Abstract
Purpose
Endothelial cell proliferation in angiogenesis is active in conditions such as cancers and diabetic retinopathy. Tamoxifen (T) and raloxifene (R) have been compared in numerous studies as a prophylaxis for breast cancer, and T is used to treat breast cancer. T, unlike R, has been linked to an increase in uterine cancers, thrombo-embolic events, and cataract. The purpose of our study was to evaluate the efficacies of T and R in reducing estrogen-induced retinal capillary endothelial cell proliferation.
Methods
Rhesus monkey retinal capillary endothelial cells (ATCC RF/6A) were used to assay cell proliferation when treated with 0.0, 0.1, 1.0, and 10.0 nM 17 β estradiol (E2) for 24 and 48 h. Viable cells were counted using a Neubauer hemocytometer with a trypan blue exclusion method to determine the number of viable cells. Cell counts were also performed using 1.0 nM E2 with 0.01, 0.1, 1.0, and 10.0 nM concentrations of either T or R. Cell medium, collected at 24 h, was evaluated for vascular endothelial growth factor and pigment epithelium-derived factor.
Results
Viable cells were significantly greater in cultures treated with 1.0 or 10.0 nM E2, compared to cells treated with 0.0 or 0.1 nM E2 both at 24 and 48 h. Viable cell counts were reduced significantly in cultures treated with 0.1, 1.0, or 10.0 nM T or R in addition to the 1.0 nM E2. Cell counts were not significantly different when comparing equal concentrations of T and R, that is, 1.0 nM E2+1 nM T or R. Vascular endothelial growth factor and pigment epithelium-derived factor protein/10,000 cells was reduced by 1.0 nM E2, but returned to higher levels with the introduction of T and R to growth media.
Conclusions
T and R showed similar potency in inhibiting estrogen-induced retinal capillary endothelial cell proliferation. Considering drug safety profiles, our results, when extended to animals and humans, suggest that R is preferable to T in treating angiogenic retinal diseases. Further studies on the signaling mechanism of estrogen-induced endothelial cell proliferation may lead to new treatment strategies in the treatment of ocular angiogenic diseases.
Introduction
Sex hormones, such as estrogen, have long been investigated as a contributing factor in proliferative diseases such as breast cancer.1–3 In 1937 Lacassagne suggested “that a therapeutic antagonist to the congestion of oestrone in the breast should be found to prevent breast cancer.3” However, it was not until 1962 that Jensen and associates proposed an assay to determine the presence of estrogen receptor (ER) and suggested that it be used to determine which breast cancers would be responsive to estrogen.4 The first nonsteroidal anti-estrogens were discovered in the 1950s.5 The selective estrogen receptor modulator (SERM) tamoxifen was first described as a treatment for breast cancer by Cole and associates in 1971.6
SERMs, such as tamoxifen (T) and raloxifene (R), can act as either an ER antagonist or agonist, depending on the cell type, and the tissue environment.7 Their actions can be either genomic or nongenomic. T has been approved by the U.S. FDA for reducing the incidence of breast cancer in women at high risk for developing the disease, and in the treatment of metastatic breast cancer.8 While it has been used for almost 40 years as a treatment for estrogen-sensitive breast cancer it was studied as a breast cancer preventative by the National Surgical Adjuvant Breast and Bowel Project and the results were published in 1998.9 R (Evista©) was originally approved (1997) for the treatment and prevention of postmenopausal bone loss and, more recently, for reducing the risk of invasive breast cancer in postmenopausal women with osteoporosis as well as in postmenopausal women at high risk for invasive breast cancer.10 R was originally developed to treat breast cancer, but was found to be less effective than T for that usage.11,12 Both T and R inhibit cell proliferation in breast tissue, but only R inhibits cell proliferation in the uterus; in fact, tamoxifen has been linked to an increase in uterine cancer.13 Recently, the Study of Tamoxifen and Raloxifene (STAR), a large clinical study done by the National Surgical Adjuvant Breast and Bowel Project, was completed in 2006, along with a follow-up publication in 2010, which compared the preventative effects of T versus R in the risk of developing invasive breast cancer.12,14 STAR was a double-blind study in which 19,747 women at risk for breast cancer were randomly assigned to use either T 20 mg/day or R 60 mg/day for 5 years.14 The update extended the time period to a median 81 months.12 The initial STAR study reported that R was as effective as T in reducing the risk of developing invasive breast cancer, but had a lower risk of endometrial cancers, hysterectomy, thrombo-embolic events, and cataract. Zhang and colleagues in 1994 speculated that T increased cataract formation by nongenomic blocking of chloride channels, which are essential for maintaining normal lens hydration and clarity.15 Investigation of R at 60 or 120 mg/day compared to placebo in a study of 7,705 women for a mean of 3.3 years has demonstrated no increased risk of cataract.16 The updated STAR report demonstrated a slightly higher risk of invasive breast cancer with R when compared to T, but once again revealed an enhanced safety profile with R as was demonstrated in the first study. R was reported to maintain 76% of the effectiveness of T, which correlates to a 38% reduction in invasive breast cancer compared to no treatment.12 This can be compared to a 50% reduction when T usage is compared to placebo.12 Even though T had been investigated for use as a breast cancer preventative in high-risk patients in 1998, very few primary care physicians have prescribed it in this fashion. One conclusion of the initial STAR study and its 2010 update was that physicians might consider prescribing R as a preventative in those patients at a high risk of developing invasive breast cancer, due to its favorable side-effect profile, even though it was slightly less effective than T. Typical human steady-state drug plasma concentrations in individuals taking 20 mg/day of T are about 328 nM/L and with R at 60 mg/day are about 3 nM/L.8,17
There is evidence that estrogen plays a role in the development of angiogenic eye diseases, such as proliferative diabetic retinopathy (PDR). Both ERα and ERβ have been identified in human retina.18,19 In fact, Ogueta and colleagues in 1999 found that ERα concentrations decreased with age (35>49>74 years) in women, whereas males had ERα levels that were between those of 49- and 74-year-old women.20 In addition, some studies do report a difference in the occurrence of PDR, which can be attributed to puberty.18,21,22 Olsen and colleagues in 2004 found that in type 1 diabetes, the duration of the postpubertal diabetes contributed twice more than the duration of the prepubertal diabetes toward the development of retinopathy.22 A case study of agonadal female twins showed that retinopathy did not develop after a long duration of very poorly controlled diabetes, that is, A1c's (glycolyslated hemoglobin) as high as 15.6%.23 Further, it is often found that diabetic retinopathy worsens during pregnancy.24 In 1999 Suzuma and colleagues, using cultures of bovine retinal endothelial cells, found an increase in the amount of total DNA in those cell cultures exposed to 17β estradiol (E2) in their growth media, compared to those not exposed to E2, and further discovered that this increase in the amount of total DNA could be blocked by the presence of T in the cell media.25 These results suggest that the level of E2 may play a role in the development of retinal angiogenic eye diseases.
