Abstract
Purpose
The human corneal endothelium has a very low mitotic rate, and with aging there is a decrease in the number of cells. 15-epi-LXA4 is an anti-inflammatory, bioactive lipid formed when aspirin acetylates cyclooxygenease-2 and redirects cyclooxygenease-2 catalytic activity away from prostaglandins. The purpose of the current study was to evaluate the action of 15-epi-LXA4 in the endothelium viability of human corneas stored in Optisol-GS.
Methods
Human corneal endothelial (HCE) cells along with the Descemet's membrane were isolated from fresh human eyes obtained from National Disease Research Interchange. Cell phenotype was identified by using the tight junctions cell marker ZO-1. LXA4 receptor (FPR2/ALX) was detected by immunostaining of HCE cells and human corneal tissue using a polyclonal antibody. Cell proliferation was evaluated with Ki-67 antibody. To measure cell migration, confluent HCE cells were wounded by a linear scraping with a sterile pipette tip in the center of the well and incubated for 24 h with or without 15-epi-LXA4. To evaluate the reparative capacity of 15-epi-LXA4, 7 pairs of human corneas were incubated in Dulbecco's modified Eagle's medium/F12 media at 37°C with or without 100 nM 15-epi-LXA4 for 24 h and then stored at 4°C in Optisol-GS for 12 days. Endothelial viability was assessed by 2 staining techniques: a viability/cytotoxicity kit and trypan blue combined with alizarin red S.
Results
HCE cells and the endothelium of human corneal sections strongly expressed the LXA4 receptor. There was a 3-fold increase in cell proliferation when HCE cells were incubated with 100 nM 15-epi-LXA4 for 24 h. No significant migration was observed after 24 h incubation with 15-epi-LXA4. Corneas incubated for 24 h in Dulbecco's modified Eagle's medium/F12 media in the presence of 15-epi-LXA4 and then stored for 12 days in Optisol-GS had a 36% to 56% increase in viability compared with controls without 15-epi-LXA4.
Conclusions
15-epi-LXA4 is an important mediator that protects the integrity of the human endothelium during corneal preservation in Optisol-GS.
Introduction
The main function of corneal endothelial cells is to provide a barrier to the cornea and maintain tissue transparency. These functions are accomplished through tight junctions and specialized pumps that avoid swelling of the stroma.1 Since human corneal endothelial (HCE) cells have a poor proliferation rate, any disease or trauma that damages them will induce a compensatory enlargement of the remaining cells that could be accompanied by cell dysfunction.
Cornea transplant or keratoplasty is the most common transplanted surgery worldwide. Corneal scarring and keratitis of varied origins (eg, trachoma) are the main complications resulting from corneal transplant in developing countries, whereas keratoconus and corneal edema are the principal causes of penetrating keratoplasty in developed nations.2
A very important factor for a successful corneal transplant is the accessibility to appropriate corneal tissue, as one of the main causes of graft failure is endothelial cell loss.3,4 It has been shown that endothelial cells of transplanted human corneas are lost at higher rates, about 7.8%/year for 3–5 years, compared with about 0.5%/year for normal corneas.5 Late endothelial failure represents 90% of the failures between 5 and 10 years after corneal transplant.6 It is, therefore, important to use donor corneas with sufficient endothelial cell density to decrease the risk of late endothelial failure.
Two approaches to cornea storage are currently in common use: hypothermic and organ culture storage. Hypothermic storage, which is used in the United States, consists of keeping the cornea at 2°C–6°C in a commercially available medium, such as Optisol-GS, that contains (in addition to nutrients) osmotically active substances such as dextran and choidroitin sulfate to maintain the normal physiological thickness and clarity of the cornea.7 This helps decrease cell metabolism and preserve the original condition of the cornea for as long as possible. Although studies claim that cornea storage in Optisol-GS maintains endothelial integrity for 14–16 days,8 degenerative changes still occur. Realistically, the average time stored corneas can be used for transplant is 4 days.3
Corneal storage under organ culture conditions is used in European countries. This approach consists of maintaining the tissue in a culture medium supplemented with fetal bovine serum (FBS), antibiotics, and antimytotics between 31°C and 37°C. The purpose of organ culture is to maintain cellular metabolism and viability as closely as possible to physiological conditions. In these conditions, the corneas can be stored for up to 35 days,9 but the method is technically more difficult, requiring careful control for contaminations and time for the cornea to spend in a de-swelling media before transplantation.
