Abstract
Repeated phencyclidine (PCP) administration induces cognitive disruptions resembling those seen in schizophrenia. Alterations in glutamate transmission and γ-aminobutyric acid (GABA) function in the prefrontal cortex (PFC) have been implicated in these PCP-induced deficits, as well as in cognitive symptoms of schizophrenia. PCP-induced cognitive deficits are reversed by chronic treatment with the atypical antipsychotic clozapine in rats. We investigated the effects of a single injection vs. repeated administration of PCP on glutamate levels in the PFC using in vivo microdialysis. Furthermore, we examined how these PCP regimens affect GABA neuronal markers in the PFC. Finally, we investigated the effects of clozapine on disruptions in glutamate levels and GABA neuronal markers induced by repeated PCP administration. Acute PCP administration (2 mg/kg) increased extracellular PFC glutamate; this increase appeared blunted, but was not eliminated, after repeated PCP pretreatment. PCP administration also strongly decreased levels of parvalbumin and glutamic acid decarboxylase-67 (two markers of GABA function) in the PFC, an effect that was maintained after a 10 day drug-free washout period and unaltered by the resumption of repeated PCP injections. All of the observed PCP effects were attenuated by chronic treatment with clozapine, an atypical antipsychotic that has partial effectiveness on cognitive impairment in schizophrenia. These findings suggest that abnormal cortical glutamate transmission, possibly driven by pathological changes in GABA function in parvalbumin-positive fast-spiking interneurons, may underlie some of the cognitive deficits in schizophrenia. A better understanding of glutamate and GABA dysregulation in schizophrenia may uncover new treatment targets for schizophrenia-related cognitive dysfunction.
Keywords: schizophrenia, phencyclidine, glutamate, GABA, clozapine, antipsychotic, cognition
1. Introduction
Glutamate abnormalities reported in schizophrenia patients (Tamminga, 1998) are proposed to be involved in schizophrenia pathology (Olney and Farber, 1995), including cognitive deficits that are core features of the disorder (Elvevag and Goldberg, 2000). N-methyl-d-aspartate (NMDA) glutamate receptor antagonists induce a psychosis-like state in healthy humans that mimics most major symptoms of schizophrenia (Javitt and Zukin, 1991), including cognitive impairment (Krystal et al., 1994). Acute NMDA receptor antagonist administration increases extracellular glutamate levels in the prefrontal cortex (PFC; Moghaddam et al., 1997), a brain region critically involved in cognition (Fuster, 1973; Robbins, 1996) and implicated in cognitive deficits in schizophrenia (Weinberger et al., 1986). Thus, excessive glutamate transmission, possibly through non-NMDA glutamate receptors, has been hypothesized to underlie schizophrenia pathology, including cognitive deficits (Mathé et al., 1998; Moghaddam et al., 1997).
The increase in PFC glutamate efflux induced by NMDA receptor antagonists may be attributable to blockade of excitatory NMDA receptors located on inhibitory γ-aminobutyric acid (GABA) interneurons, leading to disinhibition of glutamate release from neurons targeted by those interneurons (Adams and Moghaddam, 1998; Farber et al., 1998; Olney and Farber, 1995). NMDA receptor antagonist treatment has been shown to decrease markers of GABA function both in vitro and in vivo (Behrens et al., 2007; Cochran et al., 2003; Kinney et al., 2006; Morrow et al., 2007; Paulson et al., 2003). Two important markers that are reduced after NMDA receptor antagonist exposure are parvalbumin, a calcium binding protein located within a subpopulation of fast-spiking GABAergic interneurons centrally involved in information processing in the brain (Bartos et al., 2007; Cardin et al., 2009; Sohal et al., 2009), and glutamic acid decarboxylase-67 (GAD67), the main isoform of the synthesizing enzyme for GABA in the brain (Asada et al., 1997). The reductions in these two markers occur in the same neuronal population, the parvalbumin-positive fast-spiking inhibitory interneurons. Interestingly, disturbances in GABA systems, including reductions in parvalbumin and GAD67 in the PFC, have also been found in schizophrenia patients (Benes and Berretta, 2001; Guidotti et al., 2000; Hashimoto et al., 2003; Lewis et al., 2004; Olney and Farber, 1995). Disruption of GABA function in parvalbumin-positive fast-spiking interneurons may thus drive the glutamate disinhibition and cognitive impairment induced by NMDA receptor antagonists, as well as glutamate dysregulation and cognitive deficits in schizophrenia.
The schizophrenia-like state evoked by NMDA receptor antagonists in humans is present only during intoxication and dissipates after clearance of the drug from the body (Adler et al., 1998; Cho et al., 2005; Krystal et al., 1994, 2003; Malhotra et al., 1996; Meltzer et al., 1972; Pradhan, 1984; Rosenbaum et al., 1959). However, a single administration of an NMDA receptor antagonist, such as phencyclidine (PCP), induces severe nonspecific behavioral disruptions (for review, see Amitai and Markou 2010a; Jentsch and Roth, 1999), which often preclude the precise quantification of cognitive deficits that are likely induced by the first PCP administration. Repeated administration of PCP allows tolerance to develop to the initial nonspecific behavioral disruptions induced by PCP (Melnick et al., 2002). Upon administration of acute re-challenges with PCP, selective cognitive deficits can be observed (Podhorna and Didriksen, 2005). We therefore developed a repeated PCP administration regimen in which two initial PCP injections are followed by two drug-free weeks, after which several additional daily PCP injections are administered. This PCP regimen has been shown to induce robust, significant, and selective cognitive deficits with relevance to schizophrenia in the 5-choice serial reaction time task (Amitai et al., 2007; Amitai and Markou 2009a, 2009b, 2010b), as well as impulsive-like response disinhibition in the intracranial self-stimulation procedure (Amitai et al., 2009). These PCP-induced deficits were sensitive to partial attenuation by chronic treatment with the atypical antipsychotic clozapine (Amitai et al., 2007, 2009). By examining the potential of an atypical antipsychotic medication to prevent the disruptive effects of a re-challenge with a psychotomimetic drug, this experimental design parallels the prevention of recurrence of a psychotic episode in schizophrenia by antipsychotic treatment.
Repeated PCP administration alters the effects of PCP on neurotransmitter levels compared with a single acute administration. For example, while a single acute administration of PCP strongly increases dopamine levels in the PFC, repeated PCP administration decreases baseline PFC dopamine levels in the drug-free state and blunts the increase in PFC dopamine release immediately after acute re-challenges with PCP (Jentsch et al., 1998). However, little is known about the effects of repeated PCP exposure on glutamate function. To address this question, we investigated the effects of repeated PCP administration on glutamate in the PFC using in vivo microdialysis combined with the repeated PCP administration regimen that we developed previously (Amitai et al., 2007) and compared the resulting glutamate levels with the effects of a single PCP injection. Furthermore, we treated an independent group of rats with the repeated PCP regimen, harvested their brains at different time points during treatment, and assessed levels of the GABA markers parvalbumin and GAD67 in the PFC using immunohistochemistry. Finally, we assessed the effects of clozapine, an atypical antipsychotic that partially ameliorates cognitive deficits in schizophrenia, on the changes in PFC glutamate efflux and GABA markers induced by repeated PCP administration.
