at 24 and 48 µg/m2/day dose levels, the PK parameters on C1D5 showed more variability than those on C1D1 (Figure 3A).
Table 4.
Pharmacokinetic parameters of SJG-136 following daily × 5 intravenous administration of 6, 12, 24 or 48 µg/m2/day SJG-136 (Schedule A).
| SJG-136 Dose Level (µg/m2/day) |
|||||
|---|---|---|---|---|---|
| 6 (n=1) | 12 (n=1) | 24 (n=3) | 48 (n=2) | ||
|
Cmax (ng/mL) |
C1D1 | 1.02 | 3.72 | 9.14 (1.57) | 7.64 |
| C1D5 | 0.98 | 4.83 | 6.77 (2.57) | 7.54 | |
|
AUCinf (ng•min/mL) |
C1D1 | 46.7 | 163 | 470 (123) | 333 |
| C1D5 | 51.8 | 193 | 470 (304) | 340 | |
|
Half-life (min) |
C1D1 | 125 | 82.6 | 124 (10) | 81.7 |
| C1D5 | 120 | 97.7 | 122 (11) | 116 | |
|
CL (mL/min/m2) |
C1D1 | 129 | 73.5 | 53.2 (12.1) | 144 |
| C1D5 | 115 | 62.2 | 66.0 (35.9) | 144 | |
|
Vss (mL/m2) |
C1D1 | 11500 | 4130 | 4350 (795) | 10300 |
| C1D5 | 10800 | 4050 | 7170 (2660) | 12100 | |
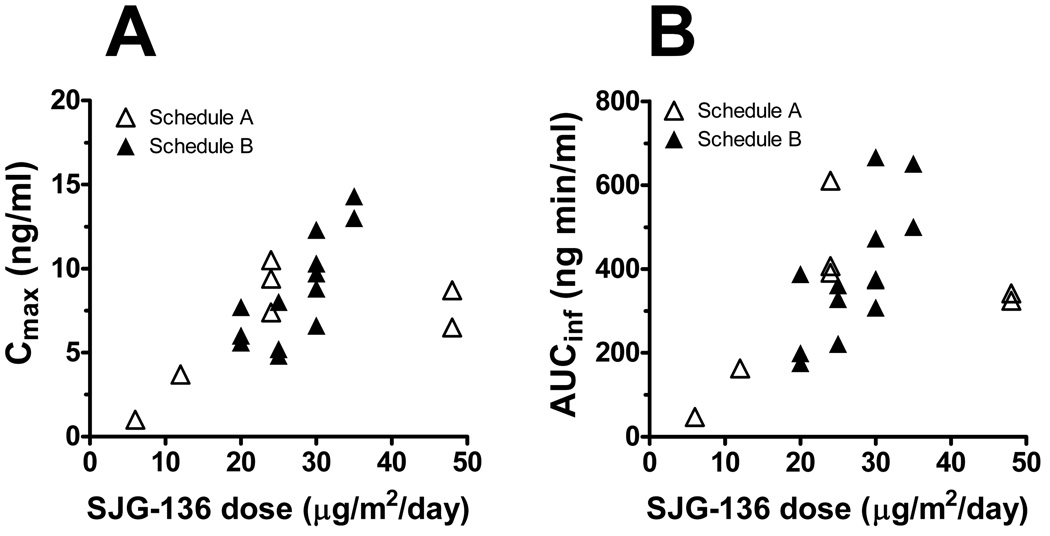
Figure 2.
Systemic exposure to SJG-136 following the first dose of SJG-136 via intravenous administration as assessed by Cmax (A) and AUCinf (B).
Figure 3.
Correlation of pharmacokinetic parameters of SJG-136. (A) Systemic exposure to SJG-136 on Schedule A following the first (C1D1) and fifth (C1D5) dose of SJG-136 in Cycle 1 as assessed by Cmax, AUCinf and VSS; (B) Systemic exposure to SJG-136 on Schedule B following the first (C1D1) and third (C1D3) dose of SJG-136 in Cycle 1 and the first (C2D1) and third (C2D3) dose of SJG-136 in Cycle 2 as assessed by Cmax, AUCinf and VSS.
For schedule B, the PK parameters were obtained on Days 1 and 3 during Cycles 1 and 2 for 13 patients (Table 5). The systemic exposure to SJG-136 (assessed by AUC and Cmax) following the first dose (C1D1) showed a dose-dependent increase (Figure 2, closed symbols and Table 5). Substantial differences in the PK parameters were observed following the first and third doses during Cycle 1 (C1D1 vs C1D3). In three out of five patients treated at the 30 µg/m2/day dose level, the AUC and Cmax values on C1D3 were lower (up to ~52%) than those on C1D1. Conversely, the Vss values on C1D3 were larger (up to ~150%) than those on C1D1. These changes in the PK parameters during Cycle 1 appear to be reversible in that the PK parameters following the first dose during Cycle 2 (C2D1) were comparable to those obtained on C1D1 (Figure 3B). Similar to the results in Cycle 1, the repeated dosing of SJG-136 during Cycle 2 led to substantial changes in the PK parameters (Figure 3B).