Lipoxins (LX) are lipid mediators derived from arachidonic acid by the action of a 15-lipoxygenease (15-LOX).10 15-epi-LXA4 [5(S), 6(R), 15(R)-trihydroxy-7, 9, 13-trans-11cis eicosatetraenoic acid] is a bioactive lipid formed when aspirin acetylates cyclooxygenease-2 and redirects catalytic activity away from prostaglandins. The main effect of LXA4 and 15-epi-LXA4 is to promote resolution of inflammation by inhibiting superoxide generation and polymorphonuclear leukocyte transmigration and to promote uptake of cellular debris, microbes, and apoptotic polymorphonuclear leukocytes by macrophages.11,12 Both LXA4 and 15-epi-LXA4 act through a receptor (FPR2/ALX), a classical 7 transmembrane G-protein coupled receptor.13,14 The advantage of 15-epi-LXA4 is that it is more stable than LXA4 and, therefore, has a longer bio-half life than LXA4.15 We found that the human corneal endothelium expresses FPR2/ALX. Therefore, the purpose of the current study was to investigate whether 15-epi-LXA4 has a protective effect in the preservation of the endothelium of human corneas stored in Optisol-GS media.
Methods
Materials
Dulbecco's modified Eagle's medium (DMEM)/F12, antibiotic-antimycotic (100×) solution, FBS, bovine pituitary extract, phosphate-buffered saline (PBS), Hanks' balanced salt solutions, Live/Dead Viability/Citotoxicity Kit, monoclonal mouse anti-ZO-1, and the secondary antibodies goat anti-mouse Alexa Fluor 488 and donkey anti-goat Alexa Fluor 488 were purchased from Invitrogen (Carlsbad, CA). Trypan blue solution (0.4%), alizarin red S, gentamicin solution, epidermal growth factor, goat serum, 4’, 6-diamidino-2-phenylindole dihydrochloride (DAPI), and insulin were obtained from Sigma (Saint Louis, MO). Optisol-GS was purchased from Bausch & Lomb (Rochester, NY). Aspirin-triggered lipoxin A4 (15-epi-LXA4) was from EMD Chemicals (Gibbstown, NJ). Monoclonal mouse Ki-67 antibody was from Dako North America (Carpinteria, CA). Goat polyclonal anti-LXA4-receptor (FPR2/ALX) antibody was from Santa Cruz (Santa Cruz, CA). FNC coating mix was from AthenasES (Baltimore, MD).
HCE cell culture
To prepare endothelial cell cultures, twelve human eyes were obtained from the National Disease Research Interchange (Philadelphia, PA). Donor age ranged from 40 to 79 years old, and postmortem time ranged from 2 to 6 h. The eyes were sent in a wet chamber with enough ice to keep them between 0°C and 4°C during the shipping. After arriving, the eyes were washed with Hanks' balanced salt solutions containing a 3×antibiotic-antimycotic mix. The corneas were carefully excised under a dissecting microscope, leaving 1 mm of sclera. The Descemet's membrane with the attached endothelium was removed from the stroma and cut into small pieces for explant culture. The pieces were placed endothelium-side down in 6-well plates, which had been coated with FNC Coating Mix, and cultured in complete medium (DMEM/F12, supplemented with 15% FBS, 5 μg/mL insulin, 10 ng/mL epidermal growth factor, 100 μg/mL bovine pituitary extract, 50 μg/mL gentamicin, and antibiotic antimycotic solution diluted 1:100). The explants were incubated at 37°C in a 5% CO2 humidified atmosphere for 2 weeks. The medium was changed every 3 days. After the primary cultures reached confluence, cells were sub-cultured at a 1:3 split ratio. Only the first passage of HCE cell was used for the experiments.