2. Materials and Methods
2.1. Subjects
Eighty male Wistar rats (Charles River Laboratories, Wilmington, MA) were housed two per cage on a 12 h/12 h reverse light/dark cycle (lights off at 8:00 am). All behavioral testing was conducted during the animals’ dark cycle. Rats were allowed to reach a body weight of at least 300 g before being restricted to 20 g of food per day and before initiating drug treatment. Food restriction was introduced to match as closely as possible the experimental conditions of previous studies in which cognitive deficits induced by repeated PCP administration had been demonstrated (Amitai et al., 2007, 2009; Amitai and Markou, 2009a, 2009b, 2010b). Water was available ad libitum. Animals were treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals. All experiments were approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
2.2. Drugs
d-phencyclidine hydrochloride (PCP) was obtained from the National Institute on Drug Abuse (Rockville, MD), dissolved in sterile 0.9% saline solution, and administered subcutaneously at a concentration of 2 ml/kg. Clozapine was generously provided by Novartis Pharma AG (Basel, Switzerland), dissolved in a small amount of 0.1 M hydrochloric acid (HCl) and diluted with saline. Matched vehicle solution was prepared by dissolving the same amount of HCl in saline. Clozapine and vehicle were administered using two successive 7 day subcutaneous osmotic minipumps (Alzet, Palo Alto, CA). The limited solubility of clozapine at the concentration that was required to deliver 4 mg/kg/day in the maximal volume that could be contained in the minipumps prevented the use of a single 14 day osmotic minipump; thus, two successive 7 day minipumps were used. All drug doses are reported as the salt.
2.3. Surgeries
2.3.1. Osmotic minipump implantation and removal
Rats were anesthetized with a 1–3% isoflurane/oxygen vapor mixture, and an osmotic minipump (Alzet model 2ML1 7-day pump, Alza Corporation, Palo Alto, CA, USA) was inserted subcutaneously on the back of the animal parallel to the spine, with the flow moderator directed posteriorly. The wound was stapled, and an antibacterial ointment was applied to the incision area. On day 7, the first minipump was removed, and a second minipump was inserted under anesthesia contralateral to the first minipump (see above). On day 14, the second minipump was removed. For minipump removal, an incision was made under anesthesia, the minipump was removed, the wound was closed with surgical staples, and an antibacterial ointment was applied.
2.3.2. Microdialysis cannula implantation
Rats were anesthetized (1–3% isoflurane/oxygen vapor mixture) and placed in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the incisor bar set to zero. The skull was exposed, and a polyurethane guide cannula (CMA/12 Guide Cannula, CMA Microdialysis, Solna, Sweden) was implanted with the cannula tip pointing at the surface of the PFC (coordinates in millimeters: anterior/posterior [A/P] +3.2, medial/lateral [M/L] ± 0.7, dorsal/ventral [D/V] −2.5 from bregma, according to the rat brain atlas of Paxinos and Watson, 1986). The active membrane of the microdialysis probe extended 3 mm beyond the cannula tip when inserted. The cannula was permanently secured to the skull using dental cement anchored with four skull screws. A slotted tether screw was secured in the cement, posterior to the cannula, to allow attachment of a tether during microdialysis sampling. Cannula placements were counterbalanced, with half of the rats receiving cannulae in the right and half in the left brain hemisphere.
2.4. Microdialysis
2.4.1. Apparatus
Microdialysis sampling was performed in freely moving rats in previously described custom-designed testing chambers located inside sound-attenuating ventilated cabinets (Kuczenski and Segal, 1989). Each chamber was outfitted with a food hopper and water bottle to allow access to food and water. An Instech miniature fluid swivel (Instech Laboratories, Plymouth Meeting, PA) was located on top of the sound-attenuating cabinet. A hole in the ceiling of the testing chamber and cabinet allowed microdialysis probe inflow tubing (ethyl vinyl acetate tubing, Cole-Parmer, Vernon Hills, IL) to be connected to the swivel, and microdialysis probe outflow tubing to be extended outside the testing chamber. Dialysate was collected into vials outside the sound-attenuating cabinets, which permitted sample collection without disturbing the animal. A microinfusion pump was connected to the swivel to allow perfusion of the tubing with artificial cerebrospinal fluid (aCSF). The tubing and swivel were supported by a counterweight assembly, thereby allowing unrestricted movement of the rat. A stainless steel spring tether (Head Block Tether for Rats model M115S, CMA Microdialysis, Solna, Sweden) connected the swivel to the tether screw that was secured to the rat’s head to minimize tension on the probe tubing.
2.4.2. Microdialysis procedure
Rats were placed in the microdialysis chamber, and a dialysis probe with a 3 mm exposed dialysis membrane (CMA 12 Elite Microdialysis Probe, CMA Microdialysis, Solna, Sweden) was inserted into the guide cannula the evening prior to the experimental manipulation, resulting in dialysate sampling from medial PFC coordinates A/P + 3.2, M/L ± 0.7, D/V −2.5 to −5.5 (in mm). Twenty grams of food were provided to each rat to match the food restriction of the rats that participated in the behavioral and microdialysis experiments; water was available ad libitum. Artificial cerebrospinal fluid (aCSF) solution (147 mM NaCl, 2.3 mM CaCl2, 0.9 mM MgCl2, 4.0 mM KCl) was perfused through the probe at a rate of 1.5 µl/min, and a test sample was collected for 20 min to ensure proper flow. The perfusion rate was then reduced to 0.2 µl/min Fifteen to 18 h after probe insertion, the perfusion rate was raised back to 1.5 µl/min and allowed to stabilize for 30 min, after which sample collection commenced. After conclusion of sample collection (160 min), rats were euthanized with an overdose of pentobarbital and decapitated, and their brains were removed and placed into a 10% formalin solution. After at least 48 h, the brains were removed from the formalin solution, frozen, and sectioned into 60 µm slices for verification of probe placements. Data were included only from rats with confirmed accurate probe placements (31 of 33 rats). Probe traces in all rats included in the experiment were located in the prelimbic and infralimbic cortices between +3.2 and +3.7 mm from bregma.
2.4.3. Biochemical analyses
Glutamate levels in the dialysates were determined using reverse-phase high-performance liquid chromatography with electrochemical detection. Samples were derivatized using O-phthaldialdehyde/sulfite derivatizing agent and loaded into an autosampler for injection onto a reverse-phase C18 column. The mobile phase was based on the methods of Wang et al. (2008) and consisted of 100 mM sodium phosphate buffer containing 10% methanol and 5% acetonitrile, pH 4.40, and the flow rate was set at 1 ml/min, with the column temperature maintained at 40°C. The glutamate derivatization product was detected with an electrochemical detector (Waters, Milford, MA) set at 0.65 V relative to an Ag-AgCl reference electrode. Concentrations were estimated from peak heights using a Waters Maxima 820 data station (Waters, Milford, MA).
2.5. GABA marker assessment
2.5.1. Intracardial perfusions
Rats were deeply anesthetized using sodium pentobarbital. Once anesthesia was deep enough that the foot pinch reflex was absent, rats were perfused intracardially through the ascending aorta at 10 ml/min with approximately 50 ml of ice-cold saline followed by 200 ml of 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS). The brains were then removed and post-fixed in 4% PFA in 0.1 M PBS at 4°C. After 48 h, brains were transferred to 2% PFA in 0.1 M PBS and stored at 4°C.
2.5.2. Immunohistochemistry
Brains were sliced using a vibratome into 50 µm coronal sections. Six sequential slices, encompassing the PFC region (2.0–1.3 mm from bregma), were processed for floating-section fluorescence double immunohistochemistry for the detection of parvalbumin and GAD67. Antigen retrieval was performed by incubating the slices in 1% sodium borohydride for 15 min as described by Stanley and Shetty (2004), followed by washing in PBS and incubating in 10% normal goat serum in PBS for 16 h at 4°C. Primary antibodies (parvalbumin: 1:3,000 rabbit polyclonal; GAD67: 1:2,000 mouse monoclonal) were diluted in 2% normal goat serum in PBS and applied to the slices for 18 h at 4°C. After several washes in PBS, the slices were incubated in a 1:1,000 dilution of AlexaFluor conjugated goat anti-rabbit (568) and goat anti-mouse (488) antibodies for 1 h at room temperature. Slices were washed in PBS and mounted sequentially on glass slides using Vectashield, covered with a coverslip, and allowed to dry for ≥ 24 h before confocal imaging.
