Abstract
Appropriate levels of phosphate in the body are maintained by the coordinated regulation of the bone-derived growth factor FGF23 and the membrane-bound protein Klotho. The endocrine actions of FGF23, in association with parathyroid hormone and vitamin D, mobilize sodium–phosphate cotransporters that control renal phosphate transport in proximal tubular epithelial cells. The availability of an adequate amount of Klotho is essential for FGF23 to exert its phosphaturic effects in the kidney. In the presence of Klotho, FGF23 activates downstream signaling components that influence the homeostasis of phosphate, whereas in the absence of this membrane protein, it is unable to exert such regulatory effects, as demonstrated convincingly in animal models. Several factors, including phosphate and vitamin D, can regulate the production of both FGF23 and Klotho and influence their functions. In various acquired and genetic human diseases, dysregulation of FGF23 and Klotho is associated with vascular and skeletal anomalies owing to altered phosphate turnover. In this Review, I summarize how the endocrine effects of bone-derived FGF23, in coordination with Klotho, can regulate systemic phosphate homeostasis, and how an inadequate balance of these molecules can lead to complications that are caused by abnormal mineral ion metabolism.
Introduction
Phosphorus, a major mineral ion that is routinely consumed through food, is usually associated with oxygen in the form of phosphate. Phosphate is widely distributed in the body and is an important factor in bone formation, but is also involved in cell signaling, energy metabolism, nucleic acid synthesis, and the maintenance of acid–base balance (urinary buffering).1,2 The physiologic balance of phosphate is maintained by the coordinated interactions of the small intestine, bone, parathyroid gland and kidneys;3–8 functional impairments in any of these organs can lead to abnormal phosphate levels (Box 1). For example, in most chronic renal diseases,9–11 impaired renal function perturbs the homeostasis of phosphate, as well as that of physiologic water, electrolytes, and mineral ion balance.
Box 1 Potential causes of serum phosphate imbalance.
-
▪
Acidosis (respiratory or lactic acidosis, diabetic ketoacidosis)
-
▪
Alkalosis
-
▪
Cortical hyperostosis
-
▪
Drug treatment (for example amphotericin B or bisphosphonate)
-
▪
Glucocorticoid deficiency
-
▪
Impairment of growth hormone secretion (acromegaly)
-
▪
Impairment of thermoregulation (hyperthermia or hypothermia)
-
▪
Hemolysis
-
▪
Infections
-
▪
Intestinal impairment (bowel infarction)
-
▪
Magnesium deficiency
-
▪
Milk–alkali syndrome
-
▪
Impairment of parathyroid hormone secretion (hypoparathyroidism or pseudo-hypoparathyroidism)
-
▪
Phosphate-containing laxatives or enemas
-
▪
Renal impairment
-
▪
Rhabdomyolysis
-
▪
Sarcoidosis
-
▪
Trauma (for example, burns or crush injuries)
-
▪
Tumors (leukemia, lymphoma, bone tumors)
-
▪
Tumoral calcinosis
-
▪
Vitamin D intoxication
As high as 70% of dietary phosphate can be absorbed from the upper half of the intestine and then taken up by the cells that need it; the remaining amount is mostly excreted through urine. Of particular interest, in response to the intestinal phosphate administration, phosphaturia can occur without measurable changes in plasma concentrations of phosphate and independent of parathyroid hormone, as similar responses have also been detected in parathyroidectomized animals. These observations implicate the putative existence of an intestinal ‘phosphate sensor’, which might send a hormonal signal to stimulate urinary phosphate excretion immediately after an intestinal phosphate load.12
Transepithelial phosphate transport in the intestine (through enterocytes) and in the kidney (through proximal epithelial cells) is primarily mediated by proteins in the sodium/phosphate cotransporter family (NaPi-2a, NaPi-2b and NaPi-2c) that are expressed in the apical membrane of the epithelial cells. More than 80% of the filtrated phosphate in the kidneys is reabsorbed in the proximal tubules through NaPi-2a and NaPi-2c. Various endocrine factors, including parathyroid hormone, active vitamin D metabolites and FGF23, can directly or indirectly control NaPi activities to influence systemic phosphate balance (Figure 1). In addition to parathyroid hormone and vitamin D, numerous other hormones can affect renal phosphate handling. Growth hormone, insulin, and thyroid hormone can all increase phosphate reabsorption, whereas calcitonin, glucocorticoids, and atrial natriuretic factor can decrease it, primarily by in fluencing the activity of NaPi-2a.13,14
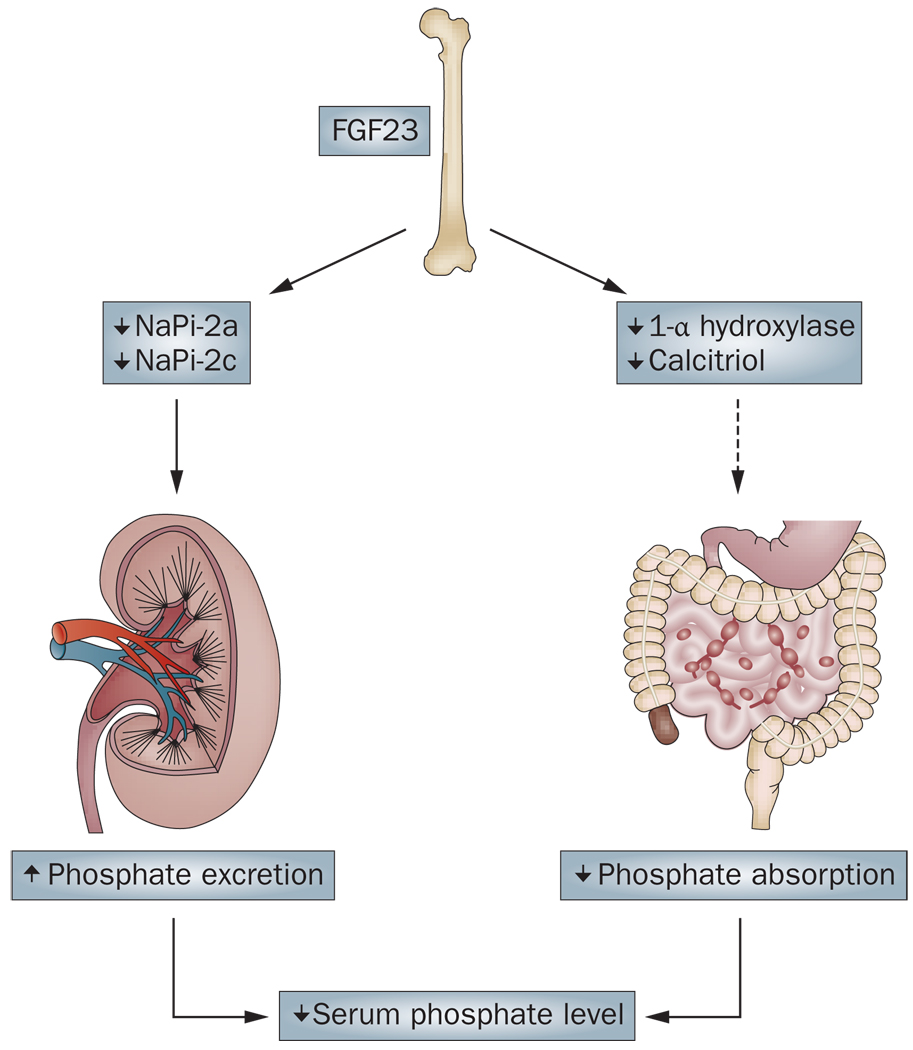
Figure 1.