Immunofluorescence staining
HCE cells, sub-cultured in 12-well plates, were fixed with 2% paraformaldehyde for 30 min at room temperature, blocked with 10% goat serum and 1% bovine serum albumin, and permeabilized with 0.2% Triton X-100 in PBS for 10 min. Cells were incubated overnight at 4°C with primary antibodies (FPR2/ALX, 1:200 dilution; ZO-1, 1:500 dilution; Ki-67, 1:500 dilution). Next, they were incubated with the appropriate secondary antibodies Alexa Fuor 488 for 1 h at 4°C. DAPI was performed to stain the nuclei. After each step, the cells were washed thrice with PBS. Cells were examined with a fluorescence microscope (Nikon, Tokyo, Japan), and the images were captured with a Photometric camera (Cool Snap HQ, Tucson, AZ). No staining was observed when the primary antibodies were omitted.
To detect the expression of FPR2/ALX receptor in corneal tissue, human corneas were fixed in 4% paraformaldehyde, embedded in optimal cutting temperature compounds and 6-μm serial cryostat sections were prepared, air-dried, and stored at −80°C. Immunofluorescence staining for the receptor was performed as just described using goat anti-FPR2/ALX polyclonal antibody.
Cell proliferation
HCE cells were sub-cultured at 60% confluence, then starved for 24 h in DMEN/F12, and incubated for an additional 24 h in the presence of different concentrations of 15-epi-LXA4 dissolved in ethanol. The same amount of ethanol (0.1%) was used in controls without LXA4. Cell proliferation was assessed by immunofluorescence staining with mouse monoclonal Ki-67 antibody as just explained, and proliferation was expressed as a percentage of Ki-67-positive cells versus the total cells stained with DAPI.
Cell migration
HCE cells were sub-cultured in 12-well plates to complete confluence and starved in DMEM/12 media without supplementation for 24 h. The cultures were wounded by a linear scraping in the center of the well with a sterilized pipette tip and then washed twice to remove all loose or dead cells. Plates were carefully checked under the microscope to confirm complete removal of the cells and photographed (0 h). Afterward, the cells were incubated in DMEN/F12 for 24 h at 37°C with 100 nM 15-epi-LXA4. Cell migration was observed under a phase-contrast microscope (Nikon), and images were captured with a Photometrics camera (Cool Snap HQ). Cell migration was quantified by measuring the width of the wounded area at 24 h and compared with 0 h.
Human corneal storage
For these experiments, 7 pairs of human corneas obtained from National Disease Research Interchange were used. Donor age ranged from 32 to 88 years old, and the postmortem time was between 1.5 and 16.5 h. One cornea of each pair was incubated in 8 mL of DMEN/F12 (control) at 37°C with 5% CO2, and the other cornea of the pair was incubated in the same medium in the presence of 100 nM 15-epi-LXA4. After 24 h, the corneas were transferred into 20 mL Optisol-GS with 15-epi-LXA4 or ethanol and stored for 12 days at 4°C. For this purpose, a suture was placed through the sclera rim and attached to the lid of the vial so that the corneas were hanging vertically in the medium. All manipulations were performed under sterile conditions in a laminar flow hood. In addition, one pair of human corneas was directly stored in Optisol-GS with 100 nM 15-epi-LXA4 or ethanol for 12 days.
Assessment of endothelial damage
The evaluation of damage of HCE cells was assessed with a Live/Dead Viability/Cytotoxicity Kit according to the manufacturer's protocol or by the combination of 2 stains: trypan blue and alizarin red S, which stain the denude Descemet's membrane and the cell border. For the staining procedure, the corneas were placed endothelial side up, and 0.4% trypan blue was added drop wise to cover the endothelium. After 90 s, the stain was poured off, and the corneas were briefly washed with PBS. The endothelial layer was then covered with alizarin red S (0.2%, pH 4.2) for 90 s and again washed with PBS. Afterward, the corneas were fixed in 95% ethanol for 2 min.
Four radial incisions were performed on each cornea, and the tissue was mounted on a slide with the endothelium side down. The damaged endothelium was assessed using a fluorescence- or phase-contrast microscope. Endothelial cell damage was expressed as the percentage of areas of cells stained with trypan blue and areas devoid of cells over the total area in consideration when trypan blue-alizarin red staining was used, or the percentage of areas of endothelial cell loss over the total area when the Live/Dead Kit was used. The areas were calculated using Adobe Photoshop program, the perimeter of uncovered area was marked, and the number of pixels was determined. The percentage of damage was evaluated in 7 to 10 fields/cornea and compared between pairs of corneas from the same donor.
