SUMMARY
Oncogenic BRAF mutations are more frequent in cutaneous melanoma from sites with little or moderate sun-induced damage than from sites with severe cumulative solar ultraviolet (UV) damage. We studied cutaneous melanomas from geographic regions with different levels of ambient UV radiation to delineate the relative effects of cumulative UV damage, age and anatomic site on the frequency of BRAF mutations.
We show that BRAF-mutated melanomas occur in a younger age group on skin without marked solar elastosis, and less frequently affect the head and neck area, compared to melanomas without BRAF mutations. The findings indicate that BRAF-mutated melanomas arise early in life at low cumulative UV doses, whereas melanomas without BRAF mutations require accumulation of high UV doses over time. The effect of anatomic site on the mutation spectrum further suggests regional differences among cutaneous melanocytes.
Keywords: BRAF, genetics, melanoma, mutation, pathology, solar elastosis, ultraviolet exposure
INTRODUCTION
The relationship between the effects of ultraviolet radiation (UV) and BRAF mutations in melanocytic neoplasia is complex. Activating mutations of the BRAF gene have been reported in the majority of melanocytic nevi and cutaneous melanoma (Davies et al., 2002, Pollock et al., 2003, Yazdi et al., 2003, Maldonado et al., 2003), which in turn are associated with UV exposure (most commonly from exposure to sunlight) (Bauer et al., 2005, MacKie and Aitchison, 1982). In contrast, nevi that develop before birth(Bauer et al., 2007) and melanomas on sun-protected anatomic sites (Curtin et al., 2005, Maldonado et al., 2003) rarely harbor BRAF mutations. The frequency of BRAF mutations is also low in melanomas that develop on anatomic sites such as the head and neck, which frequently show histopathologic signs of cumulative sun-damage (CSD) to the skin (solar elastosis) (Maldonado et al., 2003, Curtin et al., 2005). This group of melanomas also differs from those originating on intermittently sun-exposed skin by the infrequent association with melanocytic nevi, frequent association with non-melanoma skin cancers (Whiteman et al., 2003), a 20 year delay in age-specific incidence (Lachiewicz et al., 2008), and the presence of genetic aberrations in the KIT oncogene (Curtin et al., 2006). These observations led to the conclusion that there are different molecular mechanisms leading to melanoma (Maldonado et al., 2003, Rivers, 2004, Whiteman et al., 2003), resulting in genetically distinct subgroups of melanoma (Curtin et al., 2005).
The presence of BRAF mutations in melanomas is associated with other covariates. Mutation frequency is inversely correlated with age, and higher in primaries originating from anatomic sites such as the trunk and proximal extremities (Liu et al., 2007, Maldonado et al., 2003, Viros et al., 2008). Both covariates are linked to UV exposure: the face and distal extremities are more likely to be chronically sun-exposed, and as cumulative UV exposure increases with age consequently so too does CSD increases with age. This fact has hampered previous studies that investigated whether any of the factors independently affect BRAF mutation frequency. Here we present clinical, epidemiologic, pathologic and molecular data indicating that all three factors (age, anatomic site, and degree of sun-induced damage) independently affect the frequency of BRAF mutations in cutaneous melanoma.
RESULTS
Study cohort characteristics
The Europe/USA and Australian cohorts were comparable with regard to age (mean 57.5 years, SD 16.2, and mean 61.8 years, SD 15.8, respectively) and gender (56.6% and 69.6% male, respectively). As expected, the degree of solar elastosis was higher in Australians (median CSD=6, IQR=6-8) compared to Europe/USA melanomas (median CSD=4, IQR=3-6). BRAF mutations were identified in 50.5% of Europe/USA melanomas and in 39.2% of Australian melanomas. As shown in Table 1, the frequency of BRAF mutations decreased progressively with increasing degree of solar elastosis in both cohorts and was higher in melanomas situated on the trunk or lower extremities.
Table 1.