Vascular endothelial growth factor (VEGF) is well established as a controller of the angiogenic process and has been linked to retinal angiogenesis.26,27 Suzuma and colleagues in 1999 using bovine retinal microvascular endothelial cells and Mueller and colleagues in 2000 using human primary and Ishikawa uterine cells have shown an increase in VEGF levels linked to E2 after a 24 h exposure.25,28
Pigment epithelium-derived factor (PEDF) is a 50-kDa secreted glycoprotein first found to be secreted by retinal pigment epithelial cells.29,30 PEDF has been shown to be the most potent inhibitor of angiogenesis in the mammalian eye, suggesting that decreased levels of PEDF may be a factor in the development of angiogenic eye diseases such as PDR.31,32 PEDF levels have also been found to be positively linked to retinal oxygen concentrations which have been found to be inversely related to VEGF concentrations in a balance controlling angiogenesis.33 Cheung and colleagues in 2006 found that ER is an important upstream regulator of PEDF in human ovarian cancer and ovarian surface epithelial cells.34 They found that E2 reduced PEDF levels, and that this process could be prevented by the total ER antagonist, ICI 182,780.
R has a better safety profile than T overall and T also shows an increased risk of cataract.9,12,14 If R treatment achieves a reduction in estrogen-induced cell proliferation similar to T, the use of R to treat proliferative retinal disease may be more beneficial. The purpose of our study was to evaluate, using retinal capillary endothelial cells, whether R and T have a similar potency to inhibit E2-induced cell proliferation and cell secretion of angiogenic cytokines VEGF and PEDF.
Methods
Culture of retinal capillary endothelial cells
Rhesus monkey retinal capillary endothelial cells (RhREC) were purchased from American Type Culture Collection (ATCC) catalog number CRL-1780 (RF/6A). These RhREC (Macaca mulatta) cells spontaneously transformed at an early passage and have a history of being used in scientific investigation.35,36 They were used between passages 36 and 40. Once received, the RhREC were grown in Minimum Essential Medium Alpha (Gibco #1061029) containing L-glutamine, ribonucleosides, and deoxyribonucleosides without phenol red, with 10% fetal bovine serum and a final concentration of 50 IU/mL penicillin with 50 μg/mL streptomycin (Cellgro #30-001), in a humidified atmosphere of 5% CO2 in air at 37°C. While treating cells, regular fetal bovine serum in the growth medium was replaced with 10% charcoal stripped fetal bovine serum (Gibco #12676-011) to deprive the cells of hormones. 17β Estradiol (E2), tamoxifen (T), and raloxifene (R) were prepared by dissolving them in dimethyl sulfoxide. These stocks were serially diluted with cell media to obtain the final concentrations of E2, T, and R. The amount of dimethyl sulfoxide present in the final concentrations was about 1 part in 10,000,000 and was found to have no effect on cell numbers (results not shown).
Viable cell counts and measurement of cytokine levels in cell media
To determine whether the presence of E2 had an effect on cell growth, RhREC were cultured in 24-well plates at a seeding density of 20,000 cells/mL and allowed to proliferate in 1 mL medium containing 0.0, 0.1, 1.0, or 10.0 nM E2. After 24 h, cell medium was collected and then cells were harvested using 200 μL of trypsin and 800 μL of media. Cells were counted using a Neubauer hemocytometer with a trypan blue exclusion method to determine the number of viable cells per mL in each group.
To quantify rhesus monkey VEGF and PEDF in the medium, ELISAs for human VEGF (Invitrogen) and human PEDF (Millipore) were employed according to manufacturer's instructions. The BLAST database was used to determine that M. mulatta VEGF is 99% identical to human VEGFA, and that M. mulatta PEDF is 82% identical to human PEDF.37 Absorbance was measured at 450 nm using a Dynex plate reader and analyzed using Revelation 4.25 software. Concentrations of VEGF and PEDF were quantified using standard absorbance curves.
E2-induced RhREC proliferation: time- and dose-dependency experiments
RhREC were cultured in 24-well plates at a seeding density of 20,000 cells/mL in 1 mL treatment medium containing 0.0, 0.1, 1.0, or 10.0 nM E2 to determine the effects of time and E2 concentration. The medium was changed daily. Cells were harvested at the end of 24 and 48 h for cell counting. Conditioned media were also collected for measurement of cytokine levels at 24 h.