Table 5.
Pharmacokinetic parameters of SJG-136 following daily × 3 intravenous administration of 20, 25, 30 or 35 µg/m2/day SJG-136 (Schedule B).
| SJG-136 Dose Level (µg/m2/day) |
|||||
|---|---|---|---|---|---|
| 20 (n=3) | 25 (n=3) | 30 (n=5) | 35 (n=2) | ||
|
Cmax (ng/mL) |
C1D1 | 6.41 (1.10) | 5.97 (1.76) | 9.51 (2.09) | 13.63 |
| C1D3 | 6.12 (2.47) | 5.52 (2.24) | 7.84 (3.57) | 9.85 | |
| C2D1 | 7.68 (3.70) | 6.45 (0.80) | 9.61 (2.59) | 14.17 | |
| C2D3 | 6.92 (3.68) | 4.60 (0.46) | 6.91 (1.48) | 9.23 | |
|
AUCinf (ng•min/mL) |
C1D1 | 254 (116) | 303 (73) | 439 (140) | 575 |
| C1D3 | 273 (169) | 306 (155) | 372 (152) | 450 | |
| C2D1 | 426 (247) | 335 (48) | 455 (213) | 641 | |
| C2D3 | 376 (255) | 244 (39) | 319 (120) | 402 | |
|
Half-life (min) |
C1D1 | 74.3 (17.4) | 99.5 (24.0) | 85.5 (17.4) | 65.3 |
| C1D3 | 48.5 (20.4) | 91.9 (10.4) | 96.9 (16.3) | 77.2 | |
| C2D1 | 77.3 (24.6) | 81.7 (17.8) | 82.3 (11.5) | 69.2 | |
| C2D3 | 87.8 (28.9) | 94.3 (7.1) | 76.9 (10.5) | 74.5 | |
|
CL (mL/min/m2) |
C1D1 | 88.7 (32.9) | 86.1 (23.5) | 73.2 (19.8) | 61.9 |
| C1D3 | 91.7 (46.0) | 95.2 (40.7) | 92.3 (38.3) | 78.1 | |
| C2D1 | 57.0 (26.9) | 75.7 (11.6) | 75.5 (27.2) | 55.7 | |
| C2D3 | 69.1 (36.1) | 103.9 (15.1) | 104.5 (35.3) | 89.3 | |
|
Vss (mL/m2) |
C1D1 | 4730 (553) | 7630 (2590) | 4860 (911) | 3120 |
| C1D3 | 3920 (1930) | 8070 (3700) | 7370 (2720) | 5020 | |
| C2D1 | 4540 (3180) | 6050 (1300) | 5060 (1510) | 3170 | |
| C2D3 | 5600 (3990) | 8760 (1180) | 6810 (2240) | 5570 | |
Pharmacodynamic Analysis
The formation of DNA interstrand cross-links in PBMC samples obtained from patients in Schedule B was measured as a potential PD marker following SJG-136 administration. Control PBMC samples were collected preceding the SJG-136 treatment. Substantial DNA interstrand cross-linking was observed before the third dose of Cycle 1 (expressed as % decrease in tail moment), although interpatient variation occurred at different dose levels (Figure 4A). The extent of these cross-links correlated better with the systemic exposure to SJG-136 as assessed by AUCinf or Cmax than with the dose levels (p=0.008 and p=0.035, respectively; Figure 4B and 4C). The cross-links were still detected prior to Cycle 2, indicating persistence of the lesions (data not shown).
Figure 4.
Pharmacodynamic analysis of SJG-136, Schedule B. (A) Correlation of DNA cross-link formation in PMBCs with SJG-136 dosing; (B)(C) Correlation of DNA crosslink formation in PMBCs with systemic exposure to SJG-136 as assessed by Cmax and AUCinf, respectively.
Antitumor Activity
No objective responses were observed on Schedule A. However, two patients with heavily pretreated colorectal cancer achieved stable disease (SD) by radiographic criteria, accompanied by 25–30% decreases in CEA. One patient with malignant melanoma had SD lasting for 3 months.
On Schedule B, one patient with ovarian carcinoma pretreated with carboplatin/paclitaxel and doxorubicin achieved confirmed partial response (PR) lasting eight months with a 62% decrease in tumor dimension by RECIST criteria (30 µg/m2/day; Figure 5, online). At the same dose, a patient with poorly differentiated carcinoma of unknown primary cancer achieved an unconfirmed PR. SD of over 12 weeks was observed in one patient each with small cell lung carcinoma and bladder carcinoma. At the dose of 20 µg/m2/day, one patient with leiomyosarcoma had SD for 18 weeks.