Serum phosphate lowering effects of FGF23. FGF23 (produced in the bone) can suppress NaPi-2a and NaPi-2c cotransporters, which results in increased renal excretion of phosphate. Similarly, FGF23 can suppress renal expression of 1-α hydroxylase, which leads to reduced production of calcitriol and decreased intestinal phosphate absorption, and subsequent reduced serum levels of phosphate.
Among these factors, parathyroid hormone is one of the most potent regulators of phosphate metabolism. Parathyroid hormone can suppress the reabsorption of phosphate in the proximal tubules by reducing NaPi-2a and NaPi-2c activities. This reduction is achieved by internalization of NaPi proteins from the lumen side of the proximal tubular epithelial cells.15 Parathyroid hormone can also mobilize phosphate from the bone into the bloodstream, possibly by enhancing osteoclastic bone resorption.16 In addition, parathyroid hormone can increase the production of 1,25 dihydroxyvitamin D3 (calcitriol) by inducing the renal expression of 1-α hydroxylase, which affects intestinal phosphate absorption. Two complementary systems might have a role in adapting acute or chronic changes in dietary phosphate intake: chronic changes might involve calcitriol-dependent changes in NaPi transporter expression,17 whereas acute changes might be mediated through an immediate hormonal response that is yet to be determined.12 Intestinal phosphate uptake, therefore, is a major regulatory factor that can control the physiologic balance of serum phosphate level.
Cellular, intracellular, transcellular and pericellular mineral ion transports are complex processes that are achieved by both active and passive translocation. Phosphate transport across renal proximal tubular epithelial cells is mostly driven by a high extracellular sodium concentration, which is thought to be maintained by the membrane-associated Na+,K+-ATPase. Some experts have suggested that the transmembrane protein Klotho can influence Na+,K+-ATPase activity, which results in an increased Na+ ion gradient and enhances transepithelial calcium transport in the choroid plexus and the kidneys.18–20 Active regulation of phosphate homeostasis and its relation to calcium transport and balance are evolving areas of research. In the following sections, I discuss the role of the bone-derived growth factor FGF23 and the membrane protein Klotho in the regulation of systemic phosphate homeostasis, and the consequences of the inadequate balance of these molecules.
The role of FGF23
A major breakthrough in understanding the active regulation of phosphate homeostasis was accomplished by the identification of FGF23.21,22 FGF23 is a ~30 kD protein that is proteolytically processed to a ~18 kD N-terminal fragment and a ~12 kD C-terminal fragment. The receptor-binding domain of FGF23 is present in the N-terminus.
FGF23 is able to suppress the expression of NaPi-2a and NaPi-2c cotransporters either directly, as shown by in vitro studies23 or through affecting parathyroid hormone activity, which induces urinary phosphate excretion by reducing NaPi-2a and NaPi-2c co transporter activities.24 Transgenic mice that overexpress FGF23 have hypophosphatemia owing to the suppression of renal NaPi co-transporters, as well as reduced serum calcitriol levels and skeletal mineral deposition defects in the form of rickets or osteomalacia.27–30 FGF23 can also influence systemic vitamin D activity by suppressing the renal expression of 1α hydroxylase, which results in decreased production of calcitriol.24 In addition, FGF23 can reduce the activity of calcitriol by increasing the synthesis of the catabolic enzyme 24-hydroxylase,24 and the resultant reduced vitamin D activities might induce parathyroid hormone secretion and concomitant phosphaturia. Some investigators claimed that FGF23 can directly suppress parathyroid hormone secretion,25,26 which should inhibit phosphaturia, possibly by increasing NaPi-2a and NaPi-2c cotransporter activities; moreover, secondary hyperparathyroidism develops in patients with chronic kidney diseases (CKD), despite their extremely high serum levels of FGF23. Further studies are needed to determine the molecular interactions of FGF23 and parathyroid hormone that lead to biochemical changes in various clinical disorders, including CKD.
Diseases related to increased FGF23 level
A number of other human diseases are associated with increased FGF23 levels (Table 1). Vitamin-D-resistant rickets or osteomalacia in patients with X-linked hypophosphatemia (XLH) is caused by inactivating mutations in PHEX (which encodes a phosphate-regulating endopeptidase and is homologous with other endopeptidase-genes that are located on the X-chromosome) that, in turn, increase serum levels of FGF23.31 In another inherited human disease, autosomal dominant hypophosphatemic rickets (ADHR), gain-of-function mutations of FGF23 (which result in substitutions of Arg residues at 176 and/or 179 to Gln or other residues and prevent the proteolytic cleavage of FGF23) are associated with excessive urinary phosphate wasting, which causes rickets.22 Furthermore, in some patients with epidermal nevus syndrome, which can be related to activating mutations of FGFR3,32 increased serum levels of FGF23 are associated with renal phosphate wasting.33
Table 1.
Diseases owing to dysregulation of FGF23
| Human diseases | Cause |
|---|---|
| Increased FGF23 activity | |
| X-linked hypophosphatemia | PHEX mutation |
| Autosomal dominant hypophosphatemic rickets | FGF23 mutation |
| Autosomal recessive hypophosphatemic rickets/osteomalacia | DMP1 mutation |
| McCune-Albright syndrome | GNAS1 mutation |
| Osteoglophonic dysplasia | FGFR1 mutation |
| Epidermal nevus syndrome | FGFR3 mutation |
| Tumor-induced osteomalacia | FGF23-producing tumor |
| Decreased FGF23 activity | |
| Familial tumoral calcinosis | GALNT3 or FGF23 or KL mutation |
The serum levels of both the C-terminal fragment of FGF23 and of intact FGF23 are high in patients with familial tumoral calcinosis who carry a KL mutation, whereas serum levels of C-terminal FGF23 are high and levels of intact FGF23 are low-to-normal in patients with familial tumoral calcinosis who carry GLANT3 or FGF23 mutations.