T and R inhibition of E2-induced cell proliferation and cytokine release
To approximate conditions in which E2 and an SERM would both be present, experiments were carried out in which 0.01, 0.1, 1.0, or 10.0 nM R, or 0.01, 0.1, 1.0, or 10.0 nM T were present along with 1.0 nM E2 in the growth medium. RhREC were cultured at a seeding density of 20,000 cells/mL, with 1 mL treatment medium in 24-well plates and allowed to proliferate for 24 h in each of the treatment groups. Cells were counted in a Neubauer hemocytometer with a trypan blue exclusion method to determine the total number of viable cells. Percent inhibition in the T and R groups was determined as the decrease in cell number from the 1.0 nM E2 group divided by the total difference in cell number between the 0.0 and 1.0 nM E2 group. Conditioned media were collected for ELISA determination of VEGF and PEDF levels.
Statistical methods
Data were analyzed using ANOVA and a priori comparisons between treatment groups. A P value ≤0.05 was considered to be statistically significant. All experiments were repeated a minimum of 3 times with triplicate wells.
Results
E2-induced RhREC proliferations: E2 dosage dependency
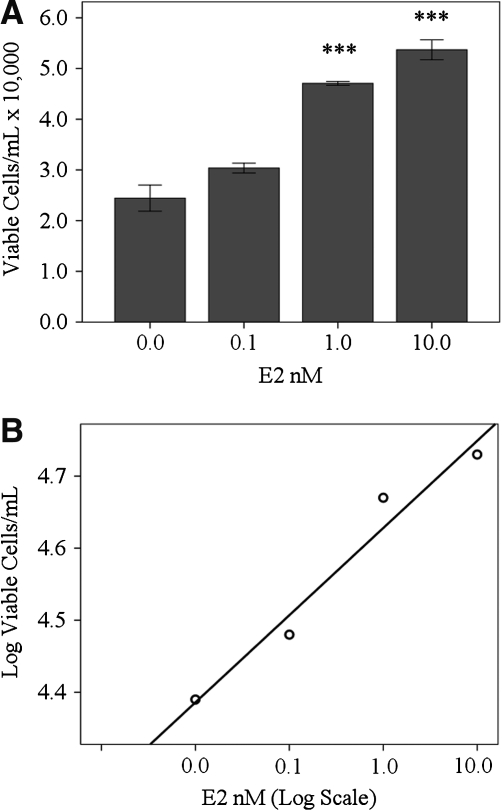
RhREC treated in media containing 0.0, 0.1, 1.0, or 10.0 nM E2 for 24 h were counted to determine viable cell counts (Fig. 1A). Total viable cell numbers were 24% greater when treated with 0.1 nM E2 (30,300 cells/mL), 93% greater when treated with 1.0 nM E2 (47,000 cells/mL, SD±6,300), P<0.001, and 120% greater when treated with 10.0 nM E2 (53,700 cells/mL), P<0.001, compared to cells grown in 0.0 nM E2 (24,400 cells/mL). Viable cell counts with 1.0 nM E2 (55% greater) and 10.0 nM E2 (77% greater) were significantly greater in number than those grown in 0.1 nM E2, P<0.001, P<0.001. A double log plot of the increase in the log of the viable cell counts compared with increasing levels in the log concentrations of E2 (0.0, 0.1, 1.0, and 10.0 nM) revealed a linear increase in viable cell number (about 12,000 cells) with each log unit increase in E2 concentration (Fig. 1B), (y=0.121(log x)+4.628; R2=0.962).
FIG. 1.
Effect of E2 dosage on RhREC proliferations. (A) RhREC were seeded at a density of 20,000 cells/mL and treated with E2 for 24 h. Cells exposed to 1.0 and 10.0 nM E2 were significantly greater in number than those exposed to 0.0 nM E2, (P<0.001,∗∗∗), (P<0.001,∗∗∗), and significantly greater in number than those exposed to 0.1 nM E2, (P<0.001,∗∗∗), (P<0.001,∗∗∗). (B) A double-log plot demonstrates a linear relation of log cell counts with log E2 concentration (0.0, 0.1, 1.0, and 10.0 nM), (R2=0.962, y=0.21(log x)+4.628). Mean±standard error (n=3) from one experiment is shown. Similar experiments were completed 5 times with equivalent results. RhREC, Rhesus monkey retinal capillary endothelial cells.
E2-induced RhREC proliferations: time dependency
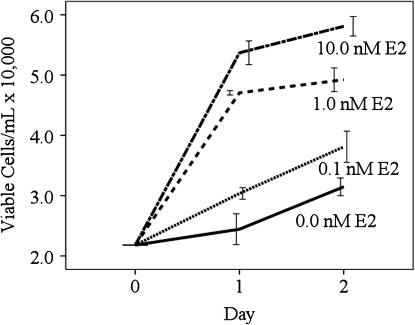
RhREC treated with 0.0, 0.1, 1.0, or 10.0 nM E2 were counted at 24 and 48 h to measure the increase in the number of viable cells (Fig. 2). At 24 h, viable cell counts were significantly greater when cells treated with 1.0 or 10.0 nM E2 were compared to those treated with 0.0 nM E2 or 0.1 nM E2 as described above. At 48 h, viable cell counts were 21% greater when treated with 0.1 nM E2 (38,100 cells/mL), 56% greater when treated with 1.0 nM E2 (49,200 cells/mL), P<0.01, and 85% greater when treated with 10.0 nM E2 (58,100 cells/mL), P<0.001, compared to those treated with 0 nM E2 (31,500 cells/mL). Viable cell counts with 1.0 (29%) and 10.0 nM E2 (52%) were greater in number than those treated with 0.1 nM E2, P<0.05, P<0.001.
