Abstract
Rationale
The remote measurement of body temperature with radiotelemetry provides a minimally invasive and robust method for larger experimental animals such as Old World monkeys. Existing literature encompasses data using intraperitoneal (IP) and subcutaneous (SC) implantation locations which may affect inferences about body temperature.
Methods
The body temperature of four adult male rhesus monkeys was monitored with radiotelemetry devices implanted both IP and SC in each subject. Animals were recorded at 5 minute intervals for five months with the two transmitters being used in sequence on a weekly basis. Additional challenge with d-methamphetamine (0.32 mg/kg ; i.m.) was conducted to compare the magnitude of the hyperthermic response measured IP and SC.
Results
Normal daily temperatures differed by about 0.5–0.8°C across implant locations with IP temperature consistently higher. The difference was consistent across the circadian cycle and when compared 1, 3 or 5 months after surgical implantation. The magnitude of the hyperthermia response to methamphetamine was about 0.75°C when measured with either IP or SC implants.
Conclusions
The study shows that data derived from the two major implantation locations used in existing literature are likely to be comparable.
Keywords: Macaca mulatta, circadian, temperature, hyperthermia, hypothermia
1. Introduction
Experimental models of thermoregulatory responses to acute challenge with recreational or therapeutic drugs, to experimental infection with fever-inducing pathogens and other biological states use a diversity of methods to report “body temperature”. The measured body temperature for any species differs depending upon the location of the measurement device, whether rectal, tympanic, dermal, intraperitoneal or subcutaneous. The relationship between temperatures measured at two respective locations may vary tremendously between experimental species depending on body size, methods of thermoregulation, typical housing conditions, ambient temperature range and other factors. Although temperature is likely to be correlated throughout the body, systematic differences that affect the interpretation of experimental outcome may arise. Unfortunately, it is most common that a given investigation selects a single method and does not compare their experimental results across body locations.
Recent studies have shown that radiotelemetric measurement of body temperature in unrestrained nonhuman primates is a robust experimental preparation capable of reporting data with sampling resolutions of 5–10 minutes for many months duration (Almirall et al. 2001; Gauvin et al. 2006; Huitron-Resendiz et al. 2007; Taffe et al. 2006; Takasu et al. 2002; Weed and Hienz 2006). These methods offer significant advantages over the traditional preparation of chair-restraint combined with rectal thermister (Bowyer et al. 2003; Johnson and Elizondo 1979). Telemetry systems require the initial surgical implantation of a transmitter but thereafter the animal can be monitored in the freely moving state within normal laboratory housing conditions. Telemetric systems therefore permit evaluation of the animal unstressed by chairing and with continuous computer recording at any selected sampling interval, including continuously. The former may be important since monkeys that appear behaviorally adapted to chairing may exhibit physiological stress responses (Ruys et al. 2004) and the data sampling only begins once the animal is restrained. A high pre-treatment baseline due to stress may, for example, obscure a thermal response to 3,4-methylenedioxymethamphetamine (MDMA) in a chaired/rectal thermister model (Banks et al. 2007; Bowyer et al. 2003) that can readily be observed with telemetry (Crean et al. 2007; Taffe et al. 2006). Alternately it may be the case that subcutaneous temperature differs from rectal independent of whether animals are restrained or unrestrained (although comparison of the high and low ambient temperature challenge data from Banks and colleagues (2007) with those of Von Huben and colleagues (2007) shows similarity of outcome).
Prior telemetry studies in macaque monkeys have used different locations to implant the transmitter devices, most usually intraperitoneal (Gauvin et al. 2006; Takasu et al. 2002) or subcutaneous (Almirall et al. 2001; Boles et al. 2003; Von Huben et al. 2007). Some studies have reported a mixture without specifying differences of outcome (Weed and Hienz 2006), or used a location that is subcutaneous but “under a non-active muscle layer” (Barger et al. 2008). In this study we sought to directly compare the most frequently used locations, intraperitoneal and subcutaneous, using a within subjects design. For this we prepared rhesus monkeys with radiotelemetric implants in both intraperitoneal and subcutaneous locations to compare temperature measurements between the two locations under normal daily living conditions. An additional probe test with methamphetamine was included to determine how IP and SC temperature might vary under acute drug challenge conditions.