Figure 5.
(online only). Confirmed partial response of a patient with ovarian carcinoma lasting eight months with a 62% decrease in tumor dimension by RECIST criteria at 30 µg/m2/day of SJG-136 (Schedule B). (A) day −1; (B) day 152. CA125 levels decreased 72% (110 U/ml to 32 U/ml).
DISCUSSION
While many available DNA-interactive agents can effectively treat some cancers, most patients with advanced solid tumors develop resistance to chemotherapy due to the ability of cancer cells to repair or tolerate the sustained DNA damage. Development of agents with different mechanisms of action is thus essential for improvement of the outcomes for patients. SJG-136 is a sequence-selective DNA cross-linking agent derived from naturally occurring antibiotics found in various Streptomyces species. Compared with adducts formed by other DNA-interacting agents, such as platinum agents, the DNA cross-links formed by SJG-136 appear to be more resistant to recognition and repair by DNA repair enzymes.3,5 Here we have identified the MTDs and DLTs for SJG-136 administered intravenously on two different dosing schedules in patients with advanced cancer.
Recently, a phase I study evaluated SJG-136 in patients with advanced solid tumors using a three weekly schedule.7 Significant toxicities observed at each dose level (30–240 µg/m2) included vascular leak syndrome, liver toxicity and fatigue, and generally occurred with delayed onset and increasing severity with following cycles. Subsequent dose reduction to 45 µg/m2 and implementation of steroid pretreatment did not resolve this issue. Despite few cases of SD observed in the study, an alternative dosing schedule was needed for further development of SJG-136.7
In this study, an accelerated titration design was used to limit the number of patients exposed to potentially toxic and ineffective doses (Schedule A). With seven patients enrolled we were able to identify DLTs and observe PK differences associated with repeated SJG-136 dosing. In contrast to animal studies, the DLTs in humans did not include myelotoxicity or gastrointestinal toxicity, but rather delayed onset of edema, liver toxicity, dyspnea, fatigue and hypoalbuminemia. The urinary analysis did not reveal any signs of proteinuria. An alternative etiology for the hypoalbuminemia may be that the third-spacing of fluid was accompanied by albumin escape into the tissues.
Based on these results and poor tolerability on daily × 5 schedule, SJG-136 was administered on a shortened schedule of daily × 3 dosing and a more conservative dose escalation (Schedule B). With 16 patients enrolled, the MTD of SJG-136 was 30 µg/m2/day. The DLTs were similar to Schedule A, including edema, fatigue, dyspnea and elevations in liver transaminases and alkaline phosphatase; however, their severity was manageable, in part by steroid premedication. To manage the edema, early diuretic therapy was required. The traditionally used loop diuretic, furosemide, was ineffective in this study. Spironolactone was chosen based on resemblance of the symptoms with anasarca usually experienced by patients with liver disease.
The PK analyses indicated dose-dependent increases in the systemic exposure of SJG-136 after the first course in both Schedules A and B (Figure 2, Tables 4,5). Consistent with these findings, the apparent CL values were comparable across differing SJG-136 dose levels. At the MTD level, repeated SJG-136 dosing during Cycle 1 leads to substantial, but reversible, decreases in the systemic exposure to SJG-136 and increases in the Vss values. These PK parameter changes were consistent with increases in extravasation of fluid, weight gain and hypoalbuminemia. In most patients, the changes in the SJG-136 PK parameters occurred in advance of the clinical onset of edema. We cannot exclude a possibility that other factors (e.g. drug metabolism, protein binding) may contribute to the PK changes at the subsequent SJG-136 doses. However, a previous study found that SJG-136 undergoes little metabolism, is mainly bound to serum albumin (~ 90%) and does not bind to glycoprotein.12
Using a modification of the Comet assay, we found that the DNA cross-linking caused by SJG-136 in peripheral blood mononuclear cells (PBMCs) prior to dose 3 of Cycle 1 correlated with the systemic exposure assessed by AUCinf or Cmax (Figures 4B and 4C). The cross-links were detected in PBMCs prior to dose 1 of Cycle 2, indicating a prolonged effect and incomplete repair and removal of the DNA cross-links over time. This data confirm the induction and persistence of the DNA damage caused by SJG-136, supporting the hypothesis of the formation of structurally non-disruptive adducts that are capable of eluding the DNA surveillance and repair mechanisms. The formation of DNA damage was confirmed in the samples obtained from two tumor biopsies of patients treated with SJG-136.7 Evidence of DNA damage was also observed by γ-H2AX foci induction in all lymphocyte samples following drug treatment in the current study (data not shown). The demonstration of the correlation between DNA adduct formation in PBMCs and in tumor tissue would provide a non-invasive approach for predicting response to therapy.