FIG. 2.
Time-dependent increase in E2-induced RhREC proliferation. RhREC were cultured in 24-well plates at a seeding density of 20,000 cells/mL in media containing 0.0, 0.1, 1.0, or 10.0 nM E2 to determine the effects of time and E2 concentration. Total viable cell counts were determined at 24 and 48 h. At 24 h, viable cell counts were again significantly greater when cells were grown in cell media containing 1.0 or 10.0 nM E2 compared to those grown in 0.0 nM E2 or 0.1 nM E2. At 48 h, cell counts were also significantly greater in cells grown in 1.0 nM E2, (P<0.01,∗∗), and 10.0 nM E2, (P<0.001,∗∗∗), compared to those treated with 0 nM E2. Viable cell counts with 1.0, (P<0.05,∗), and 10.0 nM E2, (P<0.001,∗∗∗), were significantly greater in number than those grown in 0.1 nM E2. Mean±standard error (n=3) from one experiment is shown. Similar experiments were completed 3 times with equivalent results.
E2-induced RhREC proliferation: inhibition by T and R
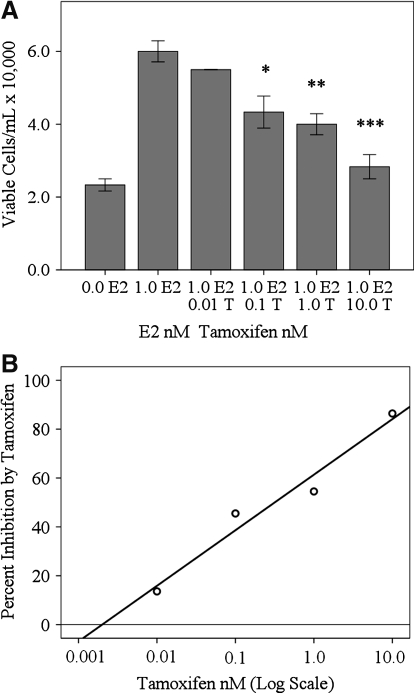
Viable cell counts were reduced by 13.6% when cells were treated with 0.01 nM T and 1.0 nM E2 (55,000 cells/mL), 45% when cells were treated with 0.1 nM T and 1.0 nM E2 (43,300 cells/mL), P<0.05, 55% when cells were grown in 1.0 nM T and 1.0 nM E2 (40,000 cells/mL), P<0.01, and 86% when cells were treated with 10.0 nM T and 1.0 nM E2 (28,300 cells/mL), P<0.001, compared to those treated with 1.0 nM E2 alone (60,000 cells/mL±5,000) (Fig. 3A). Semi-log plots (Fig. 3B) measuring percent inhibition by using 0.01, 0.1, 1.0, and 10.0 nM T revealed a linear increase (23%) in the level of T inhibition of E2-induced cell proliferation with each log unit increase in the T concentration (Fig. 3B); (y=22.74 (log x)+61.37; R2=0.961).
FIG. 3.
Tamoxifen inhibition of E2-induced RhREC proliferation. RhREC were seeded at a density of 20,000 cells/mL and viable cell counts were carried out after a 24 h treatment. Cells were treated with 0.0 and 1.0 nM E2 and also with media containing 1.0 nM E2 with 0.01, 0.1, 1.0, or 10.0 nM T (A and B). (A) Cell proliferation was inhibited by each increasing concentration of T, which reached significance, 0.1 nM T, (P<0.05,∗), 1.0 nM T, (P<0.01,∗∗), and 10.0 nM T, (P<0.001,∗∗∗). (B) A semi-log plot measuring percent inhibition resulting from the use of T in the cell media along with 1.0 nM E2 revealed a linear increase in percent inhibition with the log of T concentration. Mean±standard error (n=3) from one experiment is shown in (A). Similar experiments were completed 3 times with equivalent results.
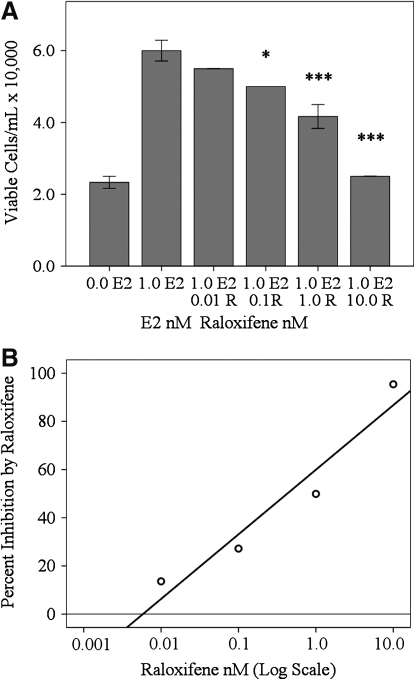
Viable cell counts were reduced by 13.6% when cells were treated with 0.01 nM R and 1.0 nM E2 (55,000 cells/mL), 27% when cells were treated with 0.1 nM R and 1.0 nM E2 (50,000 cells/mL), P<0.05, 50% when cells were treated with 1.0 nM R and 1.0 nM E2 (41,700 cells/mL), P<0.001, and 95% when cells were grown in 10.0 nM R and 1.0 nM E2 (25,000 cells/mL), P<0.001, compared to those treated in 1.0 nM E2 alone (60,000 cells/mL, SD±5,000) (Fig. 4A). Semi-log plots measuring percent inhibition using 0.01, 0.1, 1.0, and 10.0 nM R revealed a linear increase (27%) in the level of R inhibition of E2-induced cell proliferation with each log unit increase in R concentration (Fig. 4B); (y=26.81(log x)+59.93; R2=0.932).