Similarly, increased production of FGF23 by tumor cells in patients with tumor-induced osteomalacia can induce excessive renal phosphate wasting and mineralization defects in the bone. These clinical symptoms can be reversed by surgical removal of the FGF23-producing tumor.34 A pathological role for FGF23 has also been suggested in the McCune–Albright syndrome35 and in osteoglophonic dysplasia;36 in both conditions, increased serum levels of FGF23 can cause hypophosphatemia. In osteoglophonic dysplasia, an autosomal dominant disorder characterized by nonossifying bone lesions and abnormal mineral ion balance, increased FGF23 levels are caused by heterozygous, missense mutations in FGFR1 that lead to constitutive activation of the FGF receptor.36 Similarly, McCune-Albright syndrome is also a genetic disease that affects the pigmentation of the skin and induce fibrous dysplasia of the bones, and is caused by activating mutations in the GNAS1 gene.
Hypophosphatemia in patients with autosomal recessive hypophosphatemic rickets/osteomalacia (ARHR) has also been attributed to high serum FGF23 levels.37 These patients might have mutations in DMP-1, but the mechanism by which these mutations can lead to increased FGF23 production is not yet clear. In a related experimental study, increased production of FGF23 was detected in Dmp-1 knockout mice with hypophosphatemia, and genetic deletion of Fgf23 in such mutants resulted in similar hyperphosphatemia as that observed in Fgf23 single knockout mice.38 Thus, hypophosphatemia in Dmp-1 knockout mice is though to be induced by the increased FGF23 level.
Diseases related to decreased FGF23 level
Reduced FGF23 activity can also cause diseases in humans (Table 1). For instance, patients with familial tumoral calcinosis (FTC) usually develop hyperphosphatemia and ectopic calcification owing to loss-of-function mutations in FGF23.39 Similarly, mutations in GALNT3 gene, which encodes the glycosyl transferase ppGaNTase 3 have also been identified in patients with FTC.40 In these patients, serum levels of intact FGF23 are reduced, whereas levels of the processed, C-terminal FGF23 fragment are increased, which suggests an accelerated proteolysis of full-length, bioactive FGF23. Interestingly, ppGaNTase 3 has been shown to specifically induce O-glycosylation of the Thr178 residue, which is located in the proteolytic site of FGF23.41 Similarly, Galnt3-knockout mice show an impaired secretion of intact FGF23, despite an increased expression of FGF23 in the bone, which indicates an important in vivo role of GALNT3 in the processing and secretion of FGF23.42 Together, these observations imply that mutations in GALNT3 can impair O-glycosylation of FGF23 in patients with FTC, and thereby increase the susceptibility of FGF23 to proteolytic inactivation.
Studies of the human genetic disorders that influence the functionality of FGF23 have substantially increased our understanding of the regulation of systemic phosphate homeostasis. However, the exact role and mechanisms by which FGF23 regulation is perturbed in acquired human diseases should be investigated further, particularly in patients with renal diseases.43
FGF23 and chronic kidney disease
Patients with advanced stages of CKD have elevated serum levels of FGF23, which increase progressively as renal function declines. Intact FGF23 seems to be the major circulating form that is present in patients with CKD, although some studies have also reported the presence of the C-terminal fragment of FGF23. Serum measurements of FGF23 are usually performed using either the Immunotopics assay, (San Clemente, CA, USA) or the Kainos assay (Tokyo, Japan).44 Despite increased serum levels of FGF23, patients with CKD develop hyperphosphatemia.45 Inability of FGF23 to reduce or normalize serum phosphate levels in patients with CKD can trigger the development of secondary hyperparathyroidism. However, the causes of elevated serum levels of FGF23 in patients with CKD are not clear. The potential mechanisms include decreased renal clearance of FGF2346 and increased production of FGF23 that counteract hyperphosphatemia; the second possibility is supported by human studies where a high dietary phosphorus load increased serum levels of FGF23.47 Moreover, calcitriol therapy in patients with CKD might also contribute to increased serum levels of FGF23.48 Saito and colleagues have reported that both phosphorus and calcitriol independently increase circulating FGF23 levels.49 Patients with CKD tend to have low levels of calcitriol and secondary hyperparathyroidism. Whether increased levels of FGF23 can influence this dysregulation is a complex issue that should be investigated. As FGF23 can suppress vitamin D activity,50 the increased levels of FGF23 in patients with CKD might reduce vitamin D activity and eventually facilitate the development of compensatory, secondary hyperparathyroidism.51,52
The endocrine functions of parathyroid hormone con tribute to the maintenance of phosphate balance by promoting renal phosphate excretion, and might also reduce urinary calcium excretion and stimulate the renal production of active vitamin D metabolites. Nevertheless, even though serum parathyroid hormone levels are high in patients with CKD, parathyroid hormone fails to reduce serum phosphate levels in these patients. Increased production of parathyroid hormone that counteracts hyperphosphatemia could substantially contribute to the development of secondary hyperparathyroidism.53 Of particular interest, elevated level of serum FGF23 is suggested to be an important predictor of secondary hyperparathyroidism in patients who are undergoing dialysis treatment.54 The interaction between FGF23 and parathyroid hormone is a complex process that is not yet clearly understood; experimental studies have suggested that parathyroid hormone can increase FGF23 production,55,56 whereas others have found that FGF23 can inhibit parathyroid hormone synthesis.25,26 Whether increased serum parathyroid hormone levels can increase the production of FGF23 or vice versa in patients with CKD who receive hemodialysis treatment needs additional studies.
Hyperphosphatemia is an important determinant of mortality in patients with CKD, irrespective of other associated biochemical changes. However, serum phosphate level can be influenced by numerous factors, including diet, the use of phosphate-lowering drugs, or abnormal skeletal conditions. Serum phosphate level, therefore, can be misleading in risk assessment, particularly when they remain within the normal range. Some studies have suggested that under normophosphatemic conditions, serum level of FGF23 might be a better biomarker than serum phosphate level for risk assessment in patients with CKD.57
A number of studies have suggested an association between increased serum level of FGF23 and increased mortality in patients with CKD, particularly in those who undergo hemodialysis.58 The cause of this association is not clear, but some studies have found a correlation between elevated serum FGF23 level and an increased rate of left ventricular hypertrophy.59,60 Although these association studies are of interest, they do not provide enough mechanistic evidence to prove that FGF23 directly affects cardiovascular structural components, which influence cardiac functions and, eventually, mortality. The available (genetically altered) animal models might be able to show a direct effect of FGF23 on cardiovascular structure and function convincingly. Kl-deficient mice are characterized by extremely high serum levels of FGF23 compared with control mice, and early, sudden death (Figure 2), which is linked to cardiac dysfunction of the sino-atrial node.61 Determining whether high serum levels of FGF23 contribute to the cardiac dysfunction and early mortality of Kl-deficient mice might help us understand the pathologic role of elevated serum levels of FGF23 in patients with CKD.