2. Materials and Methods
2.1 Animals
Four individually housed male rhesus monkeys (Macaca mulatta) were used in these experiments. Animals were 7–7.5 years of age, weighed 9.4–13.9 kg at the start of the study and exhibited body condition scores (Clingerman and Summers 2005) of 2.0–3.0 out of 5 at the nearest quarterly exam prior to starting the study. Daily chow (230–240 g; LabDiet® 5038, PMI Nutrition International, Richmond, IN, USA; 3.22 kcal of metabolizable energy (ME) per gram) allocations were determined by a power function (Taffe 2004a; b) fit to data provided in a National Research Council recommendation (NRC/NAS 2003) and modified individually by the veterinary weight management plan. The animals' normal diet was supplemented with fruit or vegetables seven days per week and water was available ad libitum in the home cage at all times. The ambient room temperature was 25°C (+/− 0.5 °C) for these studies. Animals on this study had previously been immobilized with ketamine (5–20 mg/kg) no less than semiannually for purposes of routine care and some experimental procedures. The United States National Institutes of Health guidelines for laboratory animal care (Clark et al. 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute (La Jolla).
2.2 Apparatus
Radio telemetric transmitters (TA10TA-D70; Transoma / Data Sciences International, St. Paul, MN, USA) were implanted both subcutaneously and intraperitoneally (suture tabs are added to the devices by the manufacturer for IP implantation) in each animal. The subcutaneous placement was in the flank location, caudal to the scapula, as with our prior studies. The surgical protocol was adapted from the manufacturer's surgical manual and implantation was conducted by, or under supervision of, the TSRI veterinary staff using sterile techniques under isoflurane anesthesia. Temperature and gross locomotor activity recordings were obtained from the transmitters implanted in the monkeys via in-cage receivers (RMC-1; Transoma / Data Sciences International, St. Paul, MN, USA). Data were recorded on a 5 minute sample interval basis by the controlling computer and represented as a moving average of three samples (−5 min, current, + 5 min) for each 10 minutes. Ambient room temperature was also recorded by the system via a probe mounted near the top of the housing room.
The temperature of the animal was recorded from either the SC or IP transmitter at a given time; simultaneous recording is not possible with these devices and the DataSciences system. To compare the two measurements under normal living conditions, the animals were recorded for about a week (data comprise 5–7 days per animal) with the transmitter in each location turned on/off in sequence with a magnet passed over the implant site. Two subjects were being recorded from via the IP transmitter and two from the SC transmitter at any given time. These studies were repeated at 1, 3 and 5 months after the original surgical implantation to determine any changes associated with healing, vascular recovery, fur regrowth and/or possible encapsulation of the device.
2.3 Drug challenge studies
A dose of (+)methamphetamine HCl (0.32 mg/kg) was administered intramuscularly (i.m.) in a volume of 0.1 ml/kg saline; the dose was selected based on our previous observations (Crean et al. 2006). Drug compound was provided by the National Institute on Drug Abuse (Bethesda, MD, USA). All challenges were administered in the middle of the light cycle (at 1030 hours) and active doses were separated by no less than 1 week. For dosing conditions were included to encompass factors of drug treatment (vehicle versus MA) and transmitter location (IP vs SC) within animal.
2.4 Data Analysis
Randomized block (repeated measures) analysis of variance (ANOVA) was employed to evaluate effects on temperature. For analysis of normal daily temperature changes, individual data were averaged across a week (5–7 days) by six 4-hr bins within the day, starting at 0000 hours. Weeks of data collected from the IP and SC transmitter were analysed in the 1st, 3rd and 5th months after surgical implantation. Thus the ANOVA included factors of implant location (IP vs SC), time of day (six 4-hr bins) and months post-surgery (M1, M3, M5). For the drug study, data were first represented as a change in body temperature from the three 10-min samples collected prior to drug administration and then analyzed for timepoints from −10 min (the baseline) to 240 min post-injection using a three-way analysis using repeated measures factors of implant location (SC, IP), drug treatment condition (0.32 mg/kg MA, vehicle) and time post-injection (−10–240 minutes). A similar three-way analysis was conducted on mean hourly temperature averages in which the time post-injection factor was −1 hr to 18 hrs. Post-hoc analyses of all significant effects were conducted with the Fisher's LSD procedure. All statistical analyses were conducted using GB-STAT v7.0 for Windows (Dynamic Microsystems, Inc., Silver Spring MD) and the criterion for significance in all tests was p < 0.05.