2.5.3. Confocal microscopy and image analysis
Mounted slices were evaluated for fluorescence under settings for 488 and 568 nm excitation on a LSM510 Meta multiphoton laser confocal microscope using a 40X water-immersion objective. Each slice was imaged across the prelimbic region between bregma 1.3 and 2.0 (three images per slice). Six slices were imaged per animal. For image quantification, a z-stack of eight images was obtained (corresponding to 1.4 µm on the z-axis). All parvalbumin neurons in the images were analyzed for parvalbumin and GAD67 content using MetaMorph as described by Behrens et al. (2007), which corresponds to a mean of 300 ± 20 neurons per animal.
Confocal microscope settings remained constant for each series of experiments so that the resulting images could be analyzed by densitometry, and the treatment-dependent changes in fluorescence could be compared and expressed as a percentage of the vehicle-treated condition. For each experiment, vehicle controls were processed in parallel, such that variability attributable to antibody batch or laser power could be avoided. Neurons in the images were then analyzed for their somatic median green and red fluorescence intensity per cell using MetaMorph (scale of 0–255 arbitrary units). Values were normalized to the saline-treated conditions and are expressed as percentages of saline.
2.6. Experimental design
2.6.1. Experiment 1: Effects of single or repeated PCP administration and chronic clozapine on extracellular glutamate in the PFC
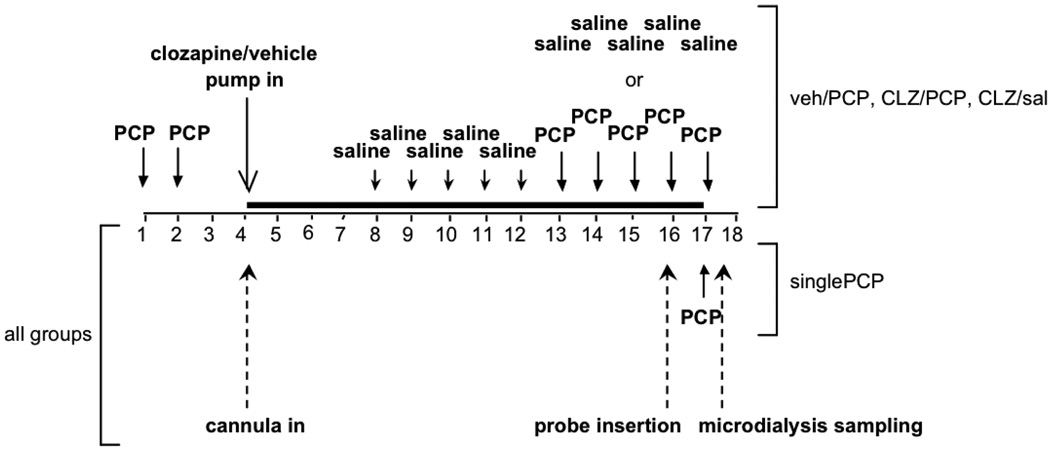
Naive rats were divided into four experimental groups: (i) Single PCP administration (n = 6): Rats received a single PCP injection during collection of microdialysis samples and received no minipump and no repeated injections. (ii) Vehicle/Repeated PCP administration (n = 7): Rats received minipumps delivering vehicle and repeated injections of PCP. (iii) Clozapine/Repeated PCP administration (n = 9): Rats received minipumps delivering clozapine and repeated injections of PCP. (iv) Clozapine/Saline (n = 9): Rats received minipumps delivering clozapine and repeated injections of saline.
Rats in the Vehicle/Repeated PCP, Clozapine/Repeated PCP, and Clozapine/Saline groups received two initial injections of 2 mg/kg PCP or saline, 24 h apart. Rats were then prepared with 7 day osmotic minipumps delivering 4 mg/kg/day clozapine (Clozapine/Repeated PCP and Clozapine/Saline groups) or vehicle (Vehicle/Repeated PCP group) and unilateral intracerebral guide cannulae directed at the surface of the PFC. Seven days after cannula/pump implantation, minipumps were removed, and new 7 day minipumps delivering clozapine or vehicle were implanted. The limited solubility of clozapine at the concentration required to deliver 4 mg/kg/day in the maximal volume that could be contained in the minipumps prevented the use of a single 14 day osmotic minipump. Beginning 3 days after cannula/pump implantation, rats received five consecutive daily saline injections to habituate them to the injection procedure. Then, after 9 days of pump treatment, the rats received five consecutive daily injections of 2 mg/kg PCP (Vehicle/Repeated PCP and Clozapine/Repeated PCP groups) or saline (Clozapine/Saline group). After receiving the fourth of these five daily injections, rats were placed in the microdialysis testing chambers, and microdialysis probes were inserted into the guide cannulae. Fifteen to 18 h after probe insertion, three 20 min baseline samples were collected, after which rats received the last of their five daily PCP or saline injections. Twenty-minute microdialysis samples were collected for 160 min after PCP/saline injection.
The administration protocol and doses of both PCP and clozapine matched those used in previous studies that showed robust, selective schizophrenia-like cognitive deficits induced by PCP and attenuation of these deficits by clozapine (Amitai et al., 2007; Amitai and Markou 2009a, 2009b, 2010b).
Rats in the Single PCP group were prepared with guide cannulae directed at the surface of the PFC but received no drug injections until the day of microdialysis sampling. Intermittent saline injections were administered to rats in this group to habituate them to the injection procedure. On day 13 after cannula implantation, rats were placed in the microdialysis testing chambers. Microdialysis probes were then inserted into the guide cannulae, and baseline samples were collected similarly to the other experimental groups. After collection of the last baseline sample, rats received a single 2 mg/kg PCP injection, and 20 min microdialysis samples were collected for 160 min after PCP injection (see Fig. 1 for a diagram of the experimental design).
Figure 1.
Diagram of Experiment 1 design. Injections and procedures in Experiment 1 are schematically represented. singlePCP, Single PCP injection group; veh/PCP, Vehicle/Repeated PCP administration group; CLZ/PCP, Clozapine/Repeated PCP administration group; CLZ/sal, Clozapine/Saline administration group. The dark line denotes the period of pump treatment.
2.6.2. Experiment 2a: Effects of repeated PCP administration on GABA markers in the PFC
Naive rats were divided into four experimental groups: (i) Single bout of PCP administration (“Initial PCP”; n = 5), (ii) Single bout of PCP administration, followed by drug-free washout (“Initial PCP/Washout”; n = 6), (iii) Single bout of PCP administration, followed by drug-free washout, then by further repeated PCP injections (“Initial PCP/Washout/PCP”; n = 6), (iv) saline injections only (“Saline”; n = 5).
Rats in the Initial PCP group received two injections of PCP, 24 h apart. These injections were analogous to the two initial PCP injections in the repeated PCP injection protocol used in Experiment 1. Twenty-four hours after the last injection, rats were perfused, and brains were harvested as described above.
Rats in the Initial PCP/Washout group received two injections of PCP, 24 h apart, followed by 10 days of drug-free washout. This washout period was analogous to the drug-free interval experienced by the rats in the Vehicle/Repeated PCP group in Experiment 1 during vehicle pump treatment and before resumption of PCP injections. During the last 5 days of this time period, rats also received daily saline injections, so as to mirror the treatment of the rats in the Vehicle/Repeated PCP group in Experiment 1. At the end of the 10 drug-free days, rats were perfused, and brains were harvested.
Rats in the Initial PCP/Washout/PCP group received two injections of PCP, 24 h apart, followed by 10 days of drug-free washout (including five consecutive daily saline injections as above), and then five consecutive daily injections of 2 mg/kg PCP. These five injections were analogous to the repeated PCP injections given to the rats in the Vehicle/Repeated PCP group in Experiment 1 toward the later part of vehicle pump treatment. Twenty-four hours after the last injection, rats were perfused, and brains were harvested.
Rats in the Saline group were treated similarly to the rats in the Initial PCP/Washout/PCP group, with the exception that they received injections of saline instead of PCP (see Fig. 2A for a diagram of the experimental design). Harvested brains were prepared for immunohistochemical analysis and examined for levels of parvalbumin and GAD67.
Figure 2.
Diagram of Experiment 2 design. Injections and procedures for (A) Experiment 2a and (B) Experiment 2b are schematically represented. The dark line denotes the period of minipump treatment.