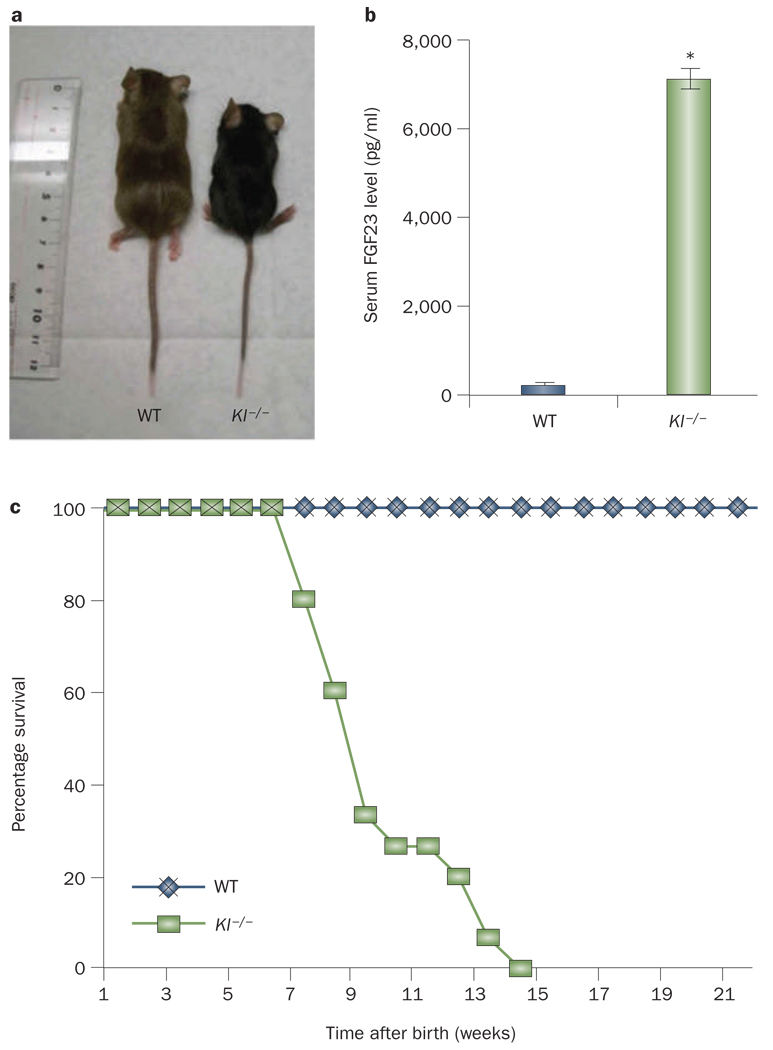
Figure 2.
Gross features, survival and serum FGF23 levels in Kl-knockout mice. Compared with WT mice, Kl−/− mice are smaller in size (a), have markedly elevated serum levels of FGF23 (b) and have a shorter lifespan (around 15–20 weeks) (c). The serum level of FGF23 was measured by enzyme-linked immunosorbent assay using a commercial kit that detects the intact form of FGF23. *P <0.001 versus WT; data presented as mean ± SEM. Abbreviations: Kl−/−, Kl-knockout; WT, wild type.
The role of Klotho
Klotho is a type 1 membrane protein, with a single transmembrane domain near its C-terminus that is hypothesized to anchor the protein to the membrane.18 Most cellular functions and cell–matrix interactions are performed through membrane proteins, which consist of transmembrane and anchored proteins. Type I membrane proteins usually have a single transmembrane stretch of hydrophobic amino acids, with the N-terminus exposed to the extracellular side of the membrane and the C-terminus exposed on the cytoplasmic side. If the short transmembrane domain of Klotho is removed, the remaining fragment (the secreted form) can be released into the circulatory system.
The gene Kl, which encodes Klotho in the mouse is located on chromosome 13q12 and has 5 exons and 4 introns.62 The transcript of this gene is about 5.2 kb. The third exon of Kl can be alternatively spliced to generate two transcripts, which encode the transmembrane and secreted forms of Klotho. The transcript of the full-length Kl encodes a protein of 1,014 amino acids and a molecular weight of 130 kD (the transmembrane form), whereas the truncated Kl encodes a protein of 550 amino acids and a molecular weight of approximately 65–70 kD (the secreted form).63–65
The full-length mouse Kl cDNA has around 93% and 80% homology with those of rat and human, respectively,64 whereas the mouse Klotho protein has around 94% and 80% homology with the rat and human proteins.45 The transmembrane form of the mouse Klotho possesses a putative signal sequence at its N-terminus, a putative transmembrane domain, and a short cytoplasmic domain at the C-terminus. The extracellular domain of Klotho consists of two internal repeats of about 550 amino acids (KL1 and KL2) that share sequence homology with β-glucosidase. Between two internal repeats (KL1 and KL2), a short stretch of basic amino acids (Lys-Lys-Arg-Lys) is included that forms a possible site for proteolytic cleavage. This short stretch of basic amino acids is present in the rat, human and mouse Klotho proteins. The secreted form of mouse Klotho only contains the N-terminal fragment, including its extracellular domain (KL1).63–65 One study has suggested that the metallo-proteinases ADAM-10 and ADAM-17 are able to cleave Klotho from the plasma membrane, and that insulin can stimulate Klotho shedding.66
Klotho expression has been detected in the distal convoluted tubules of the kidney, the parathyroid gland, and the epithelium of the choroid plexus in the brain.45 Kl-knockout mice exhibit increased renal expression of NaPi-2a and NaPi-2c protein with concomitant hyperphosphatemia (Figure 3) and develop the same physical, biochemical, and morphological characteristics as Fgf23-knockout mice.62 The identical phenotypes of these two separate knockout lines eventually led to the identification of Klotho as an essential cofactor in FGF23 signaling pathways.67–71
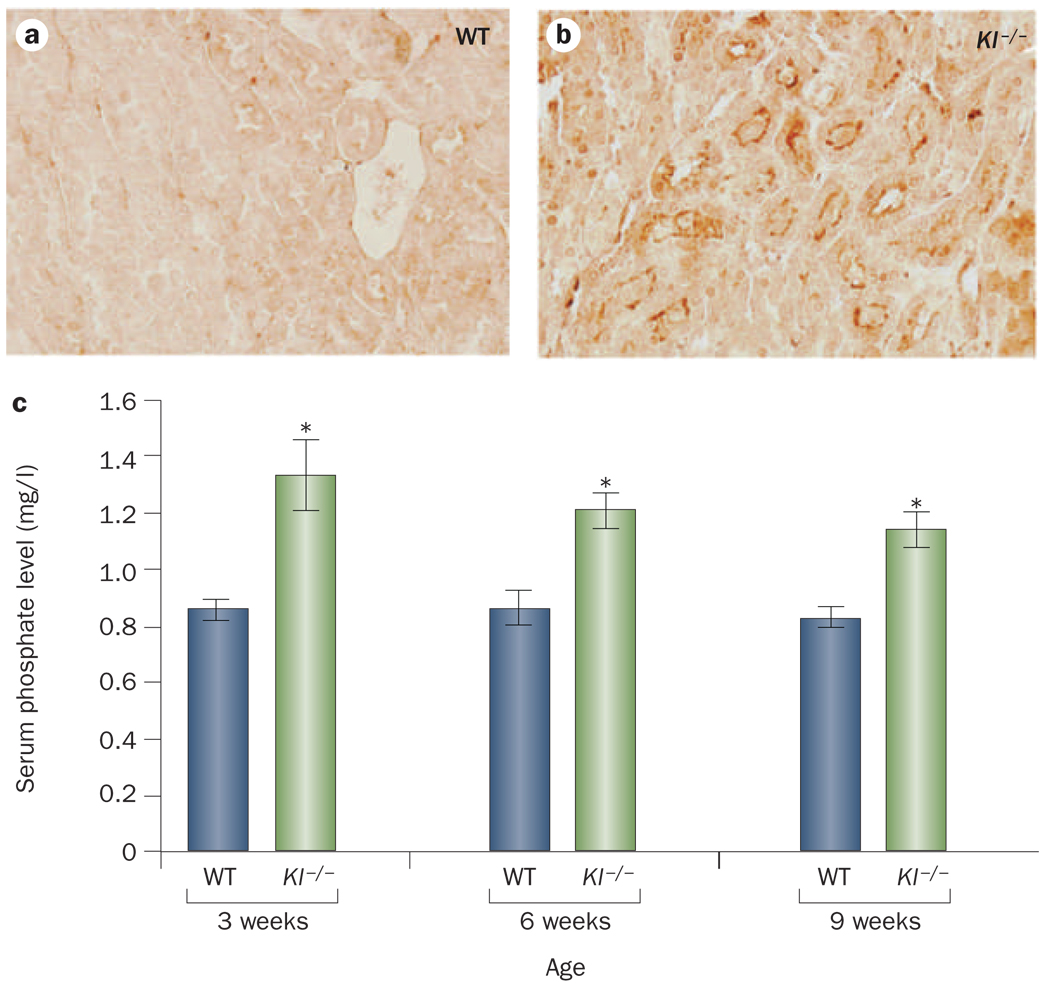
Figure 3.
Renal expression of NaPi-2a and serum levels of phosphate in Kl-knockout mice. Compared with WT mice, Kl−/− mice exhibit increased renal expression of NaPi-2a (a and b) and hyperphosphatemia (c). Note that hyperphosphatemia is observed in Kl−/− mice by 3 weeks of age and their serum phosphate level remains high for their entire lifespan. *P <0.05 versus WT; data presented as mean ± SEM. Abbreviations: Kl−/−, Kl-knockout; WT, wild type.