3. Results
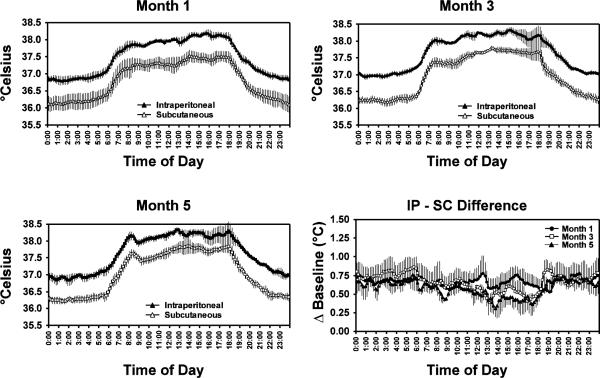
The data show that under normal living conditions the IP body temperature of rhesus macaques was higher than the SC body temperature by about 0.5–0.8°C (Figure 1). This pattern was observed across the entire 24-hr day and across five months of data collection when considered qualitatively at the level of a 10-min sampling analysis.
Figure 1.
Mean (N=4; bars indicate SEM) body temperature derived from radiotransmitters implanted intraperitoneally (IP) and subcutaneously (SC) in the same animals are presented by 10 minute bins throughout the day. Individual data were averaged across 5–7 days in each of three months following device implantation. The mean IP/SC difference is also presented for each of the three months.
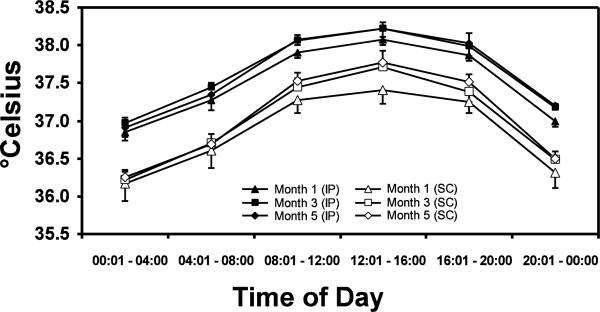
Statistical analysis of the temperature data averaged into 4-hour bins across the day (Figure 2) confirmed a significant main effect of transmitter location (F1,3 = 53.31; p<0.01) and time (bin) within the day (F5,15 = 103.64; p<0.0001). There were no significant differences in body temperature attributable to the month of sampling, nor any interaction of the three main factors. The posthoc test confirmed that IP/SC differences were significant for all 4-hr time bins in each of the three months with IP temperature consistently higher. The posthoc test also confirmed the reliability of circadian effects on body temperature since the temperature at each 4-hr time bin was significantly different from the other 5 throughout the day for almost all comparisons; i.e., six, including the three months and each transmitter location. The only exceptions were that the 0800–1200 and 1600–2000 average temperatures did not differ from each other for SC (M1, M3, M5) or IP (M1, M5) locations.
Figure 2.
Mean (N=4; bars indicate SEM) IP and SC body temperatures are presented as a 4 hour average throughout the day. Statistical analysis of these data confirmed reliable differences attributable to time of day and telemetry implant location but not of the month of sampling. See text for additional statistical outcome.