FIG. 4.
Raloxifene inhibition of E2-induced RhREC proliferation. RhREC were seeded at a density of 20,000 cells/mL and viable cell counts were carried out after a 24 h treatment. Cells were treated with 0.0 and 1.0 nM E2 and also with media containing 1.0 nM E2 with 0.01, 0.1, 1.0, or 10.0 nM R (A and B). (A) Cell proliferation was inhibited by each increasing concentration of R, which reached significance, 0.1 nM R, (P<0.05,∗), 1.0 nM R, (P<0.001,∗∗∗), and 10.0 nM R, (P<0.001,∗∗∗). (B) A semi-log plot measuring percent inhibition resulting from the use of R in the cell comma along with 1.0 nM E2 revealed a linear increase in percent inhibition with the log of R concentration (nM). Mean±standard error (n=3) from one experiment are shown in (A). Similar experiments were completed 3 times with equivalent results.
There was no significant difference in the potency of T and R inhibitions on E2-induced cell proliferation, based on the slope of the semi-log plots of T and R inhibitions on E2-induced cell proliferations (ie, increase of 23% inhibition per log unit increase of T concentrations versus increase of 27% inhibition per log unit increase of R concentrations; see text above).
E2-induced decrease in VEGF and PEDF levels in cell media
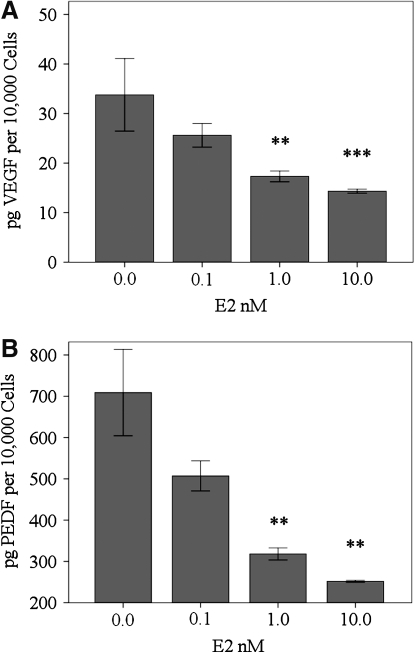
VEGF levels in the cell media were determined with RhREC treated with 0.0, 0.1, 1.0, or 10.0 nM E2. VEGF was reduced by 24% when cells were treated with 0.1 nM E2 (25.6 pg/10,000 cells), 49% when cells were treated with 1.0 nM E2 (17.3 pg/10,000 cells), P<0.01, and 58% when cells were treated with media containing 10.0 nM E2 (14.3 pg/10,000 cells), P<0.001, compared to cells treated with 0.0 nM E2 (33.8 pg/10,000 cells) (Fig. 5A). PEDF levels in the cell media were determined with RhREC grown in 0.0, 0.1, 1.0, or 10.0 nM E2. PEDF was reduced by 28% when cells were treated with 0.1 nM E2 (507 pg/10,000 cells), 55% when cells were treated with 1.0 nM E2 (318 pg/10,000 cells), P<0.01, and 64% when cells were treated with 10.0 nM (252 pg/10,000 cells) E2, P<0.01, compared to cells treated with 0.0 nM E2 (709 pg/10,000 cells) (Fig. 5B).
FIG. 5.
Effect of E2 on VEGF and PEDF level in Cell Media. RhREC were grown for 24 h in a medium containing 0.0, 0.1, 1.0, or 10.0 nM E2. ELISA was performed and the cell medium was used to quantify VEGF (A) and PEDF (B) in the cell medium. (A) VEGF levels were significantly less in the medium of cells grown in a cell medium containing 1.0 nM E2, (P<0.01,∗∗), or 10.0 nM E2, (P<0.001,∗∗∗), compared with those grown in 0.0 nM E2. (B) PEDF levels were significantly less in the medium of cells grown in a growth medium containing 1.0 nM, (P<0.01,∗∗), or 10.0 nM E2, (P<0.01,∗∗), compared with those grown in 0.0 nM E2. Mean±standard error (n=3) from one experiment is shown. Data are normalized based on the number of viable cells in the assay. Cytokine concentrations/10,000 cells were determined by dividing the VEGF or PEDF concentration in each individual well by the cell count to find cytokine concentration/cell and then multiplying this number by 10,000. Similar experiments were completed 3 times with equivalent results. VEGF, vascular endothelial growth factor; PEDF, pigment epithelium-derived factor.