Association of BRAF mutation with various clinicopathologic characteristics in 424 European and North American and 120 Australian cutaneous melanoma patients.
| Europe and USA (n=424) | Australia (n=120) | |||
|---|---|---|---|---|
| BRAF mutation | p-value | BRAF mutation | p-value | |
| Age* | P<0.001$ | P=0.052$ | ||
| <= 45 years | 69.4% (n=98) | 60.0% (n=20) | ||
| 46 – 60 years | 60.6% (n=127) | 40.6% (n=32) | ||
| 61 – 70 years | 38.5% (n=96) | 26.1% (n=23) | ||
| > 70 year | 31.7% (n=101) | 32.6% (n=43) | ||
| Gender* | P=0.083** | P=0.838# | ||
| Female | 55.6% (n=178) | 36.1% (n=36) | ||
| Male | 47.0% (n=232) | 39.0% (n=82) | ||
| CSD score | P<0.001$ | P=0.008$ | ||
| 0-3 | 66.7% (n=147) | 70.0% (n=10) | ||
| 4-6 | 51.1% (n=186) | 44.6% (n=56) | ||
| 7-10 | 23.1% (n=91) | 27.8% (n=54) | ||
| Anatomic site | P<0.001** | P=0.003# | ||
| Face or scalp | 24.3% (n=74) | 25.8% (n=62) | ||
| Upper extremities | 35.3% (n=51) | / | ||
| Trunk | 61.5% (n=200) | 53.4% (n=58) | ||
| Lower extremities | 55.6% (n=99) | / | ||
| Breslow thickness* | P=0.619** | P=0.247$ | ||
| 0.01-1.00mm | 56.2% (n=146) | 36.4% (n=66) | ||
| 1.01-2.00mm | 48.3% (n=116) | 32.0% (n=25) | ||
| 2.01-4.00mm | 50.0% (n=78) | 41.2% (n=17) | ||
| >4.00mm | 52.0% (n=50) | 60.0% (n=10) | ||
| Histologic type* | P=0.002** | P=0.002** | ||
| Superficial spreading melanoma | 56.3% (n=213) | 47.0% (n=83) | ||
| Nodular melanoma | 51.8% (n=85) | 50.0% (n=8) | ||
| Lentigo maligna melanoma | 27.7% (n=47) | 8.3% (n=24) | ||
| Other | 63.6% (n=11) | 0.0% (n=2) | ||
The total number of subjects in each category is indicated in parentheses.
Missing values for Age in 4 patients, for gender in 14 patients, for Breslow thickness in 34 patients, and for histologic type in 68 patients
p-value result of Chi-square test
p-value result of Fisher's exact test
p-value result of exact Chi-square test for trend.
When cases were stratified by anatomic site of the primary, the BRAF mutation frequency remained inversely correlated with the degree of solar elastosis in the Europe/USA cohort for the face/scalp and upper extremities. The trend remained, but was not significant for trunk and lower extremity lesions (Table 2a). The Australian melanomas showed the same association for melanomas on the trunk (p=0.048). By contrast, cases on the face and scalp did not show this trend, possibly because the number of primary tumors of the face or scalp with low solar elastosis was very small (n=2), as expected for a geographic region with high solar exposure. There was an inverse correlation between BRAF mutation frequency and age in the Europe/USA cohort (Table 2b). In summary, in the Europe/USA patients, young age and low amounts of sun-damage were associated with an increased frequency of BRAF mutations, independent of the anatomic location of the melanoma.
Table 2a.
Association between cumulative sun-damage score (CSD) and BRAF mutation stratified by anatomic site.
| BRAF mutation (%) | ||||
|---|---|---|---|---|
| CSD 0-3 | CSD 4-6 | CSD 7-10 | p-value* | |
| European and North American melanomas | ||||
| Face or scalp (n=74) | 100% 4 of 4 |
31% 4 of 13 |
18% 10 of 57 |
P=0.002 |
| Upper extremities (n=51) | 64% 9 of 14 |
33% 7 of 21 |
13% 2 of 16 |
P=0.004 |
| Trunk (n=200) | 68% 62 of 91 |
57% 56 of 99 |
50% 5 of 10 |
P=0.08 |
| Lower extremities (n=99) | 61% 23 of 38 |
53% 28 of 53 |
50% 4 of 8 |
P=0.51 |
| Australian melanomas | ||||
| Face or scalp (n=62) | 0% 0 of 2 |
32% 6 of 19 |
24% 10 of 41 |
P=1.0 |
| Trunk (n=58) | 88% 7 of 8 |
51% 19 of 37 |
39% 5 of 13 |
P=0.048 |
p-value of exact Chi-square test for trend.