2.6.3. Experiment 2b: Effects of chronic clozapine treatment on PCP-induced changes in GABA markers in the PFC
Naive rats were divided into five experimental groups (n = 5 per group): (i) Single bout of PCP administration (“Initial PCP”), (ii) Single bout of PCP administration, followed by chronic clozapine treatment (“Initial PCP/CLZ”), (iii) Single bout of PCP administration, followed by chronic clozapine treatment, with further repeated PCP injections during the later part of clozapine treatment (“Initial PCP/CLZ/PCP”), (iv) saline injections combined with chronic clozapine treatment (“Saline/CLZ/Saline”; n = 5), (v) saline injections combined with vehicle treatment only (“Saline/Vehicle/Saline”).
Rats in the Initial PCP group received two injections of PCP, 24 h apart. Again, these injections were analogous to the two initial PCP injections in the repeated PCP injection protocol used in Experiment 1. Twenty-four hours after the last injection, rats were perfused, and brains were harvested.
Rats in the Initial PCP/CLZ group received two injections of PCP, 24 h apart, followed by 1 day of drug-free washout, after which rats were prepared with 7 day osmotic minipumps delivering 4 mg/kg/day clozapine. Seven days after pump implantation, minipumps were removed. New minipumps delivering clozapine were then implanted, and clozapine treatment was continued for 2 more days. During the last 5 days of this time period, rats also received daily saline injections. This treatment was analogous to the period of PCP-free clozapine exposure experienced by the Clozapine/Repeated PCP group in Experiment 1 before resumption of PCP injections. At the end of the 9 days of clozapine treatment, rats were perfused, and brains were harvested.
Rats in the Initial PCP/CLZ/PCP group received two injections of PCP, 24 h apart, followed by 1 day of drug-free washout, after which rats were prepared with 7 day osmotic minipumps delivering 4 mg/kg/day clozapine. Seven days after pump implantation, minipumps were removed, and new minipumps delivering clozapine were implanted. Beginning 3 days after the initial pump implantation, rats received five consecutive daily saline injections, followed by five consecutive daily injections of 2 mg/kg PCP. This treatment was analogous to the full PCP and clozapine regimen experienced by the Clozapine/Repeated PCP group in Experiment 1. Twenty-four hours after the last injection, rats were perfused, and brains were harvested.
Rats in the Saline/CLZ/Saline group were treated similarly to the rats in the Initial PCP/CLZ/PCP group, with the exception that they received injections of saline only instead of PCP.
Rats in the Saline/Vehicle/Saline group were treated similarly to the rats in the Initial PCP/CLZ/PCP group, with the exception that they received injections of saline only instead of PCP and were prepared with minipumps containing vehicle instead of clozapine (see Fig. 2B for a diagram of the experimental design).
Harvested brains were prepared for immunohistochemical analysis and examined for levels of parvalbumin and GAD67.
2.7. Data analyses
Data from Experiment 1 were analyzed using two-way mixed-design analysis of variance (ANOVA). Glutamate levels are expressed as a percentage of the mean baseline value, calculated as the average of the three baseline samples taken before PCP or saline injection. Time Point was the within-subjects factor, and Drug Treatment (single PCP, vehicle+repeated PCP, clozapine+repeated PCP, clozapine+saline) was the between-subjects factor. Statistically significant effects found in the ANOVA were followed by post hoc comparisons among means using Bonferroni tests. Mean glutamate levels from each post-injection time point, expressed as a percentage of the mean baseline value, were compared to the mean baseline glutamate levels for each group to assess increases in extracellular glutamate levels above baseline in response to PCP or saline injection. In addition, mean extracellular glutamate levels of each treatment group were compared at each time point to assess differences in the glutamate response between groups.
Additionally, areas-under-the-curve were calculated for post-injection (PCP or vehicle) values (i.e., values after the baseline samples and after the challenge injection) in each experimental group. Values were corrected for baseline by subtracting the baseline value from each data point and then compared using one-way ANOVA. Statistically significant effects found in the ANOVA were followed by post hoc comparisons among means using Newman-Keuls tests.
All intensity values obtained in Experiment 2 were normalized by the mean obtained for the saline/vehicle control conditions for each experiment and expressed as a percentage of this mean. To obtain the mean fluorescence/cell/animal, percentage values were averaged across the six slices from the same animal, and the mean fluorescence intensity/cell/animal was used to calculate the mean and standard deviation per group. Data were analyzed using one-way ANOVA with Treatment Group as the between-subjects factor. Statistically significant effects found in the ANOVA were followed by post hoc comparisons using Newman-Keuls tests.
The level of significance was set at 0.05. Data were analyzed using GraphPad Prism software (GraphPad, San Diego, CA).
3. Results
3.1. Experiment 1: Effects of single or repeated PCP administration and chronic clozapine on extracellular glutamate in the PFC
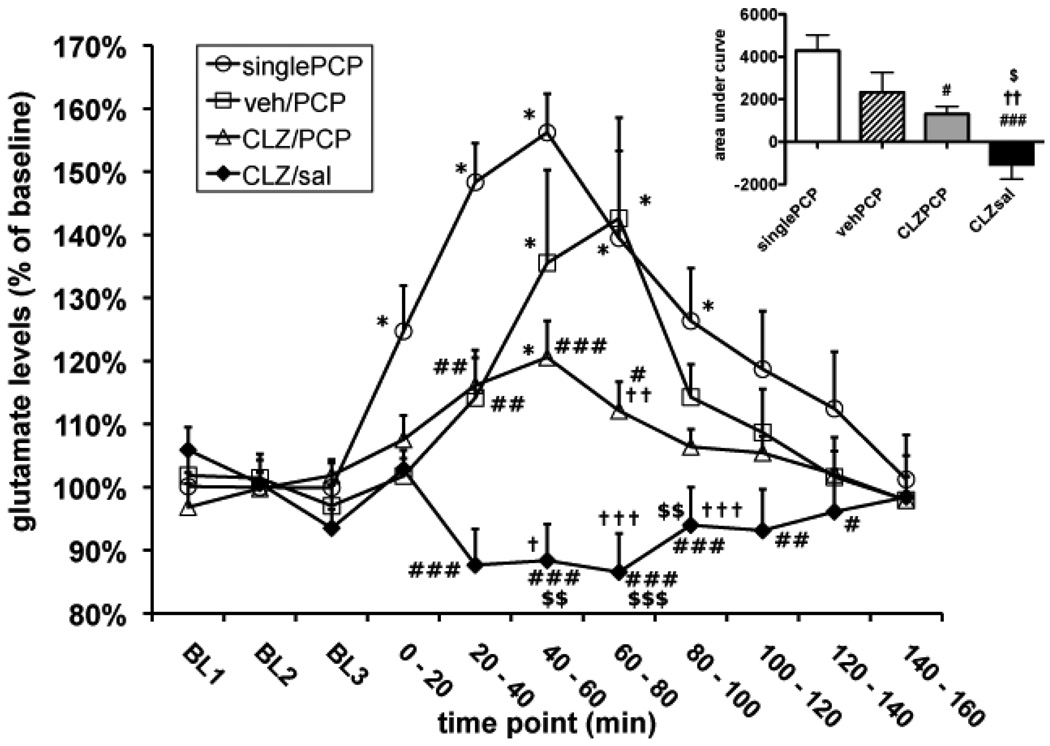
Baseline glutamate levels (Single PCP group: 2.58 ± 0.65 µM; Vehicle/Repeated PCP group: 3.50 ± 0.72 µM; Clozapine/Repeated PCP group: 4.59 ± 0.70 µM; Clozapine/Saline group: 2.92 ± 0.43 µM) did not differ significantly among groups. ANOVA of the effects of PCP/saline administration detected significant main effects of both Time Point (F8,216 = 14.95, p < 0.0001) and Drug Treatment (F3,216 = 10.23, p = 0.0001), as well as a Drug Treatment × Time Point interaction (F24,216 = 6.41, p < 0.0001). Post hoc tests comparing glutamate levels at each post-injection time point to baseline glutamate levels showed statistically significant elevations above baseline levels (p < 0.05) in the following groups:
-
-
Single PCP: at the 0–20 min, 20–40 min, 40–60 min, 60–80 min, and 80–100 min time points
-
-
Vehicle/Repeated PCP: at the 40–60 min and 60–80 min time points
-
-
Clozapine/Repeated PCP: at the 40–60 min time point
No significant elevations in glutamate levels were detected in the Clozapine/Saline group at any time point (Fig. 3).