No objective response was observed on Schedule A. However, at the MTD on Schedule B there was one confirmed PR (platinum-resistant ovarian cancer), one unconfirmed PR (poorly differentiated carcinoma), and two patients with SD lasting over 12 weeks (small cell lung carcinoma and bladder carcinoma). One patient with leiomyosarcoma had prolonged SD at 20 µg/m2/day. The antitumor activity observed at the higher doses correlated with detection of DNA cross-links in PBMCs, as well as systemic exposure to SJG-136.
Based on these results, a phase II trial of SJG-136 is being planned in platinum resistant or refractory ovarian carcinomas. It will estimate overall response rate to SGJ-136 at the MTD determined in this study and determine other parameters of efficacy, including progression-free survival, overall survival and time to progression. Response rates will be correlated with the degree of DNA cross-linking in PBMCs and tumor cells.
ACKNOWLEDGMENTS
Supported by NIH U01 CA099177, M01 RR00095 and CRUK (C2259/A9994).
This work was supported in part by the NIH cooperative agreement award 5U01 CA099177 (MLR) and UCL Experimental Cancer Medicine Centre. We thank Prof. David Thurston for his advice in the initial stages of assay development.
REFERENCES
- 1.Lawley PD, Phillips DH. DNA adducts from chemotherapeutic agents. Mutat Res. 1996;355:13–40. doi: 10.1016/0027-5107(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 2.Reddy BS, Sondhi SM, Lown JW. Synthetic DNA minor groove-binding drugs. Pharmacol Ther. 1999;84:1–111. doi: 10.1016/s0163-7258(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 3.Gregson SJ, et al. Design, synthesis, and evaluation of a novel pyrrolobenzodiazepine DNA-interactive agent with highly efficient cross-linking ability and potent cytotoxicity. Journal of Medicinal Chemistry. 2001;44:737–748. doi: 10.1021/jm001064n. [DOI] [PubMed] [Google Scholar]
- 4.Clingen PH, et al. The XPF-ERCC1 endonuclease and homologous recombination contribute to the repair of minor groove DNA interstrand crosslinks in mammalian cells produced by the pyrrolo[2,1-c][1,4]benzodiazepine dimer SJG-136. Nucleic Acids Res. 2005;33:3283–3291. doi: 10.1093/nar/gki639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartley JA, et al. SJG-136 (NSC 694501), a novel rationally designed DNA minor groove interstrand cross-linking agent with potent and broad spectrum antitumor activity: part 1: cellular pharmacology, in vitro and initial in vivo antitumor activity. Cancer Research. 2004;64:6693–6699. doi: 10.1158/0008-5472.CAN-03-2941. [DOI] [PubMed] [Google Scholar]
- 6.Alley MC, et al. SJG-136 (NSC 694501), a novel rationally designed DNA minor groove interstrand cross-linking agent with potent and broad spectrum antitumor activity: part 2: efficacy evaluations. Cancer Research. 2004;64:6700–6706. doi: 10.1158/0008-5472.CAN-03-2942. [DOI] [PubMed] [Google Scholar]
- 7.Hochhauser D, et al. Phase I study of a sequence-selective minor groove DNA binding agent SJG-136 in patients with advanced solid tumors. Clinical Cancer Research. 2009;15:2140–2147. doi: 10.1158/1078-0432.CCR-08-1315. [DOI] [PubMed] [Google Scholar]
- 8.Simon R, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Wade Calcutt M, Lee W, Puzanov I, Rothenberg ML, Hachey DL. Determination of chemically reduced pyrrolobenzodiazepine SJG-136 in human plasma by HPLC-MS/MS: application to an anticancer phase I dose escalation study. J Mass Spectrom. 2008;43:42–52. doi: 10.1002/jms.1268. [DOI] [PubMed] [Google Scholar]
- 11.Spanswick VJ, Hartley JM, Ward TH, Hartley JA. Measurement of drug-induced DNA interstrand crosslinking using the single cell gel electrophoresis (comet) assay. In: Brown R, Boger-Brown U, editors. Methods in Molecular Medicine. Vol. 28. Humana Press; 1999. pp. 143–154. Cytotoxic Drug Resistance Mechanisms. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson GP, et al. Preliminary pharmacokinetic and bioanalytical studies of SJG-136 (NSC 694501), a sequence-selective pyrrolobenzodiazepine dimer DNA -cross-linking agent. Invest New Drugs. 2004;22(3):231–240. doi: 10.1023/B:DRUG.0000026249.97007.60. [DOI] [PubMed] [Google Scholar]