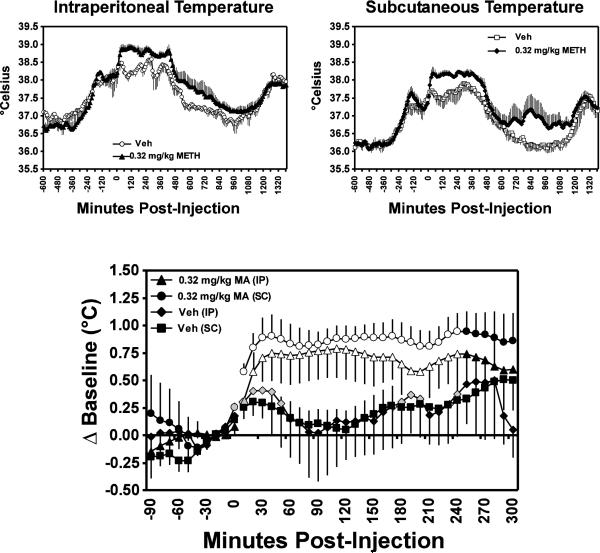
Methamphetamine challenge (Figure 3) increased body temperature, as was confirmed by a significant main effect drug condition (F1,3 = 11.67; p<0.05), time post-administration and (F25,75 = 3.16; p<0.0001) and the interaction of these two factors (F25,75 = 7.47; p<0.0001). There was no main effect of transmitter location, nor any additional interactions, confirmed in the analysis. The posthoc followup test confirmed that the change in IP temperature was significantly higher than the pre-injection baseline after either vehicle (10–50, 170–200, 230–240 min) or MA (10–240 min) injection. The change was greater after MA, since the change in IP temperature was significantly greater after MA when compared with the vehicle condition from 20–240 minutes after injection. The change in SC temp was also significantly greater than baseline in the four hours following vehicle (10–50, 160–240 min) or MA (0–240 min) challenge. As with IP temperature, the change in SC temperature was significantly greater after MA, when compared with the vehicle condition from 10–240 minutes after injection.
Figure 3.
Mean (N=4; bars indicate SEM) IP and SC body temperatures are presented for days in which animals were challenged with vehicle or 0.32 mg/kg methamphetamine (i.m.). The upper panels show raw temperatures at 10 min intervals across the entire day of challenge. The lower panel depicts 10 min samples in the hours immediately surrounding the injection, with temperature responses represented as a change from the average of the three pre-injection samples. Open symbols represent points which are significantly different from both the baseline timepoint and the respective vehicle timepoint relative to injection. Shaded symbols represent a significant difference from the pre-injection baseline (only) and the * indicates points in the drug conditions which differ significantly from the respective vehicle timepoint (only).
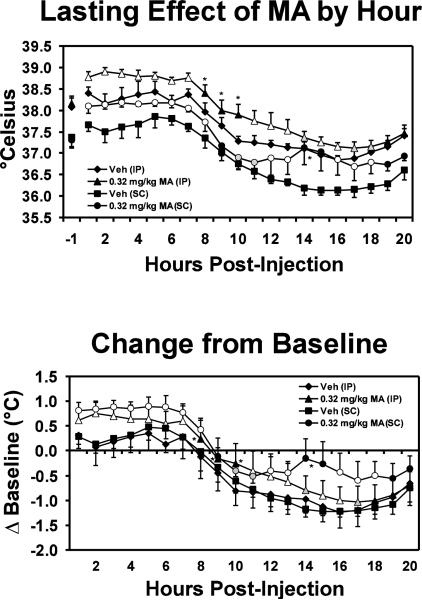
The hyperthermic effects of MA were long-lasting, as is depicted in the hourly temperature averages. The statistical analysis confirmed significant main effects of transmitter location (F1,3 = 1493.44; p<0.0001), hours post-administration (F18,54 = 55.41; p<0.0001) and the interaction of all three factors (F18,54 = 2.62; p<0.005) but not of drug condition (F1,3 = 8.81; p=0.059). The post-hoc test further confirmed that MA significantly increased either SC or IP body temperature in the 6–7 hours after dosing (i.e. until the decrease in temperature triggered by the lights going out at 1800 hrs) and then in the overnight hours.
4. Discussion
Minimally invasive radiotelemetry devices are available for sampling multiple physiological variables in most commonly used mammalian laboratory species. In macaque monkeys such devices have been used to monitor body temperature and locomotor responses to experimental infection with viral or bacterial pathogens (Boles et al. 2003; Huitron-Resendiz et al. 2007), the menstrual cycle (Barger et al. 2008), circadian manipulation of the light cycle and to acute (Crean et al. 2007; Taffe et al. 2006; Weed and Hienz 2006) or neurotoxic (Almirall et al. 2001) drug challenge. These prior studies have used a mixture of body locations to implant the recording device and it is unknown to what degree the body location affects the recorded “body temperature”.