Table 2b.
Association between age and BRAF mutation stratified by anatomic site.
| BRAF mutation (%) | |||||
|---|---|---|---|---|---|
| Age <=45 | Age 46-60 | Age 61-70 | Age>70 yrs | p-value* | |
| European and North American melanomas | |||||
| Face or scalp (n=73) | 67% 6 of 9 |
30% 3 of 10 |
27% 6 of 22 |
9% 3 of 32 |
P=0.001 |
| Upper extremities (n=51) | 62% 8 of 13 |
41% 7 of 17 |
11% 1 of 9 |
17% 2 of 12 |
P=0.008 |
| Trunk (n=200) | 74% 34 of 46 |
64% 47 of 73 |
53% 24 of 45 |
50% 18 of 36 |
P=0.013 |
| Lower extremities (n=98) | 67 % 20 of 30 |
74% 20 of 27 |
30% 6 of 20 |
43% 9 of 21 |
P=0.014 |
| Australian melanomas | |||||
| Face or scalp (n=61) | 22% 2 of 9 |
28% 5 of 18 |
29% 4 of 14 |
20% 4 of 20 |
P=0.786 |
| Trunk (n=57) | 91% 10 of 11 |
57% 8 of 14 |
22% 2 of 9 |
44% 10 of 23 |
P=0.013 |
p-value of exact Chi-square test for trend; 4 missing values for age.
When cases were stratified by age group, the inverse correlation between BRAF mutation frequency and degree of solar elastosis remained, although in only two strata was this correlation statistically significant (Table 2c).
Table 2c.
Association between cumulative sun exposure score and BRAF mutation stratified by age.
| BRAF mutation (%) | ||||
|---|---|---|---|---|
| CSD 0-3 | CSD 4-6 | CSD 7-10 | p-value* | |
| USA and Europe | ||||
| <= 45 years (n=98) | 72% 44 of 61 |
66% 23 of 35 |
50% 1 of 2 |
P=0.415 |
| 46 – 60 years (n=127) | 69% 38 of 55 |
62% 34 of 55 |
29% 5 of 17 |
P=0.012 |
| 61 – 70 years (n=96) | 53% 9 of 17 |
40% 23 of 58 |
24% 5 of 21 |
P=0.069 |
| > 70 years (n=101) | 54% 7 of 13 |
40% 15 of 38 |
20% 10 of 50 |
P=0.009 |
| Australia | ||||
| <= 45 years (n=20) | 100% 4 of 4 |
54% 7 of 13 |
33% 1 of 3 |
P=0.127 |
| 46 – 60 years (n=32) | 67% 2 of 3 |
44% 8 of 18 |
27% 3 of 11 |
P=0.251 |
| 61 – 70 years (n=23) | 0% 0 of 2 |
40% 4 of 10 |
18% 2 of 11 |
P=1.0 |
| > 70 years (n=43) | 100% 1 of 1 |
31% 4 of 13 |
31% 9 of 29 |
P=0.545 |
p-value of exact Chi-square test for trend.
Multivariate analysis
Multivariable logistic regression analysis showed that in Europe/USA melanomas, anatomic location of the melanoma in trunk or lower extremities, younger age and low solar elastosis were independently associated with BRAF mutations (Table 3). Trunk melanomas were more likely BRAF-mutated than melanomas from the face, scalp or upper extremities (OR=2.7, 95%CI=1.5-4.9). In the Australian dataset, similar associations were seen, but only the association of increased frequency of BRAF mutation with trunk melanomas was statistically significant (OR=2.8, 95%CI=1.2-6.6), likely due to the smaller sample size.
Table 3.