FGF23 signaling
In general, most FGFs bind to FGF receptors on the cell surface and activate downstream signaling events that exert diverse biological functions. FGF23 is a member of the FGF19 subfamily, which also contains FGF19 and FGF21, and has been shown to bind to multiple FGF receptors, including FGFR1c, FGFR3c, and FGFR4.67,70,72,73 Follow-up studies, however, suggested that FGFR1 is the principal mediator of the effects of FGF23 in vivo.74,75 Further research has suggested that Klotho can bind to multiple FGF receptors, and that the Klotho–FGF receptor complex binds to FGF23 with much higher affinity than either the FGF receptor or Klotho alone. The binding of this complex can then activate downstream signaling events, as demonstrated by the activation of Egr-1 and the phosphorylation of FGF receptor substrate-2a, ERK, p38, JNK, and AKT.67,70,76 Notably, these signaling phospho-proteins have been detected only when cells were treated with both FGF23 and Klotho, and not in cells that were treated with FGF23 only. These results, along with earlier observations, clearly suggest that the interaction of FGF23 and the FGF receptor and subsequent signaling activities require Klotho as a cofactor.77
In response to elevated serum phosphate levels, FGF23 is produced in the bone and exerts endocrine effects in the kidneys in coordination with Klotho, which is mostly expressed in the distal tubular epithelial cells and promotes renal phosphate excretion. The phosphate-lowering effect of FGF23 is partly mediated through the reduced expression of NaPi-2a and 1α hydroxylase in the proximal tubular epithelial cells. Despite the fact that Klotho is present only in the distal tubular epithelial cells, FGF23-mediated phosphate metabolism takes place in the proximal tubules. Of note, the FGF receptor 1 is also expressed in distal tubules.75 The interaction of proximal and distal tubules to facilitate FGF23-Klotho-mediated functions is an important and unsolved issue, and an active area of research. In one study, robust induction of phosphorylated ERK1 (a marker of FGF23 bioactivity) was detected only within Klotho-expressing distal tubules following FGF23 injection, which suggests that FGF23-mediated signaling might be initiated in the distal convoluted tubule.78 These studies have paved the way to a future, in-depth description of the role of Klotho in FGF23-mediated regulation of phosphate homeostasis.
Systemic functions of FGF23–Klotho axis
Transgenic mice that overexpress human FGF23 or mouse Fgf23 develop hypophosphatemia owing to severe urinary phosphate wasting, whereas Fgf23-knockout mice develop hyperphosphatemia owing to an increased renal uptake of filtrated phosphate. A genetic restoration of the systemic actions of human FGF23 in Fgf23-knockout mice reverses hyperphosphatemia to hypophosphatemia and prevents associated complications, including ectopic calcification.30 These genetically modified animal models have provided valuable insights into the role of FGF23 in regulating phosphate homeostasis, and some studies have clearly demonstrated in vivo the importance of Klotho in this regulation. For instance, serum phosphate levels are substantially reduced following an injection of bioactive FGF23 in either wild-type or Fgf23-knockout mice.68 As wild-type and Fgf23-knockout mice both express endogenous Klotho, the exogenous FGF23 is able to influence systemic phosphate homeostasis. In contrast, the injection of bioactive FGF23 protein into either Kl-knockout mice or Fgf23/Kl double knockout mice does not produce any obvious changes in the serum levels of phosphate.68 These observations imply that Klotho is essential for the FGF23-mediated regulation of phosphate homeostasis.