To determine the proliferation in these conditions, an additional pair of corneas was sectioned and prepared for immunostaining with Ki-67 antibody.
Statistical analysis
The significance of the data from all experiments was evaluated using the Student's t-test. P<0.05 was considered statistically significant.
Results
FPR2/ALX is strongly expressed in the corneal endothelium
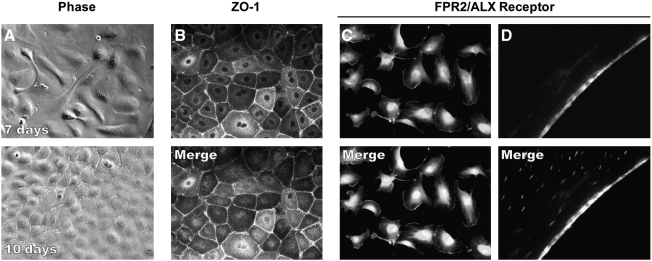
Ten days postculture, confluent HCE cells showed the typical endothelial cell polygonal mosaic morphology (Fig. 1A). Immunostaining with ZO-1 antibody demonstrated the proper location of this tight junction associated protein, implying that HCE cells in culture were able to recruit ZO-1 to the cell border (Fig. 1B). The FPR2/ALX receptor was strongly expressed in the cell membrane as well as around the nuclei (Fig. 1C). Frozen section of human corneas also showed a strong positive staining in the endothelial layer (Fig. 1D).
FIG. 1.
Expression of FPR2/ALX receptor in corneal endothelium. (A) HCE cells cultured as explained in Methods section were proliferated at 7 days, and by 10 days the cells reached confluence. (B) Confluent HCE cells expressed ZO-1, a tight junction-associated protein, showing endothelial cells boundaries in a polygonal pattern. (C) FPR2/ALX receptor is strongly expressed in HCE cells in culture. (D) Human corneal sections showing a strong positive staining for FPR2/ALX in the endothelium. The lower figures in B–D show the nuclei of the cells with 4’, 6-diamidino-2-phenylindole dihydrochloride staining. HCE, human corneal endothelial.
15-epi-LXA4 stimulates HCE cell proliferation but does not affect migration
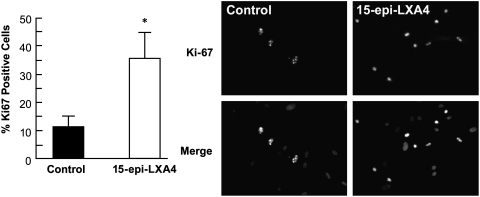
Since the lipoxin receptor is strongly expressed in HCE cells, we decided to investigate the role of 15-epi-LXA4 on corneal endothelial proliferation and migration. Cell proliferation was evaluated by immunostaining with Ki-67 antibody, a marker for actively cycling cells. 15-epi-LXA4 promotes HCE cell proliferation in a dose-dependent fashion (Fig. 2). There was a 3-fold increase in proliferation compared with controls after 24 h stimulation with 100 nM 15-epi-LXA4. Similar results were obtained using porcine corneal endothelial cells in culture (data not shown).
FIG. 2.
15-epi-LXA4 stimulates endothelial cell proliferation. First passage of HCE cells derived from a 40-year-old donor were plated in a 12-well plate and grow to 60%–70% confluence. After starving for 24 h, the cells were incubated in Dulbecco's modified Eagle's medium/F12 with different concentrations of 15-epi-LXA4 for 24 h at 37°C. Then, the cells were immunostained with Ki-67 monoclonal antibody as explained in Methods section. Cell proliferation was expressed as the percentage of Ki-67 positive cells versus the total cells. The bars represent the average±SD of 10 fields randomly chosen, *P<0.05 compared with control. The experiment was repeated 4 times.
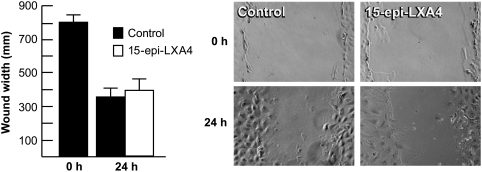
To study the role of 15-epi-LXA4 on HCE cell migration, a simple cell wound model was used, where the cells were injured by a linear scrape in the center of the well as explained in Methods section. After 24 h, there was an approximate 50% reduction of the wounded area. No significant difference in migration between control and 100 nM 15-epi-LXA4-treated cells was found (Fig. 3).