Multivariable logistic regression analyses of clinicopathologic factors associated with BRAF mutation
| BRAF wild-type | BRAF mutation | Odds-ratio (95%CI)# | p-value | |
|---|---|---|---|---|
| European and North American melanomas n=409* | N=201 | N=208 | ||
| Anatomic site of melanoma | ||||
| Face, scalp, upper extremities | 84 | 33 | 1 | |
| Trunk | 76 | 121 | 2.7 (1.5-4.9) | 0.001 |
| Lower Extremities | 41 | 54 | 2.2 (1.1-4.1) | 0.018 |
| Solar elastosis score | ||||
| ≥6 | 100 | 43 | 1 | |
| 0 to 5 | 101 | 165 | 1.8 (1.1-3.1) | 0.024 |
| Age at diagnosis | ||||
| ≤ 60 years | 78 | 140 | 1 | |
| > 60 years | 123 | 68 | 0.4 (0.3-0.7)## | <0.001 |
| Australian melanomas n=118** | N=73 | N=45 | ||
| Anatomic site of melanoma | ||||
| Face and scalp | 46 | 15 | 1 | |
| Trunk | 27 | 30 | 2.8 (1.2-6.6) | 0.014 |
| Solar elastosis score | ||||
| 9 or 10 | 19 | 2 | 1 | |
| 0 to 8 | 54 | 43 | 4.3 (0.91-20.0) | 0.067 |
| Age at diagnosis | ||||
| > 60 years | 46 | 20 | 1 | |
| ≤ 60 years | 27 | 25 | 2.1 (0.91-4.8) | 0.085 |
Sample size reduced because of missing values for age and sex
sample size reduced because of missing values for age
95%-CI = 95% confidence interval; both models were adjusted for the confounding effects of gender.
If the >60 years group was used as the reference category, the odds ratio (95%CI) was 2.4 (1.5-3.7).
Note: For the Australian samples, the cutoff used to dichotomize CSD was optimized due to the smaller sample size. For the same cutoff used for the European and Australian samples see Supplementary Table 2.
The contribution of the variables solar elastosis, anatomic site and age is illustrated in the CART analysis (Figs. 1a and 1b). The trees show subgroups represented by boxes whose sizes correspond to the number of represented cases and whose center indicates the proportion of BRAF mutation shown on the x-axis. CART analyses for both cohorts show similar patterns of branching, with the European/American group branching first by CSD, followed by body site and finally by age, while the Australian group, limited by the smaller sample size, showed branching by body site or CSD followed by age.
Figure 1.
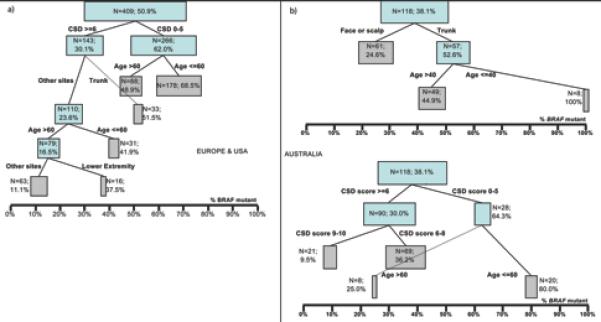
a) Result of classification and regression tree (CART) analysis of 409 melanomas from Europe and North America. b) 118 melanomas from Australia. The % indicates the percentage of BRAF-mutated melanomas in each subgroup. The boxes represent sub-groups of the data; the size of the boxes represent the sample size of the sub-groups; the location of the boxes is according to the % BRAF-mutated melanomas in the sub-groups; final sub-groups were represented by grey-highlighted boxes.