E2-induced decrease in VEGF and PEDF: effect of T and R
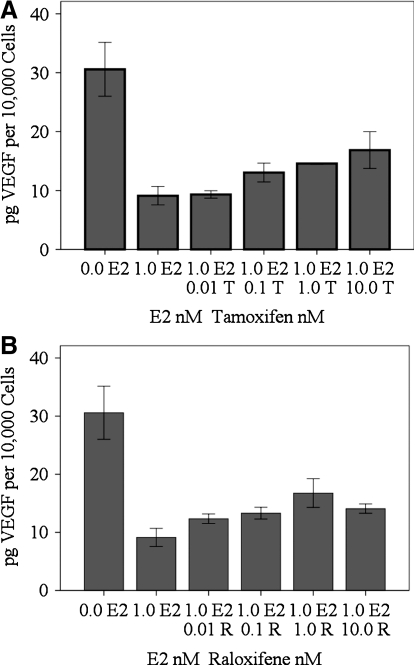
There was no difference between RhREC treated with 0.01 nM T plus 1.0 nM E2 (9.3 pg/10,000 cells) and RhREC treated with 1.0 nM E2 without T (9.1 pg/10,000 cells). VEGF was increased by 43% when cells were treated with 0.1 nM T and 1.0 nM E2 (13.1 pg/10,000 cells), 60% when cells were treated with 1.0 nM T and 1.0 nM E2 (14.6 pg/10,000 cells), and 85% when cells were treated with 10.0 nM T and 1.0 nM E2 (16.9 pg/10,000 cells), compared to those treated with 1.0 nM E2 alone (Fig. 6A). VEGF levels were increased by 35% when RhREC were treated with 0.01 nM R and 1.0 nM E2 (12.3 pg/10,000 cells), 46% when cells were treated with 0.1 nM R and 1.0 nM E2 (13.3 pg/10,000 cells), 84% when cells were treated with 1.0 nM R and 1.0 nM E2 (16.8 pg/10,000 cells), and 54% when cells were treated with 10.0 nM R and 1.0 nM E2 (14 pg/10,000 cells), compared to those treated with 1.0 nM E2 alone (9.1 pg/10,000 cells) (Fig. 6B). Although there was a trend toward greater VEGF levels with increasing levels of T or R, none of these increases were statistically significant. The addition of T and R did not restore the level of VEGF to control (0.0 E2, 30.6 pg/10,000 cells).
FIG. 6.
Effect of T and R on E2-induced changes in VEGF levels in cell media. RhREC were grown for 24 h in a medium containing either 0.0 or 1.0 nM E2 alone, or 1.0 nM E2 with either 0.01, 0.1, 1.0, or 10.0 nM T (A) or R (B). ELISA was performed and the cell medium was used to quantify VEGF. (A) VEGF levels increased with increasing concentrations of T, but did not reach a level of significance compared to VEGF produced by cells grown in 1.0 nM E2 alone. (B) VEGF levels increased with increasing concentrations of R, but did not reach the level of significance compared to VEGF produced by cells grown in 1.0 nM E2 alone. Mean±standard error (n=3) from one experiment is shown. Data are normalized based on the number of viable cells in the assay. Cytokine concentrations/10,000 cells were determined by dividing the VEGF concentration in each individual well by the cell count to find cytokine concentration/cell and then multiplying this number by 10,000. Similar experiments were completed 3 times with equivalent results.
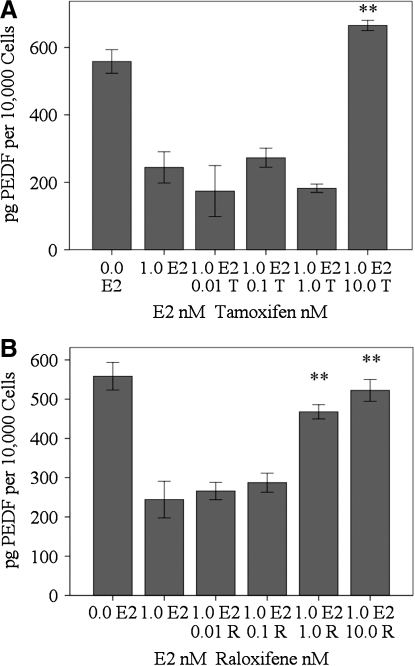
PEDF levels were not changed when RhREC were treated with 0.01 (173 pg/10,000 cells), 0.1 (272 pg/10,000 cells), or 1.0 nM T (182 pg/10,000 cells) with 1.0 nM E2 compared to 1.0 nM E2 alone (244 pg/10,000 cells). PEDF was increased by 172% when cells were treated with 10.0 nM T and 1.0 nM E2 (665 pg/10,000 cells), P<0.01, compared to those treated with 1.0 nM E2 alone (Fig. 7A). PEDF levels were not changed when RhREC were treated with either 0.01 (266 pg/10,000 cells) or 0.1 nM R (287 pg/10,000 cells) with 1.0 nM E2, compared to media from cells treated with 1.0 nM E2 (244 pg/10,000 cells) alone. PEDF was increased by 92% when cells were treated with 1.0 nM R and 1.0 nM E2 (468 pg/10,000 cells), P<0.01, and 114% when cells were treated with 10.0 nM R and 1.0 nM E2 (522 pg/10,000 cells), compared to those treated with 1.0 nM E2 alone, P<0.01 (Fig. 7B). T and R at 10 nM restored PEDF levels to control (0.0 E2, 558 pg/10,000 cells).
FIG. 7.
Effect of T and R on E2-induced changes in PEDF levels in cell media. RhREC were grown for 24 h in a medium containing either 0.0 or 1.0 nM E2 alone or 1.0 nM E2 with 0.01, 0.1, 1.0, or 10.0 nM T (A) or R (B). ELISA was performed and the cell medium was used to quantify PEDF. (A) PEDF levels were significantly greater in the 1.0 nM E2 group with 10.0 nM R group compared to the 1.0 nM E2 alone group (P<0.01,∗∗). (B) PEDF levels were significantly increased in the 1.0 nM E2 with 1.0 nM R, (P<0.01,∗∗), and in the 1.0 nM E2 group with 10.0 nM R group, (P<0.01,∗∗), compared to the 1.0 nM E2 alone group. Mean±standard error (n=3) from one experiment is shown. Data are normalized based on the number of viable cells in the assay. Cytokine concentrations/10,000 cells were determined by dividing the PEDF concentration in each individual well by the cell count to find cytokine concentration/cell then multiplying this number by 10,000. Similar experiments were completed 3 times with equivalent results.