The essential in vivo role of Klotho has been also demonstrated in a genetically engineered, hypophosphatemic mouse model.79 These mice possess a mutation that in activates Phex, a phosphate-regulating gene that is homologous to the endopeptidase-encoding genes that are located on the X chromosome. This mutation is associated with severe hypophosphatemia secondary to excessive urinary phosphate wasting, which is caused by increased serum accumulation of FGF23. In vivo genetic manipulation studies have shown that the inactivation of Klotho in Phex–deficient mice results in hyperphosphatemia, not hypophosphatemia, even though mice that are deficient of both Phex and Kl have markedly elevated serum levels of FGF23.79 The opposing phenotypes of these single mutant and double mutant mice suggest that the disruption of Klotho-mediated pathways abrogates the hypophosphatemic phenotype that is normally caused by the increased serum levels of FGF23.80,81
Furthermore, genetic inactivation of Kl in FGF23 transgenic mice results in a phenotype that is consistent with Klotho-deficiency, which again emphasizes the in vivo importance of Klotho in the function of FGF23.82 In humans, a homozygous, loss-of-function mutation in KL causes tumoral calcinosis, severe hyperphosphatemia and ectopic calcification despite high serum levels of FGF23 in the affected patients.83 Together, these human and mouse genetic studies provide compelling evidence that klotho is essential in the FGF23-mediated regulation of systemic phosphate homeostasis in vivo.
Nevertheless, under pathological conditions where the concentration of FGF23 is extremely high, FGF23 might exert nonspecific effects without klotho, as FGF23 can bind to FGF receptors with low affinity in the absence of klotho.70 Several in vitro studies also support the possibility of such nonspecific responses. For instance, FGF23 has been shown to suppress osteoblast differentiation and bone mineralization in fetal rat calvaria cells.84 As Kl is not expressed in osteoblasts, any effect of FGF23 on these cells would indicate that Klotho-independent effects of FGF23 exist, unless bone cells express extremely low levels of Klotho that are undetectable with existing tools. In a similar study, FGF23 was shown to exhibit weak proliferative effects on a murine bone-marrow-derived pro-B cell line that overexpresses FGF receptors but does not express Klotho.85
Future studies should determine whether extremely high serum levels of FGF23 can lead to ectopic activation of FGF receptors and induce cardiac morbidity in patients with CKD. In this scenario, patients with CKD might benefit from therapy that lowers FGF23 level. However, a thorough understanding of the role of elevated serum FGF23 levels in patients with CKD is needed before any therapeutic strategy can be proposed. For example, whether increased FGF23 level is a protective response (in early stages) or an adverse effect (in later stages) in patients with CKD is not clear. Moreover, vitamin D deficiency has been linked to increased mortality in advanced CKD patients,86 and, as FGF23 can suppress the production of active vitamin D metabolites, any detrimental effect of FGF23 in these patients might be influenced by reduced vitamin D activity.
As discussed above, the creation of Phex/Kl double mutant mice has clearly demonstrated that the FGF23-mediated hypophosphatemia in Phex-deficient mice is Klotho-dependent. These genetic studies have provided in vivo evidence that suggests that Klotho might be a potential therapeutic tool to manipulate FGF23 function, and direct manipulation of Klotho might be used as a novel therapeutic strategy for FGF23-related hypo phosphatemic diseases.43,80 The clinical application of a controlled reduction of FGF23 might be of therapeutic benefit for patients with excessive urinary phosphate wasting diseases, including ADHR, ARHR, and XLH. Currently, treatments for these genetic hypophosphatemic diseases, such as oral phosphate replacement, are mostly palliative, and the prolonged use of these therapies can lead to complications, notably secondary hyperparathyroidism. Treatment of Phex-deficient mice with anti-FGF23 antibodies inhibited the endogenous FGF23 activities and resulted in increased serum levels of phoshate.87 In a similar study, inactivation of endogenous FGF23 activity in Phex-deficient mice by manipulation of Klotho’s function resulted in hyperphosphatemia, even though Phex/Kl double mutant mice have markedly elevated serum levels of FGF23.79 Finally, in contrast to anti-FGF23 therapy, administration of exogenous, bioactive FGF23 protein might help restore phosphate balance and delay associated complications, such as the ectopic calcifications in patients with FTC that are usually caused by reduced FGF23 activity.
Exogenous FGF23 treatment has been shown to delay the progression of renal failure induced by experimental nephritis. However, this treatment also aggravated renal osteodystrophy owing to reduced levels of calcitriol, which demonstrates a potential limitation of FGF23 therapy.88 Renal osteodystrophy is often described as a CKD and mineral and bone disorder. Of particular interest, calcitriol can exert opposing effects on serum phosphate levels: it can induce both FGF23 and Klotho to increase urinary excretion of phosphate and lower serum phosphate levels, but can also facilitate increased intestinal absorption of phosphate to increase serum phosphate levels (Figure 4).
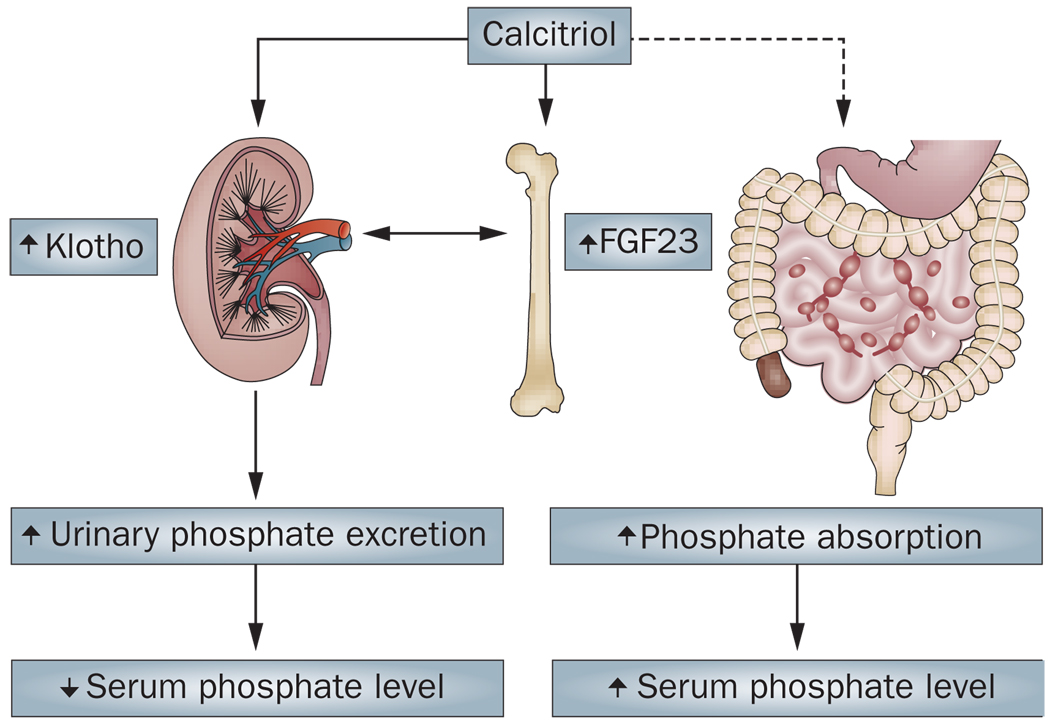
Figure 4.