DISCUSSION
Growing evidence indicates that melanoma is a heterogeneous group of biologically distinct disease subsets. Precise understanding of the defining characteristics of different melanoma subcategories is imperative to better understand risk factors, to devise prevention, treatment and follow-up strategies, and to evaluate new therapies. Previous studies revealed that BRAF mutations in melanoma are associated with a particular subtype of melanoma that shows distinctive epidemiologic, clinical, histologic and molecular characteristics. Specifically, BRAF mutations were found to be inversely correlated with patient age (Liu et al., 2007, Maldonado et al., 2003, Shinozaki et al., 2004, Edlundh-Rose et al., 2006) and the degree of UV-exposure, the latter assessed by the amount of solar elastosis (Maldonado et al., 2003, Viros et al., 2008, Landi et al., 2006) or inferred from the anatomic site of the primary tumor (Deichmann et al., 2006, Liu et al., 2007). The close inter-relationships of patient age, anatomic site, and measures of sun-induced damage have made it difficult to segregate their individual associations with (and possible contribution to) BRAF mutations in melanomas. This is because age and the degree of solar elastosis, at least in sun-exposed skin, are closely linked. Solar elastosis reflects cumulative UV exposure and thus develops over the lifetime of an individual as a function of exposure time and sun intensity. Thus in geographic regions with low to moderate sun intensity, elastosis is expected to develop later in life, while solar elastosis is likely to develop significantly earlier in life in geographic regions with high sun intensity such as Australia. The comparison of the two cohorts from geographic regions with different sun intensities thus could help to separate the associations of age with BRAF mutation frequency from those with CSD. As illustrated by the results of the CART analysis (Fig. 1), there is an inverse association between BRAF mutation frequency and degree of solar elastosis by age (Table 1); this association is statistically significant in the US/Europe cohort but not in the Australian cohort, the latter probably due to the smaller sample size (Table 1). However, solar elastosis remained independently associated with BRAF mutation frequency in multivariate analysis including across strata of age groups (Table 3), suggesting that it does have an independent effect. Hence our study confirms that patient age, solar elastosis, and the anatomic site of primary melanoma (with the possible exclusion of the face/scalp in the Australian group) are each independently associated with BRAF-mutation frequency in cutaneous melanoma.
The results of this study should be interpreted with the methods of selection of the study cohorts in mind. The Europe/USA cohorts were selected to be representative of the general populations from which they were derived. In contrast, the Australian cohort was enriched for melanomas from the face without marked solar elastosis and from the trunk with marked solar elastosis, for reasons elaborated in the Methods section. Therefore, it is very difficult to compare the prevalence of findings in these two cohorts, and the results in the Australian cohort may not be applicable to the general Australian population. Study of unselected Australian cohorts is required to further investigate the findings of this study.
The role of UV exposure early in life as a melanoma risk factor is well documented. Decades ago, epidemiologic studies showed that migrants to Australia arriving before the age of 10 years had a melanoma risk similar to that of native-born Australians, whereas the incidence in those arriving later was significantly lower (Cooke and Fraser, 1985, Holman and Armstrong, 1984). A recent pooled analysis of 5700 melanoma cases confirmed childhood sunburns as a risk factor for melanoma development (Chang et al., 2009), and ambient UV exposure early in life has been shown to be associated with the development of BRAF-mutant melanoma.(Thomas et al., 2007) Acquired nevi, which also show frequent BRAF mutations, usually arise during the first two decades of life, supporting the notion that BRAF mutations occur early in life. Our data also demonstrate an independent association of younger age with the presence of BRAF mutations in melanoma. In aggregate, these observations strongly suggest that it is the subset of BRAF-mutant nevi and melanomas that are most associated with effects of UV exposure early in life. However, there are some limitations to this study. For example, we did not analyze NRAS mutation status in our patient cohort, and we were not able to control for phenotypic factors (such as tanning response, tendency to burn, epidemiological measures of cumulative sun exposure, propensity for nevi etc.). Future studies will have to clarify whether BRAF plays a specific role in melanocyte differentiation or proliferation in the developing and growing organism, and whether activating mutations in BRAF explain the specific vulnerability of younger patients with regard to develop melanocytic tumors.
Our data also shows that BRAF mutations are most common on melanomas on the trunk in both cohorts, confirming the findings of previous studies (Maldonado et al., 2003, Viros et al., 2008). This is at least in part due to the fact that the relative proportion of BRAF–mutated melanomas is decreased in highly sun-exposed sites because of an increase of melanomas that arise through other mechanisms and which require high cumulative doses of UV (and therefore is more common in anatomic sites such as the face). These melanomas have been shown to harbor KIT mutations in about 20% of cases, which in general are mutually exclusive of BRAF mutations (Curtin et al., 2006), implicating KIT pathway activation as one characteristic of this other type of melanoma. However, KIT mutations are also more common in melanomas arising in other special sites such as the glabrous skin and mucosa. Therefore other factors such as biological differences between melanocytes of these anatomic regions may play a role in melanoma pathogenesis at these sites (Whiteman et al., 2003, Green, 1992). Some degree of biological difference among melanocytes in different body sites is evident; for example, glabrous sites by definition have no hair and are therefore devoid of a presumed stem cell niche in the bulge region of the hair follicle present on the remainder of the body (Yang and Peng, 2010).