Discussion
It has been determined that E2 levels range from 0.2 to 3 nM in postpubescent females and slightly <0.2 nM in postpubescent males.38,39 The results obtained in this study indicate that E2 at 0.1, 1.0, and 10.0 nM increased cell proliferation in RhREC compared to 0.0 nM E2 and increased cell proliferation induced by 1.0 nM E2 is blocked by either T or R at 0.1, 1.0, or 10.0 nM concentrations. This is in agreement with Suzuma and colleagues 1999 work with primary retinal capillary bovine cells, in which increased cell proliferation induced by E2 was blocked with T.25 We are the first to demonstrate that R is as effective as T in reducing the E2-induced proliferation in a primate retinal endothelial cell line. SERMs are an interesting category of pharmaceuticals, in that they may stimulate some estrogen-modulated activities while blocking others.40,41 Since SERMs can stimulate or depress estrogen effects depending on the type of ER or co-activators present in a particular cell, a SERM, or a co-activator blocker might be designed to suppress only that ER found in the retinal microvascular endothelial cell environment.7
Previous comparisons between T and R are of interest. While the 1988 work by Buzdar and colleagues pointed out that a drug with “fewer side effects” than T was needed, R was ineffective in their group of 14 patients with disseminated breast cancer who had demonstrated primary or secondary resistance to T in a phase II study.11 Hopes were raised by the conclusions of the initial STAR study that perhaps R was a drug with “fewer side effects,” which could be as effective as T in preventing breast cancer in high-risk individuals.14 Even though the STAR update found that R was effective, although not quite as effective as T, in reducing breast cancer, its superior safety profile compared to T made it attractive to primary care physicians who had been reluctant to prescribe T for their high-risk patients.12 Results from our cell study on T and R inhibition of E2-induced cell proliferations suggest that T and R have similar potency to block estrogen-mediated retinal angiogenesis. This conclusion is not only consistent with the STAR study on T and R inhibition on estrogen effect in high-risk cancer patients, but also suggests that in vitro cell study may be appropriate as a model for drug potency before animal research and clinical studies.
Retinal angiogenesis is a much studied, but not fully understood, orderly process by which retinal capillary endothelial cells proliferate and migrate through a damaged vessel basement membrane and form tubules that can circulate blood.42,43 This pattern is a response of the retina to hypoxia following vascular damage as can occur with long-term diabetes. A similar process of cell proliferation is found in other angiogenic retinal or choroidal diseases of different etiologies such as neovascular age-related macular degeneration. Newly formed vessels are prone to leak and hemorrhage due to a reduced number of pericytes and incomplete intercellular junctions.44 SERM reduction of retinal capillary endothelial cell proliferation in vitro using T or R suggests a means of controlling retinal or choroidal angiogenesis. However, once again, the safety profile of T has perhaps discouraged its investigation for this use in the eye, especially considering that T has also been correlated with cataract formation.15 R has not been linked to cataract formation.12,14,16
Results from the present study using RhREC show that E2 induced a reduction of the level of VEGF and PEDF. Suzuma and colleagues25 reported an increase of VEGF in response to E2 treatment of bovine retinal endothelial cells, and Mueller and colleagues28 reported an increase in VEGF in human endometrial cells in response to E2. Gao and colleagues found PEDF to be inversely related to VEGF levels in the retina of Brown Norway rats with oxygen-induced retinopathy.33 In our experiments, RhREC were tested under normoxic conditions. The variables in our study were not how cells would respond to hypoxia, but how do these primate retinal capillary endothelial cells respond to E2 exposure and ER blockage. These E2-induced decreases in PEDF may be mitigated by T and R. However, the relative level of change (in response to T and R) does not exactly correspond to our cell growth patterns. T and R (co-treated with E2) restored the PEDF level (at a higher T and R concentration of 10.0 nM) to that of the control (0.0 E2). However, T and R did not similarly restore VEGF levels. Therefore, it is likely that different cytokine pathways may be involved in E2-induced proliferation of RhREC.
In summary, we studied the effect of E2 alone and in combination with either T or R on the proliferation of RhREC. We found that E2 at normal physiological levels does increase the proliferation of these cells and that this proliferation is effectively inhibited (with similar potency) by the addition of either T or R to the cell media. Further animal and clinical studies should reveal the efficacy of T and R treatment for E2-induced angiogenic eye diseases. Detailed biochemical studies will elucidate how T and R inhibit E2 receptors that would lead to cell signaling and cytokine release.