FIG. 3.
15-epi-LXA4 has no effect on endothelial cell migration. First passage of HCE cells derived from a 63-year-old donor were cultured to complete confluence. After starving for 24 h, a linear wound was made as explained in Methods section. Then, the cells were incubated in Dulbecco's modified Eagle's medium/12 with or without 100 nM 15-epi-LXA4 for 24 h at 37°C. Cells were observed under the microscope and photographed. The migration was expressed as wound width, calculated with an Adobe Photoshop program. Each bar represents the average±SD of 8 pictures of areas randomly chosen. The experiment was repeated thrice with similar results.
Effect of 15-epi-LXA4 on endothelial preservation of human corneas
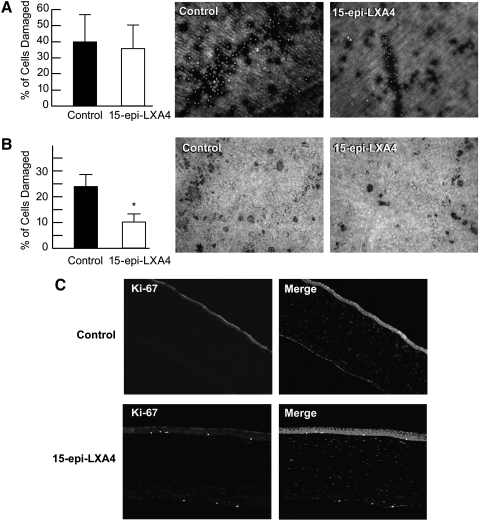
To determine the action of 15-epi-LXA4 in the endothelium of corneas after storage, one pair of human corneas was kept in Optisol-GS at 4°C for 12 days with 100 nM LXA4 or ethanol. A second pair was cultured for 24 h at 37°C in DMEM/12 with or without 100 nM 15-epi-LXA4 and then preserved in Optisol-GS for 12 days at 4°C. No significant differences in cell damage with or without 100 nM 15-epi-LXA4 were found when the corneas were stored directly in Optisol-GS (Fig. 4A). However, corneas incubated in DMEM/12 in the presence of 100 nM 15-epi-LXA4 for 24 h and then stored for 12 days in Optisol-GS showed a significant decrease in endothelial damage (Fig. 4B). In these conditions, cell damage decreased from >23% in storage without 15-epi-LXA4 to 11% when 15-epi-LXA4 was added.
FIG. 4.
Decrease in endothelia cell damage in corneas stored in Optisol-GS after organ cultured with 15-epi-LXA4. (A) A pair of corneas from a 47-year-old donor was stored in 20 mL Optisol-GS with or without 100 nM 15-epi-LXA4 for 12 days at 4°C as explained in Methods section. At the end of storage, the corneas were stained with a Live/Dead Viability/Cytotoxicity Kit. A fluorescence microscope was used to evaluate the cell damage. There were areas of endothelial cell loss with multiple red fluorescent cells representing dead cells; 8–10 pictures were taken from each cornea and the average±SD of the damaged area was calculated. (B) A pair of corneas from a 58-year-old donor was organ cultured with or without 100 nM 15-epi-LXA4 for 24 h at 37°C. Afterward, the corneas were transferred to Optisol-GS with or without 15-epi-LXA4 and stored for 12 days at 4°C. The endothelial cell damage was detected with a combination of 2 stains: trypan blue and alizarin red S. The percentage of cell damage was calculated as explained in Methods section. (C) One pair of corneas from a 59-year-old donor incubated as in (B) was fixed with 4% paraformaldehyde, embedded with optimal cutting temperature (OCT), cut into 6 μm serial sections, and immunostained with Ki-67 antibody. Ki-67 positive cells are shown in the endothelium. *Significant difference with respect to control.
A third pair of human corneas was incubated as described in Fig. 4B after tissues were sectioned and immunostained with the proliferation marker Ki-67. Positive staining was observed when the corneas were treated with 15-epi-LXA4 (Fig. 4C).