The endocrine effects of vitamin D on phosphate metabolism. Calcitriol increases urinary excretion of phosphate by inducing the expression of both FGF23 (in bone) and Klotho (in kidney), which results in decreased serum phosphate levels. Calcitriol can also facilitate increased intestinal absorption of phosphate, which increases serum phosphate levels.
Conclusions
The regulation of systemic phosphate homeostasis seems to be strictly controlled by a limited number of factors, as demonstrated by the opposing phenotypes of transgenic and knockout Fgf23-mutant mice, their similarities with Kl-mutant mice, and—more importantly—the corresponding clinical phenotype in hereditary diseases that are caused by FGF23 or KL mutations in humans (Box 1).22,27,68,89–92 The overlapping phenotypes and the lack of redundancy in phenotypes of Fgf23-mutant mice with Kl-mutant mice, suggest that the biological network that actively regulates phosphate homeostasis consists of a limited number of essential factors. Our understanding of the essential in vivo role of FGF23 in maintaining systemic phosphate homeostasis has laid the foundation for future work to determine the therapeutic benefit of manipulating the FGF23–Klotho network in patients with excessive urinary phosphate-wasting diseases. In addition, serum FGF23 measurements might have both diagnostic and prognostic relevance in these patients, and might be used to determine the underlying causes of diseases that are associated with abnormal mineral ion metabolism. For instance, serum FGF23 levels can aid the diagnosis of tumor-induced osteomalacia, and pretreatment serum level of FGF23 might be a good predictor of the efficacy of vitamin D therapy in patients who receive dialysis, as well as for the future development of refractory hyperparathyroidism.54
Hypophosphatemia in the early posttransplant period in patients who receive renal transplant is more strongly associated with increased level of FGF23 than that of parathyroid hormone.93 Similarly, another study suggests that FGF23 is independently and negatively associated with the calcification of arteries in the hand, but not of the aorta in patients with CKD who undergo hemodialysis, and proposed that plasma FGF23 levels can be a reliable marker for medial, peripheral artery calcification in these patients.94 Furthermore, extensive cardiovascular calcification, which is a leading cause of death in patients with CKD who undergo hemodialysis, is associated with high serum FGF23 levels.58,95–98
In conclusion, experimental studies have provided compelling evidence of the in vivo importance of Klotho in FGF23-mediated regulation of systemic phosphate homeostasis. Translation of this research to new therapies for patients who suffer from the complications of abnormal mineral ion metabolism will be a challenging yet clinically rewarding effort.99
Key points.
-
▪
FGF23 is a bone-derived growth factor that can influence the homeostasis of phosphate and vitamin D
-
▪
Systemic regulation of phosphate homeostasis by FGF23 is dependent on the activity of the membrane protein Klotho
-
▪
Determination of serum FGF23 levels might improve the diagnosis and prognosis of various diseases associated with abnormal mineral ion metabolism, including tumor-induced osteomalacia and chronic kidney diseases
-
▪
Restoration of normal FGF23 activity, by targeting FGF23 or Klotho, might have therapeutic benefits in diseases associated with abnormal mineral ion metabolism
-
▪
Hyperphosphatemia might have more serious consequences in both skeletal and nonskeletal tissues than usually appreciated
Acknowledgments
The author thanks T. Nakatani and M. Ohnishi (Harvard School of Dental Medicine, Boston) for technical assistance and T. Taguchi (Nagasaki University, Japan) for continued help and encouragement. Some of the original research that formed the basis of this Review is supported by a grant (R01-DK077276 to M.S.R.) from the National Institute of Diabetes and Digestive and Kidney Diseases, and institutional support from Nagasaki University School of Biomedical Science and Harvard School of Dental Medicine.
Footnotes
Competing interests
The author declares associations with the following companies: Daiichi Sankyo, Genzyme and Kyowa Hakko Kirin Pharmaceuticals. See the article online for full details of the relationships.
References
- 1.Rastegar A. New concepts in pathogenesis of renal hypophosphatemic syndromes. Iran J. Kidney Dis. 2009;3:1–6. [PubMed] [Google Scholar]
- 2.Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am. J. Med. 2005;118:1094–1101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 4.Imel EA, Econs MJ. Fibroblast growth factor 23: roles in health and disease. J. Am. Soc. Nephrol. 2005;16:2565–2575. doi: 10.1681/ASN.2005050573. [DOI] [PubMed] [Google Scholar]
- 5.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe PS. The wrickkened pathways of FGF23, MEPE and PHEX. Crit. Rev. Oral Biol. Med. 2004;15:264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drezner M. In: Principles in Bone Biology. Bilezikian J, Raisz L, Rodan G, editors. New York: Academic Press; 2002. pp. 321–338. [Google Scholar]
- 8.Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am. J. Nephrol. 2007;27:503–515. doi: 10.1159/000107069. [DOI] [PubMed] [Google Scholar]
- 9.Razzaque MS, Taguchi T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med. Electron Microsc. 2002;35:68–80. doi: 10.1007/s007950200009. [DOI] [PubMed] [Google Scholar]
- 10.Razzaque MS. Does renal ageing affect survival? Ageing Res. Rev. 2007;6:211–222. doi: 10.1016/j.arr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi T, Razzaque MS. The collagen-specific molecular chaperone HSP47: is there a role in fibrosis? Trends Mol. Med. 2007;13:45–53. doi: 10.1016/j.molmed.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Berndt T, et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc. Natl Acad. Sci. USA. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol. Rev. 2000;80:1373–1409. doi: 10.1152/physrev.2000.80.4.1373. [DOI] [PubMed] [Google Scholar]
- 14.Faroqui S, Levi M, Soleimani M, Amlal H. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int. 2008;73:1141–1150. doi: 10.1038/ki.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006;70:1548–1559. doi: 10.1038/sj.ki.5001813. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S. Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am. J. Nephrol. 2007;27:466–478. doi: 10.1159/000106484. [DOI] [PubMed] [Google Scholar]
- 17.Capuano P, et al. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am. J. Physiol. Cell Physiol. 2005;288:C429–C434. doi: 10.1152/ajpcell.00331.2004. [DOI] [PubMed] [Google Scholar]
- 18.Nabeshima Y, Imura H. alpha-Klotho: a regulator that integrates calcium homeostasis. Am. J. Nephrol. 2008;28:455–464. doi: 10.1159/000112824. [DOI] [PubMed] [Google Scholar]
- 19.Razzaque MS. Klotho and Na+, K+-ATPase activity: solving the calcium metabolism dilemma? Nephrol. Dial. Transplant. 2008;23:459–461. doi: 10.1093/ndt/gfm702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imura A, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 22.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 23.Berndt TJ, et al. Biological activity of FGF-23 fragments. Pflugers Arch. 2007;454:615–623. doi: 10.1007/s00424-007-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada T, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Dov IZ, et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krajisnik T, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 27.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23(R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 28.Shimada T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson T, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 30.DeLuca S, et al. Amelioration of the premature aging-like features of Fgf-23 knockout mice by genetically restoring the systemic actions of FGF-23. J. Pathol. 2008;216:345–355. doi: 10.1002/path.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson KB, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 32.Hafner C, et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J. Clin. Invest. 2006;116:2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman WH, Jueppner HW, Deyoung BR, O’Dorisio MS, Given KS. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am. J. Med. Genet. A. 2005;134:233–236. doi: 10.1002/ajmg.a.30599. [DOI] [PubMed] [Google Scholar]