Figure 3.
Effects of chronic clozapine and single or repeated PCP administration on extracellular glutamate levels in the PFC. Glutamate levels are expressed as mean ± SEM. Asterisks (*p < 0.05, **p < 0.01, ***p < 0.001) denote statistically significantly differences compared with baseline glutamate values in the same group. Pound signs (#p < 0.05, ##p < 0.01, ###p < 0.001) denote statistically significant differences compared with the Single PCP group. Dagger signs (†p < 0.05, ††p < 0.01, †††p < 0.001) denote statistically significant differences compared with the Vehicle/Repeated PCP group. Dollar signs ($p < 0.05, $$p < 0.01, $$$p < 0.001) denote statistically significantly differences compared with the Clozapine/Repeated PCP group. SinglePCP, Single PCP injection group; veh/PCP, Vehicle/Repeated PCP administration group; CLZ/PCP, Clozapine/Repeated PCP administration group; CLZ/sal, Clozapine/Saline group. The inset shows the area-under-the-curve for each treatment group, defined as the time integral of the glutamate concentrations observed for each group.
The increases in glutamate levels seen after PCP injection were not attributable to the injection procedure itself. No changes in neurotransmitter levels in the PFC were observed in our laboratory after systemic saline injections, and the existing literature reports no effects of such saline injections on extracellular glutamate levels in the PFC and other brain areas (e.g., Abekawa and Koyama, 2006; Abekawa et al., 2007; Adams and Moghaddam, 1998; Lopez-Gil et al., 2007). Moreover, no changes in glutamate levels were observed after saline injections in the Clozapine/Saline group. While this group was treated with chronic clozapine at the time of saline injection, it is highly unlikely that the lack of effect of the saline injection on extracellular glutamate levels was attributable to clozapine treatment because significant, albeit attenuated, increases in glutamate levels above baseline were still seen in clozapine-treated animals after PCP injection in the Clozapine/Repeated PCP group.
The Single PCP group exhibited the most profound increase in extracellular glutamate. The glutamate increase in the Vehicle/Repeated PCP group appeared less pronounced than that of the single PCP group, despite significant elevations above baseline (see above); the glutamate levels of the Vehicle/Repeated PCP group were significant lower than those of the Single PCP group at the 20–40 min time point.
In the Clozapine/Repeated PCP group, the glutamate increase in response to PCP was further attenuated than that seen in the Vehicle/Repeated PCP group. While extracellular glutamate rose above baseline after PCP injection (see above), glutamate levels in this group were significantly lower than those of the Vehicle/Repeated PCP group at the 60–80 min time point, and significantly lower than those of the Single PCP group at the 20–40 min, 40–60 min, and 60–80 min time points.
Finally, no increases in glutamate levels above baseline were seen in the Clozapine/Saline group. Glutamate levels in this group differed from the Clozapine/Repeated PCP group at the 20–40 min, 40–60 min, and 60–80 min time points; from the Vehicle/Repeated PCP group at the 20–40 min, 40–60 min, and 60–80 min time points; and from the Single PCP group at the at the 20–40 min, 40–60 min, 60–80 min, 80–100 min, and 100–120 min time points (Fig. 3).
An ANOVA on the areas-under-the-curve (AUC) confirmed a significant effect of Drug Treatment (F3,27 = 10.62, p < 0.0001). While post-hoc tests found no significant difference in AUC between the Single PCP and Vehicle/Repeated PCP groups, a t-test comparing the two groups found a strong trend towards a smaller AUC in the Vehicle/Repeated PCP group (p = 0.068). Post-hoc tests also confirmed that AUC for the Clozapine/Repeated PCP group was smaller than for the Single PCP group, and AUC for the Clozapine/Saline group was smaller than for all other treatment groups (p < 0.05; Fig. 3).
3.2. Experiment 2a: Effects of repeated PCP administration on GABA markers in the PFC
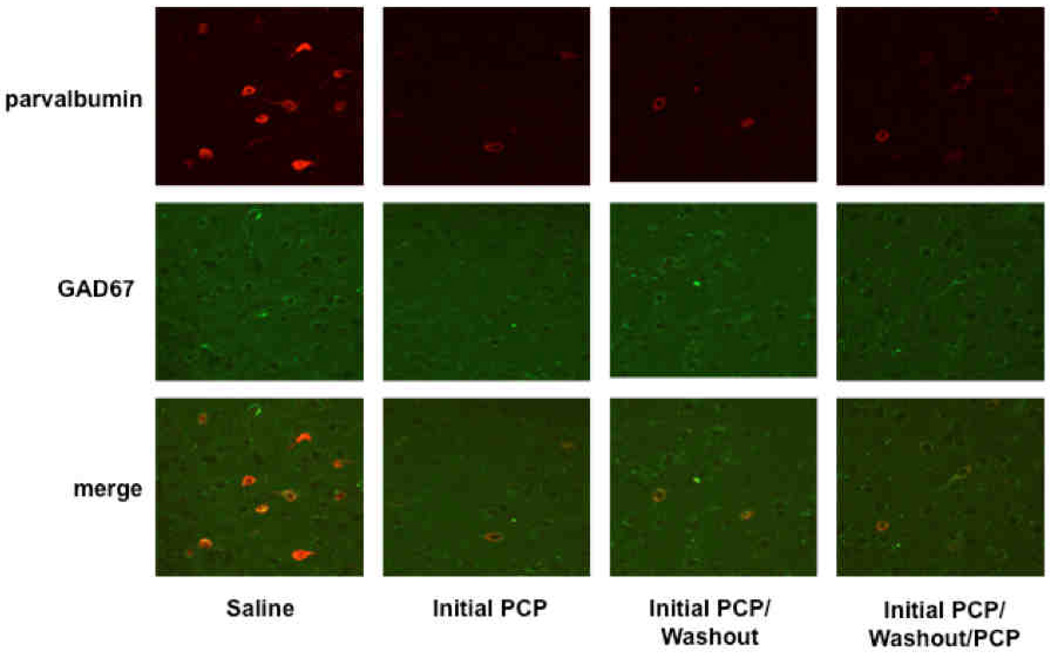
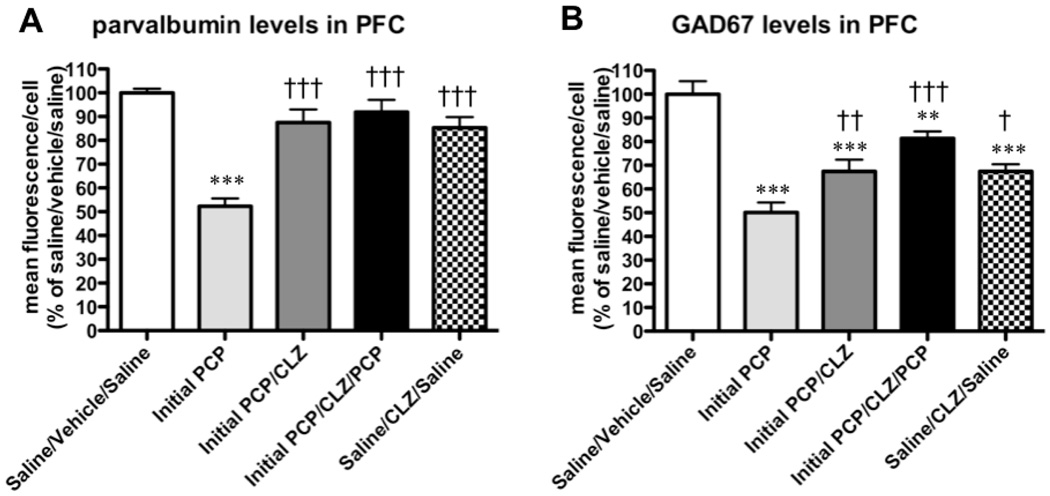
PCP administration decreased levels of GABA markers (Fig. 4). ANOVAs detected a significant effect of Treatment Group on levels of both parvalbumin (F3,18 = 36.73, p < 0.0001; Fig. 5A) and GAD67 (F3,18 = 30.81, p < 0.0001; Fig. 5B). Post hoc tests confirmed that levels of both parvalbumin and GAD67 decreased in all of the PCP-treated groups (Initial PCP, Initial PCP/Washout, Initial PCP/Washout/PCP) compared with the Saline group (p < 0.001 for all comparisons). No significant differences in parvalbumin and GAD67 levels were found between any of the PCP-treated groups.