The independent association of high solar elastosis with melanomas without BRAF mutations indicates a pathogenetic role of high cumulative UV exposure for some melanoma types. Solar elastosis is a marker of UV-induced damage, but it may also be a measure of UV tolerance. In this case, low solar elastosis on exposed body sites would represent a phenotypic indicator of sun-avoidance due to a tendency to burn easily (the latter being a consequence of a genetically-impaired tanning response), whereas high solar elastosis would be a phenotypic indicator of intact protection against the immediate consequences (i.e. sunburn) of excessive sun-exposure. In this scenario, BRAF mutations favor individuals with a genetically impaired tanning response reflected by the presence of low solar elastosis. This hypothesis is supported by prior studies finding an association between BRAF mutation status and inherited polymorphisms of the melanonocortin-1 receptor (MC1R) (Landi et al., 2006) in some populations. MC1R is a critical component of the tanning response. However, as the ability to tan is encoded by several genes, the phenotype of low solar elastosis may constitute a more sensitive read-out of the integrity of the tanning response than polymorphisms, even common ones, in a single gene such as MC1R. This may explain why solar elastosis or high cumulative sun-exposure is found to be “protective” of melanoma in some studies (MacKie and Aitchison, 1982, Osterlind et al., 1988, Elwood and Jopson, 1997). High UV-tolerant individuals may thus be at risk for CSD melanomas, but as the chronically exposed body surface area is typically small, the number of melanocytes at risk for transformation is significantly smaller resulting in a comparatively smaller risk.
In conclusion, the results of the present study indicate that patient age, CSD and anatomic site-specific effects independently influence the frequency of BRAF mutations in melanoma.
METHODS
Study cohorts
The study comprised two patient cohorts. The first cohort (from regions with relatively low solar/UV exposure) consisted of 424 primary invasive melanomas from non-glabrous skin of patients from Central Europe and the USA and was assembled in part from cases that have been described in prior studies (Curtin et al., 2005, Landi et al., 2006, Viros et al., 2008) as well as 162 invasive melanomas from patients of the Department of Dermatology in Vienna, Austria. The tumors were consecutive melanomas retrieved from pathology archives. A second cohort (from regions with relatively high solar/UV exposure) of 120 patients with primary invasive melanoma occurring on non-glabrous skin was selected from patients of Melanoma Institute Australia (Sydney, Australia), and was enriched for melanomas from the face without marked solar elastosis and from the trunk with marked solar elastosis. The Australian cohort was selected in this manner in order to enhance the power of the study to address the question of whether anatomic site and sun-damage were independent risk factors for BRAF mutations. Tumors of any thickness were included. All study sites procured samples under approval of their respective Ethics Committees. Anatomic site was determined by the information provided in the pathology request form and then categorized into face and scalp, trunk, upper extremities, and lower extremities.
BRAF mutation analysis
DNA was extracted from micro-dissected paraffin sections and BRAF exon 15 was amplified as described previously (Maldonado et al., 2003). PCR products were purified using ExoSAP-IT® (USB Corporation, Cleveland, OH) and sequenced directly using an ABI PRISM® 3700 DNA Analyzer (Applied Biosystems, Foster City, CA).
Cumulative sun damage
Solar elastosis was scored using an 11-point score as previously described [Landi et al. 2006]. In brief, representative areas were examined at 100-200x magnifications on hematoxylin-and-eosin (H&E) stained sections of the normal skin surrounding the melanomas. The degree of solar elastosis (CSD score) was scored as described in Supplementary Table 1. For statistical analysis scores were recoded as an ordinal scale from 0 to 10.
Statistics
The analysis was stratified by region into melanoma patients from Europe/USA (n=424) and melanoma patients from Australia (n=120). Age of patients was described using arithmetic mean and standard deviation (SD); CSD score distribution was skewed and was therefore described using median and inter-quartile range (IQR). Age was categorized using the quartiles of the distribution. BRAF-mutant and BRAF-non-mutant melanomas were compared with other categorical, continuous and ordinal data using chi-square test, chi-square test for trend, Fisher's exact test, t-test and non-parametric Wilcoxon test, respectively. Comparisons between BRAF and categorized CSD score (0-3, 4-6, 7-10) in various anatomic sites were conducted using exact chi-square tests for trend.