The present study provides direct evidence, within the same subjects, that body temperature of rhesus macaques assessed with subcutaneous (SC) and intraperitoneal (IP) implants is closely correlated. In these data the subcutaneous measurements were consistently lower than intraperitoneal measurements by about 0.5–0.8 degrees Celsius, as would be expected. This difference was very consistent as it was maintained across the circadian cycle, which encompassed a day to night range of approximately 1.0–1.25 degrees Celsius.
The data also confirmed that temperature recordings do not change in the first five months after surgical implantation of the devices. This is important methodologically, particularly with the SC implant since hair regrowth (after shaving to minimize surgical contamination) continues in some individuals for many months after surgery. Similarly, it might be expected that encapsulation and revascularization of the implant may change slightly with time. These data show that such theoretical concerns do not greatly affect observed temperature. One minor caveat is that although there was no significant main effect of the month of measurement confirmed by the statistical analysis, temperature was quantitatively lower in the first month compared with the third and fifth months. Therefore there may be an effect which the present study lacked the power to detect. It is interesting that this difference was seen in both the SC and IP measurements (Figure 2), which probably rules out hair regrowth as a likely factor.
As we have previously shown (Crean et al. 2007; Crean et al. 2006), acute challenge with 0.32 mg/kg methamphetamine significantly increases subcutaneous body temperature with effects that last hours after intramuscular injection. The present data replicate those findings and further confirm that intraperitoneal temperature increases by the same amount and with the same duration of effect as does subcutaneous temperature. This provides initial evidence that vasoconstrictor properties of methamphetamine (Frewin et al. 1969; Polesskaya et al. 2011) do not interact with the measurement of temperature at SC and IP locations.
The present data do not specifically address whether a given implantation location would be preferred for a given experimental application. Indeed, the reported literature seems to confirm that either location is well tolerated in macaque monkeys with minimal post-surgical or long-term complications. Nevertheless these data help to guide decision making with respect to the experimental protocol. One clear advantage for the subcutaneous location is that the IP surgery is considered a major survival surgery under the NIH Guide (Clark et al. 1996) because it “penetrates a major body cavity”. From a protocol perspective therefore, the SC implantation better addresses refinement goals in minimizing distress in the use of laboratory animals. This consideration is particularly acute when contemplating exceptionally long term studies in a multi-decade laboratory animal, such as the several macaque species used in the relevant recent literature (Almirall et al. 2001; Taffe et al. 2006; Takasu et al. 2002; Weed and Hienz 2006). Radiotelemetry devices have a finite battery life; for example the ones used in this study have a warranteed life of 11 months continual use (“nominal” of 16 months, which we have found to be a good-to-conservative estimate). It is possible to implant a second transmitter upon expiration of the first transmitter, thereby continuing studies for up to 32 months in the same animals (the duration of study can be further extended by intermittently turning off the transmitter if the experimental goals permit). Multiple minor surgical procedures are similarly preferable to multiple major survival surgical procedures assuming the experimental goals permit the SC placement.
In conclusion, the present study provides specific evidence on which to base inferences between datasets or studies which employ different locations for radiotransmitter implants. The close correlation across the circadian cycle and the consistency of these differences across five months of experimental recording time provide confidence when assessing longitudinal data such as those associated with neurotoxic insults or experimental infection with pathogens. Furthermore, our acute challenge data with methamphetamine provide additional confidence that data may be compared for acute drug studies as well. These data together provide additional evidence for the utility and robustness of the minimally invasive radiotelemetry procedure for measuring physiological variables in awake, unrestrained nonhuman primates.
Figure 4.
Mean hourly (N=4; bars indicate SEM) IP and SC body temperatures are presented for days in which animals were challenged with vehicle or 0.32 mg/kg methamphetamine (i.m.). The upper panel depicts the temperature averages for up to 20 hours after dosing. The lower panel illustrates the data represented as the average of individual changes from the pre-injection baseline to highlight the magnitude of the drug effect observed from IP and SC locations. Statistical conventions are as in Figure 3 but are provided for the methamphetamine conditions only for clarity.