- 34.Shimada T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riminucci M, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrow EG, et al. Extended mutational analyses of FGFR1 in osteoglophonic dysplasia. Am. J. Med. Genet. A. 2006;140:537–539. doi: 10.1002/ajmg.a.31106. [DOI] [PubMed] [Google Scholar]
- 37.Lorenz-Depiereux B, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, et al. Pathogenic role of Fgf23 in Dmp1-null mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 40.Frishberg Y, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J. Mol. Med. 2005;83:33–38. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- 41.Garringer HJ, et al. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J. Clin. Endocrinol. Metab. 2006;91:4037–4042. doi: 10.1210/jc.2006-0305. [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa S, et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150:2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razzaque MS. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol. Dial Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imel EA, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J. Clin. Endocrinol. Metab. 2006;91:2055–2061. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 45.Fukagawa M, Nii-Kono T, Kazama JJ. Role of fibroblast growth factor 23 in health and in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2005;14:325–329. doi: 10.1097/01.mnh.0000172717.49476.80. [DOI] [PubMed] [Google Scholar]
- 46.Imanishi Y, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–1946. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 48.Nishi H, et al. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin. Pract. 2005;101:c94–c99. doi: 10.1159/000086347. [DOI] [PubMed] [Google Scholar]
- 49.Saito H, et al. Circulating FGF-23 is regulated by 1alpha, 25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 51.Shigematsu T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am. J. Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 52.Gutierrez O, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 53.Felsenfeld AJ, Rodríguez M, Aguilera-Tejero E. Dynamics of parathyroid hormone secretion in health and secondary hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2007;2:1283–1305. doi: 10.2215/CJN.01520407. [DOI] [PubMed] [Google Scholar]
- 54.Nakanishi S, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 55.Kawata T, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 56.Saji F, et al. Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron Physiol. 2009;111:p59–p66. doi: 10.1159/000210389. [DOI] [PubMed] [Google Scholar]
- 57.Gutiérrez OM, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fliser D, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 59.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am. J. Med. Sci. 2009;337:116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 60.Gutiérrez OM, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeshita K, et al. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 62.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 63.Matsumura Y, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 64.Shiraki-Iida T, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 65.Ohyama Y, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 66.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl Acad. Sci. USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurosu H, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakatani T, et al. In vivo genetic evidence of klotho-dependent functions of FGF23 in regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 71.Lanske B, Razzaque MS. Premature aging in klotho mutant mice: cause or consequence? Ageing Res. Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Gattineni J, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Renal Physiol. 2009;297:F282–F291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J. Am. Soc. Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamazaki Y, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 77.Medici D, et al. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J. Cell Biol. 2008;182:459–465. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am. J. Physiol. Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J. Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bai X, Dinghong Q, Miao D, Goltzman D, Karaplis AC. Klotho ablation converts the biochemical and skeletal alterations in FGF23 (R176Q) transgenic mice to a Klotho-deficient phenotype. Am. J. Physiol. Endocrinol. Metab. 2009;296:E79–E88. doi: 10.1152/ajpendo.90539.2008. [DOI] [PubMed] [Google Scholar]
- 83.Ichikawa S, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H, et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J. Bone Miner. Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 85.Yu X, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolf M, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 87.Aono Y, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 88.Kusano K, Saito H, Segawa H, Fukushima N, Miyamoto K. Mutant FGF23 prevents the progression of chronic kidney disease but aggravates renal osteodystrophy in uremic rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2009;55:99–105. doi: 10.3177/jnsv.55.99. [DOI] [PubMed] [Google Scholar]
- 89.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature ageing-like phenotype in fibroblast growth factor 23 null mice is a vitamin-D mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masi L, et al. A novel recessive mutation of fibroblast growth factor-23 in tumoral calcinosis. J. Bone Joint Surg. Am. 2009;91:1190–1198. doi: 10.2106/JBJS.H.00783. [DOI] [PubMed] [Google Scholar]
- 92.Lammoglia JJ, Mericq V. Familial tumoral calcinosis caused by a novel FGF23 mutation: response to induction of tubular renal acidosis with acetazolamide and the non-calcium phosphate binder sevelamer. Horm. Res. 2009;71:178–184. doi: 10.1159/000197876. [DOI] [PubMed] [Google Scholar]
- 93.Bhan I, et al. Post-transplant hypophosphatemia: Tertiary ‘Hyper-Phosphatoninism’? Kidney Int. 2006;70:1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 94.Inaba M, et al. Role of fibroblast growth factor-23 in peripheral vascular calcification in non-diabetic and diabetic hemodialysis patients. Osteoporos. Int. 2006;17:1506–1513. doi: 10.1007/s00198-006-0154-6. [DOI] [PubMed] [Google Scholar]
- 95.Stompór T. An overview of the pathophysiology of vascular calcification in chronic kidney disease. Perit. Dial. Int. 2007;27 Suppl. 2:S215–S222. [PubMed] [Google Scholar]
- 96.DeLoach SS, Berns JS. Arterial stiffness and vascular calcification in dialysis patients: new measures of cardiovascular risk. Semin. Dial. 2007;20:477–479. doi: 10.1111/j.1525-139X.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 97.Negri A. FGF23 in chronic kidney disease and kidney post-transplant patients [Spanish] Nefrologia. 2009;29:196–202. doi: 10.3265/Nefrologia.2009.29.3.5290.en.full. [DOI] [PubMed] [Google Scholar]
- 98.Ibrahim S, Rashed L. Serum fibroblast growth factor-23 levels in chronic haemodialysis patients. Int. Urol. Nephrol. 2009;41:163–169. doi: 10.1007/s11255-008-9466-0. [DOI] [PubMed] [Google Scholar]
- 99.Razzaque MS. Can fibroblast growth factor 23 fine-tune therapies for diseases of abnormal mineral ion metabolism? Nat. Clin. Pract. Endocrinol. Metab. 2007;3:788–789. doi: 10.1038/ncpendmet0667. [DOI] [PubMed] [Google Scholar]