Classification and regression tree (CART) analysis was performed to identify subgroups of the data set that were similar in BRAF mutation status. This was achieved by a stepwise procedure. In the first step, the entire data set was analyzed by chi-square tests of all characteristics against BRAF mutations. The strongest influencing variable was identified and the dataset was divided into subgroups according to this variable. In all subsequent steps of the analysis, the first step was repeated using the newly defined subgroups as the entire data set. The procedure stopped when sample size was too small or no further significant division was identified.
Two multivariable logistic regression analyses were performed to identify independent associations between demographic and tumor-specific factors and BRAF mutation status separately for both cohorts. CSD was dichotomized into CSD<6 and CSD≥6 groups on the basis of the discriminatory value of this cut-off in the CART analysis. All variables were recoded as ordinal numbers in preparation for these analyses. After a logistic model was established using backward and forward selection procedures, confounding was assessed for all variables not participating in the model. Results were presented as odds ratios with 95% confidence intervals (95%CI).
All analyses were performed using SPSS for Windows, version 17.0 (SPSS Inc, Chicago, Illinois, USA).
SIGNIFICANCE.
The association of oncogenic BRAF mutations with low-moderate sun-induced damage may be indirect because the degree of cumulative sun-induced damage is highly correlated with patient age and anatomic site. However, we show that age, solar elastosis, and anatomic site contribute independently to the occurrence of BRAF mutations. The findings are consistent with the view that BRAF-mutated lesions arise early in life at low cumulative UV doses, whereas melanomas without BRAF mutations require accumulation of high UV doses over time resulting in solar elastosis. The effect of anatomic site on the mutation spectrum further suggests regional differences among cutaneous melanocytes.
Supplementary Material
Acknowledgments
Sources of support: Dr. Okamoto is supported by Intendis Austria and the Österreichische Nationalbank (project number 13036). Dr. Scolyer is a Cancer Institute New South Wales Clinical Research Fellow. Dr. Bastian is supported by the National Cancer Institute (RO1-CA131524).
REFERENCES
- Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Risk Factors Of Incident Melanocytic Nevi: A Longitudinal Study In A Cohort Of 1,232 Young German Children. International Journal Of Cancer. 2005;115:121–6. doi: 10.1002/ijc.20812. [DOI] [PubMed] [Google Scholar]
- Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital Melanocytic Nevi Frequently Harbor Nras Mutations But No Braf Mutations. J Invest Dermatol. 2007;127:179–82. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- Chang YM, Barrett JH, Bishop DT, Armstrong BK, Bataille V, Bergman W, Berwick M, Bracci PM, Elwood JM, Ernstoff MS, et al. Sun Exposure And Melanoma Risk At Different Latitudes: A Pooled Analysis Of 5700 Cases And 7216 Controls. Int J Epidemiol. 2009;38:814–30. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke KR, Fraser J. Migration And Death From Malignant Melanoma. Int J Cancer. 1985;36:175–8. doi: 10.1002/ijc.2910360208. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic Activation Of Kit In Distinct Subtypes Of Melanoma. J Clin Oncol. 2006;24:4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, Leboit PE, et al. Distinct Sets Of Genetic Alterations In Melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations Of The Braf Gene In Human Cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Deichmann M, Krahl D, Thome M, Wust K, Hassanzadeh J, Helmke B. The Oncogenic B-Raf V599e Mutation Occurs More Frequently In Melanomas At Sun-Protected Body Sites. Int J Oncol. 2006;29:139–45. [PubMed] [Google Scholar]