Table 1 summarizes the results of 6 pairs of human corneas incubated in DMEM/F12 with or without 100 nM 15-epi-LXA4 for 24 h at 37°C and then stored in Optisol-GS with or without 15-epi-LXA4 for 12 days at 4°C. Cell damage was assessed by the combination of trypan blue-alizarin red S staining as explained in Methods section. Endothelial cell damage decreased between 36% and 56% in the presence of 100 nM 15-epi-LXA4. Although all pairs of corneas showed a significant decrease in endothelial cell damage with 15-epi-LXA4, postmortem preservation time and possible aging seemed to influence the protective effect of 15-epi-LXA4.
Table 1.
Decrease in Endothelial Cell Damage in Human Corneas Stored in Optisol-GS After Incubation with 15-epi-LXA4
| |
|
|
|
Endothelial damage |
|
|
|---|---|---|---|---|---|---|
| Age | Gender | Postmortem Time (h) | Preservation in wet chamber (h) | Control | 15-epi-LXA4 | Decrease in damage (%) |
| 32 | M | 12 | 43 | 19±6 | 8±1.6a | 43 |
| 35 | M | 12 | 23 | 21±8 | 10±3.0a | 52 |
| 52 | F | 16.5 | 27.5 | 14±5 | 9±3.8a | 36 |
| 58 | F | 6 | 32 | 23±5.7 | 11±2.6a | 51 |
| 65 | F | 1.5 | 42.5 | 18±3.2 | 10±2.1a | 56 |
| 88 | F | 4 | 44 | 16±3.8 | 9±1.8a | 44 |
Pairs of human corneas from the same donor were preincubated in Dulbecco's modified Eagle's medium/F12 media with or without 100 nM 15-epi-LXA4 for 24 h at 37°C, then stored in Optisol-GS with or without 15-epi-LXA4 for 12 days at 4°C. Endothelial cell damage was evaluated by a combination of trypan blue-alizarin red S staining.
Significant differences with regard to control.
Discussion
One of the main causes of graft failure is endothelial cell loss due to inadequate tissue preservation. It is well documented that endothelial cells in transplanted human corneas are lost at a higher rate than normal corneas.5,6 The cause is not clear, but it had been suggested that breakdown of the blood-ocular barrier and an increase in the inflammatory response could cause an apoptotic response.16
Here we show that incubation for 24 h with 15-epi-LXA4, a lipid mediator involved in the resolution of inflammation, stimulates the proliferation of human endothelial cells without affecting the migration. Human endothelial cells have very limited proliferation and are arrested in the G1 phase of the cell cycle.17 Several methods have been used to enhance HCE cell proliferation, such as addition of growth factors to the cultured media, EDTA, to destroy the tight junctions and viral oncogene transformation.18–22 All of these techniques have limitations, for example, long-term safety of transfected HCE cells, low efficiency, and so on. In a more recent report, HCE cells were treated with mouse embryonic-stem cell conditioned media, which increase cell proliferation.23 The authors postulate that the media promote cell-cycle entrance, in part, by down regulation of p21 expression. Although interesting, the extract derived from mouse could be a health risk factor in clinical application.
We found a strong expression of FPR2/ALX by immunofluorescence in the human corneal endothelium and in the isolated cells. An earlier study had reported mRNA expression of FPR2/ALX in epithelial cells derived from human corneas.24 A few recent studies have shown that LXA4 stimulates corneal epithelial wound healing,25,26 is a potent inhibitor of corneal neovascularization by directing inhibiting vascular endothelial growth factor,27,28 and reduces inflammation in endotoxin-induced uveitis models.29,30 To our knowledge, this is the first study showing an effect of a lipoxin in corneal endothelial cells.