Figure 4.
Fluorescence confocal images of representative fields depicting expression of parvalbumin (top), GAD67 (middle), or both (bottom) in parvalbumin interneurons in the prelimbic region of rats treated with saline or PCP.
Figure 5.
Effects of repeated PCP administration on GABA markers in the PFC: quantification of parvalbumin and GAD67 mean fluorescence/cell for the region normalized by the means of saline-treated animals. (A) Parvalbumin and (B) GAD67 immunofluorescence levels are expressed as mean ± SEM. Asterisks (***p < 0.001) denote statistically significantly differences compared with the Saline group.
3.3. Experiment 2b: Effects of chronic clozapine treatment of PCP-induced changes in GABA markers in the PFC
Chronic Clozapine attenuated the PCP-induced decreases in GABA markers levels (Fig. 6). ANOVAs detected a significant main effect of Treatment Group on parvalbumin levels (F4,20 = 18.58, p < 0.0001; Fig. 7A). Post hoc tests showed that parvalbumin levels decreased in the Initial PCP group compared with the Saline/Vehicle/Saline group (p < 0.001). None of the clozapine-treated groups (Initial PCP/CLZ, Initial PCP/CLZ/PCP, Saline/CLZ/Saline) differed from the Saline/Vehicle/Saline group in terms of parvalbumin levels, regardless of whether they were also treated with PCP or with only saline injections. Furthermore, none of the clozapine-treated groups differed significantly from each other. All clozapine-treated groups had significantly higher parvalbumin levels than the Initial PCP group (p < 0.001 for all comparisons).
Figure 6.
Fluorescence confocal images of representative fields depicting expression of parvalbumin (top), GAD67 (middle), or both (bottom) in parvalbumin interneurons in the prelimbic region of rats treated with saline or PCP in combination with chronic clozapine or vehicle.
Figure 7.
Effects of chronic clozapine treatment on PCP-induced changes in GABA markers in the PFC: quantification of parvalbumin and GAD67 mean fluorescence/cell for the region normalized by the means of saline-treated animals. (A) Parvalbumin and (B) GAD67 immunofluorescence levels are expressed as mean ± SEM. Asterisks (**p < 0.01, ***p < 0.001) denote statistically significantly differences compared with the Saline/Vehicle/Saline group. Dagger signs (†p < 0.05, ††p < 0.01, †††p < 0.001) denote statistically significant differences compared with the Initial PCP group.
ANOVAs also found a significant main effect of Treatment Group on GAD67 levels (F4,20 = 19.28, p < 0.0001; Fig. 7B). Post hoc tests confirmed that, similar to parvalbumin, GAD67 levels decreased in the Initial PCP group compared with the Saline/Vehicle/Saline group (p < 0.001). GAD67 levels also decreased compared with the Saline/Vehicle/Saline group in all of the clozapine-treated groups (p < 0.001 for Initial PCP/CLZ or Saline/CLZ/Saline vs. Saline/Vehicle/Saline; p < 0.01 for Initial PCP/CLZ/PCP vs. Saline/Vehicle/Saline). However, all clozapine-treated groups had significantly higher GAD67 levels than the Initial PCP group (p < 0.001 for Initial PCP/CLZ/PCP vs. Initial PCP; p < 0.01 for Initial PCP/CLZ vs. Initial PCP; p < 0.05 for Saline/CLZ/Saline vs. Initial PCP). None of the clozapine-treated groups differed significantly from each other in terms of GAD67 levels.
4. Discussion
Confirming previous studies (Abekawa et al., 2003, 2006, 2007; Adams and Moghaddam, 1998, 2001), acute PCP exposure significantly elevated extracellular glutamate levels in the PFC of rats. This PCP-induced glutamate efflux was observed after a single PCP injection as well as after repeated PCP administration.
Interestingly, the increase in PFC glutamate in response to PCP appeared blunted after repeated PCP exposure compared to that seen after a single acute PCP injection into previously PCP-naive rats (Fig. 3), indicating that partial tolerance may develop with repeated exposure to PCP. While extracellular glutamate levels after a single PCP injection only differed significantly from those seen after repeated PCP administration at one time point (20–40 min after injection), inspection of the data reveals a strong tendency towards lower glutamate levels in the repeated PCP group compared to the single PCP injection group at the 0–20 min and 40–60 min time points also. Moreover, it is notable that glutamate levels in the repeated PCP group were elevated above baseline only at two time points (40–60 min and 60–80 min post-injection), whereas significant increases over baseline glutamate levels were found in the single PCP injection group at five time points, starting from the 0–20 min time point and continuing through the 80–100 min time point. Finally, there was a trend towards a smaller area under the curve for the repeated PCP groups compared to the single PCP injection group. Although the maximum glutamate efflux reached by the repeated PCP group (~140% of baseline) is not much lower than that seen in the single PCP injection group (~155% of baseline), the onset and peak of the PCP-induced glutamate increase appear considerably delayed in the repeated PCP group. Glutamate level increases above baseline begin in the first sample after injection in the single PCP injection group, and the maximum concentration is reached at the 40–60 min time point. In the repeated PCP group, increases in glutamate levels above baseline are not observed until 40–60 min after injection, and the peak elevation occurs during the 60–80 min time point (Fig. 3). Repeated exposure to PCP may therefore alter the time course of glutamate efflux in response to PCP; partial tolerance may develop specifically to mechanisms underlying the early phase of PCP-induced glutamate release, while later effects are relatively unaltered.
This finding suggests that the profound behavioral abnormalities observed after a single PCP injection may be attributable to excessive or rapid-onset PFC glutamate efflux. These extreme, sudden increases in PFC glutamate release may disrupt brain function so severely that potential cognitive deficits are obscured by nonspecific general behavioral suppression, and thus cannot be measured or quantified. In contrast, a less extreme and more slowly developing increase in extracellular glutamate after repeated PCP administration, compared with the increases seen after a single PCP administration, may lead to less profound disruptions in brain function that are behaviorally quantifiable because they allow the subjects to perform the task and reveal selective cognitive deficits. Nevertheless, because the statistical findings on differences in glutamate efflux seen after a single PCP injection vs. repeated PCP administration are not completely unequivocal, caution must be taken in making inferences about the functional significance of these changes.
Chronic clozapine attenuated the glutamate increase induced by a PCP challenge administered after repeated PCP exposure (Fig. 3). These findings are consistent with previous studies that reported clozapine-induced attenuation of glutamate increases induced by acute NMDA receptor antagonist administration (Abekawa et al., 2006, 2007; López-Gil et al., 2007; however, see Adams and Moghaddam, 2001), an effect that parallels amelioration of NMDA receptor antagonist-induced cognitive disruptions by clozapine (Abdul-Monim et al., 2006; Amitai et al., 2007; Didriksen et al., 2007; Grayson et al., 2007; Hashimoto et al., 2005; Idris et al., 2005). Altogether, these observations suggest that at least part of the clozapine-induced attenuation of NMDA receptor antagonist-induced cognitive deficits, and by extension the beneficial effects of clozapine on cognitive dysfunction in schizophrenia (Meltzer and McGurk, 1999), may be attributable to a reduction of abnormally elevated PFC glutamate levels.