- Edlundh-Rose E, Egyhazi S, Omholt K, Mansson-Brahme E, Platz A, Hansson J, Lundeberg J. Nras And Braf Mutations In Melanoma Tumours In Relation To Clinical Characteristics: A Study Based On Mutation Screening By Pyrosequencing. Melanoma Res. 2006;16:471–8. doi: 10.1097/01.cmr.0000232300.22032.86. [DOI] [PubMed] [Google Scholar]
- Elwood JM, Jopson J. Melanoma And Sun Exposure: An Overview Of Published Studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Green A. A Theory Of Site Distribution Of Melanomas. Vol. 3. Cancer Causes Control; Queensland, Australia: 1992. pp. 513–6. [DOI] [PubMed] [Google Scholar]
- Holman CD, Armstrong BK. Cutaneous Malignant Melanoma And Indicators Of Total Accumulated Exposure To The Sun: An Analysis Separating Histogenetic Types. J Natl Cancer Inst. 1984;73:75–82. [PubMed] [Google Scholar]
- Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Epidemiologic Support For Melanoma Heterogeneity Using The Surveillance, Epidemiology, And End Results Program. J Invest Dermatol. 2008;128:1340–2. doi: 10.1038/jid.2008.18. [DOI] [PubMed] [Google Scholar]
- Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, Calista D, Kanetsky PA, Pinkel D, Bastian BC. Mc1r Germline Variants Confer Risk For Braf-Mutant Melanoma. Science. 2006;313:521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- Liu W, Kelly JW, Trivett M, Murray WK, Dowling JP, Wolfe R, Mason G, Magee J, Angel C, Dobrovic A, et al. Distinct Clinical And Pathological Features Are Associated With The Braf(T1799a(V600e)) Mutation In Primary Melanoma. J Invest Dermatol. 2007;127:900–5. doi: 10.1038/sj.jid.5700632. [DOI] [PubMed] [Google Scholar]
- Lucas RM, Ponsonby AL, Dear K, Taylor BV, Dwyer T, Mcmichael AJ, Valery P, Van Der Mei I, Williams D, Pender MP, et al. Associations Between Silicone Skin Cast Score, Cumulative Sun Exposure, And Other Factors In The Ausimmune Study: A Multicenter Australian Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2887–94. doi: 10.1158/1055-9965.EPI-09-0191. [DOI] [PubMed] [Google Scholar]
- Mackie RM, Aitchison T. Severe Sunburn And Subsequent Risk Of Primary Cutaneous Malignant Melanoma In Scotland. Br J Cancer. 1982;46:955–60. doi: 10.1038/bjc.1982.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, Ono T, Albertson DG, Pinkel D, Bastian BC. Determinants Of Braf Mutations In Primary Melanomas. J Natl Cancer Inst. 2003;95:1878–90. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish Case-Control Study Of Cutaneous Malignant Melanoma. Ii. Importance Of Uv-Light Exposure. Int J Cancer. 1988;42:319–24. doi: 10.1002/ijc.2910420303. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al. High Frequency Of Braf Mutations In Nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Rivers JK. Is There More Than One Road To Melanoma? Lancet. 2004;363:728–30. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- Shinozaki M, Fujimoto A, Morton DL, Hoon DS. Incidence Of Braf Oncogene Mutation And Clinical Relevance For Primary Cutaneous Melanomas. Clin Cancer Res. 2004;10:1753–7. doi: 10.1158/1078-0432.ccr-1169-3. [DOI] [PubMed] [Google Scholar]
- Thomas NE, Edmiston SN, Alexander A, Millikan RC, Groben PA, Hao H, Tolbert D, Berwick M, Busam K, Begg CB, et al. Number Of Nevi And Early-Life Ambient Uv Exposure Are Associated With Braf-Mutant Melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991–7. doi: 10.1158/1055-9965.EPI-06-1038. [DOI] [PubMed] [Google Scholar]
- Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, Bastian BC. Improving Melanoma Classification By Integrating Genetic And Morphologic Features. Plos Med. 2008;5:E120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic Nevi, Solar Keratoses, And Divergent Pathways To Cutaneous Melanoma. J Natl Cancer Inst. 2003;95:806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- Yang L, Peng R. Unveiling Hair Follicle Stem Cells. Stem Cell Rev. 2010;6:658–64. doi: 10.1007/s12015-010-9172-z. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Palmedo G, Flaig MJ, Puchta U, Reckwerth A, Rutten A, Mentzel T, Hugel H, Hantschke M, Schmid-Wendtner MH, et al. Mutations Of The Braf Gene In Benign And Malignant Melanocytic Lesions. J Invest Dermatol. 2003;121:1160–2. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