We took advantage of the selective proliferative action of 15-epi-LXA4 in HCE cells to investigate whether the lipid mediator can be used to improve cornea storage. We found that corneas incubated with 100 nM 15-epi-LXA4 for 24 h in a basal media containing inorganic salts, amino acids, glucose, and vitamins without serum are enough to promote proliferation and enhance endothelial cell density after 12 days in Optisol-GS. This preincubation at 37°C is fundamental for the action of 15-epi-LXA4, as corneas stored directly in Optisol-GS with LXA4 did not show differences from controls. This suggests that at lower temperatures (2°C–6°C) 15-epi-LXA4 will not activate the FPR2/ALX receptor to promote the signaling mechanisms that induce HCE cell survival and proliferation. The short time used in this study for corneas in organ culture was not only enough to stimulate the proliferation by 15-epi-LXA4 but also allowed us to avoid the period of deswelling used in corneas incubated at 37°C. It has been shown that the presence of dextran in the deswelling media may induce endothelial alterations.31 In addition, decrease of endothelial cell density between 5% and 10% has been found after longer periods of organ culture storage.32
In the conditions of our experiments, corneas stored for 12 days in Optisol-GS after a 24 h period in organ culture with 15-epi-LXA4 had a decrease in cell damage that ranged from 36% to 66%. Increase in postmortem time seems to have an effect in the repair process by 15-epi-LXA4. In addition, we must point out that the corneas in this study were kept in a moist chamber from 23 to 44 h. Studies of corneas stored at 4°C in a moist chamber for 2 days reported significant endothelial damage33; our corneas after 24 h in organ culture and 12 days in Optisol-GS showed between 14% to 23% of endothelial damage when stained with trypan blue. This time did not seem to affect the repair process stimulated by 15-epi-LXA4.
Eye banks report the need to discard considerable amounts of tissue with low endothelial cell density.34,35 Our study reports that 15-epi-LXA4 could be useful to increase the time of storage and improve endothelial cell density, helping to minimize waste of donor tissue.
Acknowledgments
This work was supported by National Institutes of Health, National Eye Institute grant EY004928.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Edelhauser H.F. The balance between corneal transparency and edema: the Proctor lecture. Invest. Opthalmol. Vis. Sci. 2006;47:1754–1767. doi: 10.1167/iovs.05-1139. [DOI] [PubMed] [Google Scholar]
- 2.Bimbaun F. Reinhard T. Bohhringer D., et al. Endothelial cell loss after autologous rotational keratoplasty. Graefes Arch. Clin. Exp. Opthalmol. 2005;243:57–59. doi: 10.1007/s00417-004-0902-2. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelmus K.R. Stulting R.D. Sugar J. Khan M.M. Primary corneal graft failure. A national reporting system. Medical Advisory Board of the Eye Bank Association of America. Arch. Opthalmol. 1995;113:1497–1502. doi: 10.1001/archopht.1995.01100120027002. [DOI] [PubMed] [Google Scholar]
- 4.Clerhout I. Beele H. Kestelyn P. Graft failure: I. Endothelial loss. Int. Opthalmol. 2008;28:165–173. doi: 10.1007/s10792-007-9087-0. [DOI] [PubMed] [Google Scholar]
- 5.Bourne W.M. Cellular changes in transplanted human corneas. Cornea. 2001;20:560–569. doi: 10.1097/00003226-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bourne W.M. Nelson L.R. Hodge D.O. Central corneal endothelial cell changesover a ten-year period. Invest. Opthalmol. Vis. Sci. 1997;38:779–782. [PubMed] [Google Scholar]
- 7.Pels E. Beekhuis H. Volker-Dieben H.J. Long term tissue storage for keratoplasty. In: Brighbill F.S., editor. Corneal Surgery, Theory, Technique and Tissue. 3rd. St Louis: Mosby, Inc.; 1999. pp. 897–906. [Google Scholar]
- 8.Lindstrom R.L. Kaufman H.E. Skelnik D.L., et al. Optisol corneal storage medium. Am. J. Opthalmol. 1992;114:345–356. doi: 10.1016/s0002-9394(14)71803-3. [DOI] [PubMed] [Google Scholar]