Studies have suggested that the increased glutamate efflux observed in the PFC after NMDA receptor antagonist administration may be driven by disruption of inhibitory GABA circuits (Zhang et al., 2008). GABAergic interneurons in the hippocampus and limbic areas were found to be approximately 10-fold more sensitive to inhibition by NMDA receptor antagonists than adjacent glutamatergic pyramidal cells (Grunze et al., 1996; Li et al., 2002). NMDA receptor antagonist exposure, therefore, would be expected to preferentially block NMDA receptors located on inhibitory GABA interneurons and result in a net effect of disinhibited excitatory glutamatergic transmission in these brain areas (Farber et al., 1998). Electrophysiological studies confirm this prediction for several brain areas (Grunze et al., 1996; Li et al., 2002), including the PFC (Homayoun and Moghaddam, 2007; Zhang et al., 2008). Furthermore, local administration of PCP or another NMDA receptor antagonist, dizocilpine, into the PFC has been shown to decrease extracellular GABA levels in this brain region (Yonezawa et al., 1998).
The preferential blockade by NMDA receptor antagonists appears to particularly affect parvalbumin-containing GABA interneurons, possibly because of a higher ratio of NR2A to NR2B subunits in the NMDA receptors expressed on these neurons compared with glutamatergic pyramidal cells (Kinney et al., 2006). Parvalbumin-positive GABA neurons form a subpopulation of fast-spiking inhibitory interneurons (Kawaguchi and Kondo, 2002) that play a key role in the generation of synchronized gamma oscillations, which in turn are thought to be crucially important for information processing in the brain (Bartos et al., 2007; Cardin et al., 2009; Sohal et al., 2009). Disruption of parvalbumin-positive GABA interneuron function can therefore be expected to have profound effects on cognitive processes.
Importantly, evidence of disturbed GABA function in schizophrenia patients has been found in numerous studies (for review, see Benes and Berretta, 2001; Lewis et al., 2004). Examination of postmortem PFC tissue from schizophrenia patients revealed decreases in the number of cells expressing GAD67 mRNA (Akbarian et al., 1995; Guidotti et al., 2000; Hashimoto et al., 2003; Volk et al., 2000) and GAD67 protein levels (Guidotti et al., 2000). Indeed, reduced GAD67 mRNA expression in the PFC is one of the most consistent molecular pathologies found in individuals with schizophrenia (Knable et al., 2002). PFC tissue from schizophrenia patients also showed reductions in parvalbumin mRNA (Hashimoto et al., 2003) and protein (Beasley et al., 2002; Beasley and Reynolds, 1997), as well as the number of cells expressing GABA transporter-1 (GAT-1) mRNA (Volk et al., 2001). The number of axon cartridges formed by chandelier cells, a type of parvalbumin-positive GABA interneuron expressing GAT-1, was also decreased in the PFC of schizophrenia patients (Pierri et al., 1999; Woo et al., 1998).
In our study, we found that repeated PCP treatment reduced the levels of parvalbumin and GAD67 in the PFC of rats (Fig. 4, 5). This finding was consistent with previous observations of lasting downregulation of GABA function after recurring exposure to NMDA receptor antagonists. Sustained treatment with another NMDA receptor antagonist, ketamine, decreased levels of parvalbumin and GAD67 in cultured GABA interneurons in vitro (Behrens et al., 2007; Kinney et al., 2006), and in the PFC of mice (Behrens et al., 2007). Reduced levels of parvalbumin mRNA or protein were also found in the PFC of rats (Cochran et al., 2003) and monkeys (Morrow et al., 2007) after repeated administration of PCP. GAD67 mRNA was also significantly decreased in the frontal cortex of rats treated with chronic dizocilpine (Paulson et al., 2003). Suppression of GABA function may play a critical role in abnormal glutamate neurotransmission and cognitive disruption observed after NMDA receptor antagonist administration, and thus may also drive cognitive pathology in schizophrenia.
The reduction in the two markers of GABA function in our study was detectable after just two PCP injections, and did not dissipate after 10 days of drug-free washout. Additional PCP injections did not further aggravate the reduction in either of these two markers (Fig. 4, 5). These findings suggest that the initial PCP injections in our regimen result in long-term neural adaptations in the PFC, including, although not necessarily limited to, the GABA system. These adaptations may drive changes in the effects of PCP exposure after repeated administration on both behavior and PFC glutamate release compared with the effects of PCP in naive animals. The exact mechanism by which reduced GABA function in the PFC alters the effects of PCP is unclear. However, persistently reduced GABA function (and thus persistent disinhibition of PFC glutamatergic neurons) may result in compensatory changes that bring PFC glutamatergic cells under inhibitory control by other mechanisms, rendering them less sensitive to disinhibition in response to blockade of GABA interneuron function by NMDA receptor antagonists. Such adaptations would result in decreased glutamate efflux in response to NMDA receptor antagonist exposure compared with naive animals (consistent with our findings in Experiment 1; Fig. 3), and possibly in the less severe, more selective behavioral disruption seen after repeated NMDA receptor antagonist administration compared with the profound, nonspecific behavioral suppression seen in naive animals given NMDA receptor antagonists (Amitai et al., 2007; Jentsch and Roth, 1999; Melnick et al., 2002; Podhorna and Didriksen, 2005).
Chronic clozapine treatment prevented the PCP-induced reductions in parvalbumin levels in the present study (Fig. 6, 7). Clozapine itself slightly reduced GAD67 levels; however, it partially, but significantly, attenuated the profound reduction in GAD67 levels induced by PCP exposure. Given that clozapine treatment did not begin until after administration of the initial two PCP injections, at a time point when reductions in parvalbumin and GAD67 would have already developed, clozapine can apparently reverse already established alterations in GABA function induced by NMDA receptor antagonists. The attenuation by clozapine of GABA dysregulation induced by repeated PCP may underlie the ameliorative effects of clozapine on PCP-induced cognitive deficits.
If, as hypothesized above, the adaptations in the GABA system drive blunted glutamate release and milder, more selective behavioral disruption in response to NMDA receptor antagonist administration, then one might expect that the effect of clozapine of attenuating these GABAergic changes would result in clozapine-treated rats given repeated PCP injections responding more like naive rats that receive PCP for the first time (i.e., with large increases in extracellular PFC glutamate and severe, nonspecific behavioral suppression). Such a pattern, however, was not observed. Instead, chronic clozapine treatment further reduced glutamate efflux (Fig. 3) and attenuated cognitive disruption (Amitai et al., 2007, 2009) in response to repeated PCP. It must be remembered, though, that in these animals, clozapine was still on board during the repeated PCP injections. In this way, the rats differed from naive rats that received acute PCP, in which the PCP injections resulted in extreme PFC glutamate efflux and profound behavioral suppression. This sustained clozapine treatment likely exerted a protective effect against PCP-induced disruptions. Moreover, given the somewhat mixed statistical evidence for reductions in glutamate release after repeated PCP administration, caution must be taken with any interpretations of the potential role of GABAergic neuroadaptations in this possible blunting of glutamate release, and the interaction of clozapine treatment with either.
However, for some aspects of PCP-induced cognitive disruption, rats that receive chronic clozapine in addition to repeated PCP administration do indeed appear to behave more like previously naive rats that receive PCP for the first time, as may be expected if clozapine prevented neuroadaptations occurring with repeated PCP administration. Specifically, the disinhibited impulsive responding seen in response to PCP administration (i.e., increased premature responses in the 5-choice serial reaction time task and extra and timeout responses in the intracranial self-stimulation procedure) does not develop until after repeated PCP administration. Sensitization processes that drive this potentiated impulsive behavior after repeated PCP administration may be at least partially mediated by the long-lasting alterations in GAB A function seen after repeated PCP treatment. The strong attenuation of this impulsive behavior by chronic clozapine may be attributable to clozapine preventing these sensitization processes, possibly via clozapine-induced attenuation of the GABAergic changes induced by PCP administration.