Acknowledgements
The author is grateful to Sophia A Vandewater for expert technical assistance for USPHS grants DA018418 and DA024194 for support. The NIH/NIDA had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, nor in the decision to submit the paper for publication. This is publication #21167 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no conflicts of interest to report for this study.
Literature Cited
- Almirall H, Bautista V, Sanchez-Bahillo A, Trinidad-Herrero M. Ultradian and circadian body temperature and activity rhythms in chronic MPTP treated monkeys. Neurophysiol Clin. 2001;31:161–70. doi: 10.1016/s0987-7053(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35:1840–5. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Barger LK, Hoban-Higgins TM, Fuller CA. Assessment of circadian rhythms throughout the menstrual cycle of female rhesus monkeys. Am J Primatol. 2008;70:19–25. doi: 10.1002/ajp.20451. [DOI] [PubMed] [Google Scholar]
- Boles JW, Pitt ML, LeClaire RD, Gibbs PH, Ulrich RG, Bavari S. Correlation of body temperature with protection against staphylococcal enterotoxin B exposure and use in determining vaccine dose-schedule. Vaccine. 2003;21:2791–6. doi: 10.1016/s0264-410x(03)00222-6. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Young JF, Slikker W, Itzak Y, Mayorga AJ, Newport GD, Ali SF, Frederick DL, Paule MG. Plasma levels of parent compound and metabolites after doses of either d-fenfluramine or d-3,4-methylenedioxymethamphetamine (MDMA) that produce long-term serotonergic alterations. Neurotoxicology. 2003;24:379–90. doi: 10.1016/S0161-813X(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources, National Research Council; Washington D.C.: 1996. p. 125. [Google Scholar]
- Clingerman KJ, Summers L. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 2005;34:31–6. doi: 10.1038/laban0505-31. [DOI] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/−)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−)3,4-methylenedioxymethamphetamine, (+/−)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–25. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewin DB, Jellett LB, Whelan RF. Modification of the vasoconstrictor action of sympathomimetic agents by bretylium tosylate and tranylcypromine in man. Br J Pharmacol. 1969;36:602–10. doi: 10.1111/j.1476-5381.1969.tb08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Tilley LP, Smith FW, Jr., Baird TJ. Electrocardiogram, hemodynamics, and core body temperatures of the normal freely moving cynomolgus monkey by remote radiotelemetry. J Pharmacol Toxicol Methods. 2006;53:140–51. doi: 10.1016/j.vascn.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Marcondes MC, Flynn CT, Lanigan CM, Fox HS. Effects of simian immunodeficiency virus on the circadian rhythms of body temperature and gross locomotor activity. Proc Natl Acad Sci U S A. 2007;104:15138–43. doi: 10.1073/pnas.0707171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GS, Elizondo RS. Thermoregulation in Macaca mulatta: a thermal balance study. J Appl Physiol. 1979;46:268–77. doi: 10.1152/jappl.1979.46.2.268. [DOI] [PubMed] [Google Scholar]
- NRC. NAS . Nutrient Requirements of Nonhuman Primates. Second Revised Edition National Research Council of The National Academy of Sciences; Washington D.C.: 2003. [Google Scholar]
- Polesskaya O, Silva J, Sanfilippo C, Desrosiers T, Sun A, Shen J, Feng C, Polesskiy A, Deane R, Zlokovic B, Kasischke K, Dewhurst S. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011;1373:91–100. doi: 10.1016/j.brainres.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav. 2004;82:205–13. doi: 10.1016/j.physbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004a;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Erratum: “Effects of parametric feeding manipulations on behavioral performance in macaques.”. Physiol Behav. 2004b;82:589. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82:276–81. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu N, Nigi H, Tokura H. Effects of diurnal bright/dim light intensity on circadian core temperature and activity rhythms in the Japanese macaque. Jpn J Physiol. 2002;52:573–8. doi: 10.2170/jjphysiol.52.573. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/−)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–81. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Hienz RD. Effects of morphine on circadian rhythms of motor activity and body temperature in pig-tailed macaques. Pharmacol Biochem Behav. 2006 Jul 19; doi: 10.1016/j.pbb.2006.06.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]