- 9.Nejepinska J. Juklova K. Jirsova K. Organ culture, but not hypothermic storage, facilitates the repair of the corneal endothelium following mechanical damage. Acta. Opthalmol. 2010;88:413–419. doi: 10.1111/j.1755-3768.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 10.Serhan C.N. Lipoxin and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell–cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 11.Serhan C.N. Gotlinger K. Hong S. Arita M. Resolvins, docosatrienes and neuroprotectins, novel ω-3derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Serhan C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang N. Serhan C. Sven-Erik D., et al. The lipoxin receptor ALX: a potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 14.Ye R.D. Boulay F. Wang J.M., et al. International Union of Basic and Clinical Pharmacology. LXXIII nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takano T. Fiore S. Maddox J.F., et al. Aspirin-triggered 15-Epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J. Exp. Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armitage W.J. Dick A.D. Bourne W.M. Predicting endothelial cell loss and long term corneal graft survival. Invest. Opthalmol. Vis. Sci. 2003;44:3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 17.Joyce N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 18.Wilson S.E. Walker J.W. He Y.G. McCash C.S. Extended life of human corneal endothelial cells transfected with the SV40 large T antigen. Invest. Opthalmol. Vis. Sci. 1993;34:2112–2123. [PubMed] [Google Scholar]
- 19.Feldman S.T. Gjerset R. Gately D., et al. Expression of SV40 virus large antigen by recombinant adenoviruses activates proliferation of corneal endothelium in vitro. J. Clin. Invest. 1993;91:1713–1720. doi: 10.1172/JCI116381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pistov M.Y. Sadovnikova E. Danilov S.M. Human corneal endothelial cells: isolation, characterization and long term cultivation. Exp. Eye Res. 1988;47:403–414. doi: 10.1016/0014-4835(88)90051-6. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone E.W. Wong H.C. Coster D.J. Williams K.A. Factors affecting bovine corneal endothelial cell density in vitro. Br. J. Opthalmol. 1996;80:256–262. doi: 10.1136/bjo.80.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senoo T. Obara Y. Joyce N.C. EDTA: a promoter of proliferation in human corneal endothelium. Invest. Opthalmol. Vis. Sci. 2000;41:2930–2935. [PubMed] [Google Scholar]
- 23.Lu X. Chen D. Liu Z., et al. Enhanced survival in vitro of human corneal endothelial cells using mouse embryonic stem cell conditioned medium. Mol. Vis. 2010;16:611–622. doi: 10.1167/2.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronert K. Gewirtz A. Madara J.L. Serhan C.N. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interlukin (IL)-13 and interferon γ and inhibits tumor necrosis factor α-induced IL-8 release. J. Exp. Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronert K. Masheshwari N. Khan N. Hassan I.R. Dunn M. Schwartzman M.L. A role for the mouse 12/15-lipoxygenease pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 26.Biteman B. Hassan I.R. Walker E., et al. Interdependence of lipoxin A4 and heme-oxygenease in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y. Arita M. Zhang Q., et al. Anti-angiogenesis effect of the novel anti-inflammatory and proresolving lipid mediators. Invest. Opthalmol. Vis. Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leedom A.J. Sullivan A.B. Dong B., et al. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madeiros R. Rodrigues G.B. Figueiredo C.P., et al. Molecular mechanisms of topical anti-inflammatory effects of lipoxin A(4) in endotoxin-induced uveitis. Mol. Pharmacol. 2008;74:154–161. doi: 10.1124/mol.108.046870. [DOI] [PubMed] [Google Scholar]
- 30.Karim M.J. Bhattacherjee P. Biswas S. Paterson C.A. Anti-inflammatory effects of lipoxins on lipopolysaccharide-induced uveitis in rats. J. Ocul. Pharmacol. Ther. 2009;25:483–486. doi: 10.1089/jop.2008.0134. [DOI] [PubMed] [Google Scholar]
- 31.Borderie V.M. Baudrimont M. Lopez M., et al. Evaluation of the deswelling period in dextran-containig medium after corneal organ culture. Cornea. 1997;16:215–223. [PubMed] [Google Scholar]
- 32.Pels E. Schuchard Y. Organ culture preservation of human corneas. Doc. Opthalmol. 1983;15:147–153. doi: 10.1007/BF00154722. [DOI] [PubMed] [Google Scholar]
- 33.Means T.L. Geroski D.H. Hadley A., et al. Viability of human corneal endothelium following Optisol-GS storage. Arch. Opthalmol. 1995;113:805–809. doi: 10.1001/archopht.1995.01100060131047. [DOI] [PubMed] [Google Scholar]
- 34.Armitage W.J. Easty D.L. Factors influencing the suitability of organ-cultures corneas for transplantation. Invest. Opthalmol. Vis. Sci. 1997;38:16–24. [PubMed] [Google Scholar]
- 35.Engelman K. Bednarz J. Valtink M. Prospects for endothelial transplantation. Exp. Eye Res. 2004;78:573–578. doi: 10.1016/s0014-4835(03)00209-4. [DOI] [PubMed] [Google Scholar]