Overall, our findings suggest that chronic clozapine prevents some PCP-induced neural alterations that drive downregulation of PFC GABA function, increased PFC glutamate efflux, and behavioral disruptions. The exact mechanisms by which these various PCP-induced effects, and their attenuation by clozapine, are connected require further experimental investigation.
In summary, we found that acute PCP administration strongly increases extracellular glutamate levels in the PFC. In vivo microdialysis revealed that this PCP-induced glutamate efflux is maintained after repeated PCP pretreatment, but appears to be blunted in comparison to the effects of a single PCP injection. Moreover, we demonstrated that PCP induces long-lasting reductions in GABA function in the PFC after as little as two injections. Chronic clozapine treatment significantly attenuated both the increased glutamate efflux and the reduction in GABA function observed in the PFC after PCP exposure. Our findings suggest that excessive glutamate transmission and/or reduced GABA function in the PFC may underlie the cognitive-disruptive effects of PCP, and possibly some of the cognitive deficits that characterize schizophrenia. Conversely, prevention of excessive glutamate efflux in the PFC and/or protection of adequate GABA function may contribute to the attenuation by clozapine of schizophrenia-like cognitive deficits induced by PCP, and to the beneficial effects of clozapine on cognitive function in schizophrenia patients.
Acknowledgments
The authors would like to thank Ms. Jessica Benedict for outstanding technical assistance, Mr. Michael Arends for excellent editorial assistance, and Dr. Daniel Hoyer from Novartis Pharma AG for providing us with clozapine.
Role of the funding source
Supported by National Institute of Mental Health grant R01MH062527 to AM, National Institute on Drug Abuse grant R01DA001568 to RK, and Tobacco-Related Disease Research Program Individual Pre-doctoral Fellowship 15DT-0048 from the State of California to NA. The funding sources had no input on the research design, data analyses or interpretation, or writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav. Brain. Res. 2006;169:263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Abekawa T, Honda M, Ito K, Koyama T. Effects of NRA0045, a novel potent antagonist at dopamine D4, 5-HT2A, and a1 adrenaline receptors, and NRA0160, a selective D4 receptor antagonist, on phencyclidine-induced behavior and glutamate release in rats. Psychopharmacology (Berl) 2003;169:247–256. doi: 10.1007/s00213-003-1517-8. [DOI] [PubMed] [Google Scholar]
- Abekawa T, Ito K, Koyama T. Role of the simultaneous enhancement of NMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch. Pharmacol. 2006;374:177–193. doi: 10.1007/s00210-006-0115-9. [DOI] [PubMed] [Google Scholar]
- Abekawa T, Ito K, Koyama T. Different effects of a single and repeated administration of clozapine on phencyclidine-induced hyperlocomotion and glutamate releases in the rat medial prefrontal cortex at short- and long-term withdrawal from this antipsychotic. Naunyn Schmiedebergs Arch. Pharmacol. 2007;375:261–271. doi: 10.1007/s00210-007-0154-x. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol. Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol. Psychiatry. 1998;43:811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Increased impulsivity and disrupted attention induced by repeated phencyclidine are not attenuated by chronic quetiapine treatment. Pharmacol. Biochem. Behav. 2009b;93:248–257. doi: 10.1016/j.pbb.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology (Berl) 2009a;202:275–286. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by repeated administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol. Psychiatry. 2010a;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Effects of metabotropic glutamate receptor 2/3 agonism and antagonism on schizophrenia-like cognitive deficits induced by phencyclidine in rats. Eur. J. Pharmacol. 2010b;639:67–80. doi: 10.1016/j.ejphar.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Clozapine attenuates disruptions in response inhibition and task efficiency induced by repeated phencyclidine administration in the intracranial self-stimulation procedure. Eur. J. Pharmacol. 2009;602:78–84. doi: 10.1016/j.ejphar.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr. Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol. Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, D'Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, Abi-Dargham A, Belger A, Abi-Saab W, Lipschitz D, Bennet A, Seibyl JP, Krystal JH. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005;179:136–143. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- Didriksen M, Skarsfeldt T, Arnt J. Reversal of PCP-induced learning and memory deficits in the Morris’ water maze by sertindole and other antipsychotics. Psychopharmacology (Berl) 2007;193:225–233. doi: 10.1007/s00213-007-0774-3. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Farber NB, Newcomer JW, Olney JW. The glutamate synapse in neuropsychiatric disorders: focus on schizophrenia and Alzheimer’s disease. Prog. Brain. Res. 1998;116:421–437. doi: 10.1016/s0079-6123(08)60453-7. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J. Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP- induced cognitive deficit in the novel object recognition task in the rat. Behav. Brain Res. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Grunze HCR, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J. Neurosci. 1996;76:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur. J. Pharmacol. 2005;519:114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007;24:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Dazzi L, Chhatwal JP, Verrico CD, Roth RH. Reduced prefrontal cortical dopamine, but not acetylcholine, release in vivo after repeated, intermittent phencyclidine administration to rats. Neurosci. Lett. 1998;258:175–178. doi: 10.1016/s0304-3940(98)00879-9. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J. Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J. Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Bartko JJ, Webster MJ, Torrey EF. Molecular abnormalities in the major psychiatric illnesses: Classification and Regression Tree (CRT) analysis of post-mortem prefrontal markers. Mol. Psychiatry. 2002;7:392–404. doi: 10.1038/sj.mp.4001034. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J. Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity. J. Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Mathé JM, Nomikos GG, Schilström B, Svensson TH. Non-NMDA excitatory amino acid receptors in the ventral tegmental area mediate systemic dizocilpine (MK-801) induced hyperlocomotion and dopamine release in the nucleus accumbens. J. Neurosci. Res. 1998;51:583–592. doi: 10.1002/(SICI)1097-4547(19980301)51:5<583::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Melnick SM, Rodriguez JS, Bernardi RE, Ettenberg A. A simple procedure for assessing ataxia in rats: effects of phencyclidine. Pharmacol. Biochem. Behav. 2002;72:125–130. doi: 10.1016/s0091-3057(01)00727-4. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Holzman PS, Hassan SZ, Guschwan A. Effects of phencyclidine and stress on plasma creatine phosphokinase (CPK) and aldolase activities in man. Psychopharmacologia. 1972;26:44–53. doi: 10.1007/BF00421917. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr. Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology (Berl) 2007;192:283–290. doi: 10.1007/s00213-007-0708-0. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Paulson L, Martin P, Persson A, Nilsson CL, Ljung E, Westman-Brinkmalm A, Eriksson PS, Blennow K, Davidsson P. Comparative genome- and proteome analysis of cerebral cortex from MK-801-treated rats. J. Neurosci. Res. 2003;71:526–533. doi: 10.1002/jnr.10509. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. second ed. New York: Academic Press; 1986. [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am. J. Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. Performance of male C57BL/6J mice and Wistar rats in the water maze following various schedules of phencyclidine treatment. Behav. Pharmacol. 2005;16:25–34. doi: 10.1097/00008877-200502000-00003. [DOI] [PubMed] [Google Scholar]
- Pradhan SN. Phencyclidine (PCP): some human studies. Neurosci. Biobehav. Rev. 1984;8:493–501. doi: 10.1016/0149-7634(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D. Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (Amytal) sodium: I. Attention, motor function, and proprioception. Arch. Gen. Psychiatry. 1959;1:651–656. doi: 10.1001/archpsyc.1959.03590060113013. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 2004;89:204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit. Rev. Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am. J. Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical γ- aminobutyric acid neurons in subjects with schizophrenia. Arch. Gen. Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM. Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J. Neurochem. 2008;106:943–956. doi: 10.1111/j.1471-4159.2008.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal γ- aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of γ-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur. J. Pharmacol. 1998;341:45–56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J. Neurophysiol. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]