Acknowledgments
We thank Albert Muniz, Brandi Betts, Aditi Mukherjee, Ernest Heimsath, Shohreh Kalhor, Eileen Kotchan Vidro, and Pamela Forbes for their help in the completion of this project. We also thank the Kronkosky Charitable Foundation, a Faculty Development Grant (UTPB), Ashbel Smith Professorship (DMA), SALSI (San Antonio Life Science Alliance), and, RCMI grant (2G12RR013646-11), NIH Grant (GM08194) for the generous support of our work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Love R.R. Philips J. Oophorectomy for breast cancer: history revisited. J. Natl. Cancer Inst. 2002;94:1433–1434. doi: 10.1093/jnci/94.19.1433. [DOI] [PubMed] [Google Scholar]
- 2.Beatson G.T. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 3.Lacassagne A. Hormonal pathogenesis of adenocarcinoma of the breast. Am. J. Cancer. 1936;27:217–225. [Google Scholar]
- 4.Jensen E.V. On the mechanism of estrogen action. Perspect. Biol. Med. 1962;6:47–59. doi: 10.1353/pbm.1963.0005. [DOI] [PubMed] [Google Scholar]
- 5.Lerner L.J. Holthaus F.J., Jr. Thompson C.R. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958;63:295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- 6.Cole M.P. Jones C.T. Todd I.D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br. J. Cancer. 1971;25:270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C.L. O'Malley B.W. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 8.Tamoxifen. Drugs @ FDA: U.S. Food and Drug Administration Web site. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. [2010]. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist
- 9.Fisher B. Costantino J.P. Wickerham D.L., et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 10.Evista. Drugs@FDA: U.S. Food and Drug Administration Web site. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. [2010]. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist
- 11.Buzdar A.U. Marcus C. Holmes F. Hug V. Hortobagyi G. Phase II evaluation of Ly156758 in metastatic breast cancer. Oncology. 1988;45:344–345. doi: 10.1159/000226637. [DOI] [PubMed] [Google Scholar]
- 12.Vogel V.G. Costantino J.P. Wickerham D.L., et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev. Res. (Phila Pa) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuzick J. Forbes J. Edwards R., et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 14.Vogel V.G. Costantino J.P. Wickerham D.L., et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J.J. Jacob T.J. Valverde M.A., et al. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J. Clin. Invest. 1994;94:1690–1697. doi: 10.1172/JCI117514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grady D. Ettinger B. Moscarelli E., et al. Safety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluation. Obstet. Gynecol. 2004;104:837–844. doi: 10.1097/01.AOG.0000137349.79204.b8. [DOI] [PubMed] [Google Scholar]
- 17.Evista. Eli Lilley Web site. Highlights of prescribing information. Web Page. http://pi.lilly.com/us/evista-pi.pdf. [2010]. http://pi.lilly.com/us/evista-pi.pdf
- 18.Krolewski A.S. Warram J.H. Rand L.I. Christlieb A.R. Busick E.J. Kahn C.R. Risk of proliferative diabetic retinopathy in juvenile-onset type 1 diabetes: a 40-yr. follow-up study. Diabetes Care. 1986;9:443–452. doi: 10.2337/diacare.9.5.443. [DOI] [PubMed] [Google Scholar]
- 19.Giddabasappa A. Bauler M. Yepuru M. Chaum E. Dalton J.T. Eswaraka J. 17-beta estradiol protects ARPE-19 cells from oxidative stress through estrogen receptor-beta. Invest. Ophthalmol. Vis. Sci. 2010;51:5278–5287. doi: 10.1167/iovs.10-5316. [DOI] [PubMed] [Google Scholar]
- 20.Ogueta S.B. Schwartz S.D. Yamashita C.K. Farber D.B. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest. Ophthalmol. Vis. Sci. Aug. 1999;40:1906–1911. [PubMed] [Google Scholar]
- 21.Murphy R.P. Nanda M. Plotnick L. Enger C. Vitale S. Patz A. The relationship of puberty to diabetic retinopathy. Arch. Ophthalmol. 1990;108:215–218. doi: 10.1001/archopht.1990.01070040067032. [DOI] [PubMed] [Google Scholar]
- 22.Olsen B.S. Sjolie A.K. Hougaard P., et al. The significance of the prepubertal diabetes duration for the development of retinopathy and nephropathy in patients with type 1 diabetes. J. Diabetes Complicat. 2004;18:160–164. doi: 10.1016/S1056-8727(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 23.Bell D.S.H. Lack of long-term diabetic complications in spite of poor glycemic control in twins with pure gonadal dysgenesis. Diabetes Care. 1995;18:1286–1287. doi: 10.2337/diacare.18.9.1286. [DOI] [PubMed] [Google Scholar]
- 24.Moloney J.B. Drury M.I. The effect of pregnancy on the natural course of diabetic retinopathy. Am. J. Ophthalmol. 1982;93:745–756. doi: 10.1016/0002-9394(82)90471-8. [DOI] [PubMed] [Google Scholar]
- 25.Suzuma I. Mandai M. Takagi H., et al. 17 Beta-estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 1999;40:2122–2129. [PubMed] [Google Scholar]
- 26.Aiello L.P. Avery R.L. Arrigg P.G., et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 28.Mueller M.D. Vigne J.L. Minchenko A. Lebovic D.I. Leitman D.C. Taylor R.N. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10972–10977. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tombran-Tink J. Chader G.G. Johnson L.V. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp. Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 30.Tombran-Tink J. Johnson L.V. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest. Ophthalmol. Vis. Sci. 1989;30:1700–1707. [PubMed] [Google Scholar]
- 31.Takenaka K. Yamagishi S. Jinnouchi Y. Nakamura K. Matsui T. Imaizumi T. Pigment epithelium-derived factor (PEDF)-induced apoptosis and inhibition of vascular endothelial growth factor (VEGF) expression in MG63 human osteosarcoma cells. Life Sci. 2005;77:3231–3241. doi: 10.1016/j.lfs.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 32.Dawson D.W. Volpert O.V. Gillis P., et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 33.Gao G. Li Y. Zhang D. Gee S. Crosson C. Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 34.Cheung L.W. Au S.C. Cheung A.N., et al. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology. 2006;147:4179–4191. doi: 10.1210/en.2006-0168. [DOI] [PubMed] [Google Scholar]
- 35.Brar V.S. Sharma R.K. Murthy R.K. Chalam K.V. Evaluation of differential toxicity of varying doses of bevacizumab on retinal ganglion cells, retinal pigment epithelial cells, and vascular endothelial growth factor-enriched choroidal endothelial cells. J. Ocul. Pharmacol. Ther. 2009;25:507–511. doi: 10.1089/jop.2009.0028. [DOI] [PubMed] [Google Scholar]
- 36.Amrite A.C. Ayalasomayajula S.P. Cheruvu N.P. Kompella U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006;47:1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NCBI. Entrez, The Life Sciences Search Engine. Web Page. www.ncbi.nlm.nih.gov/sites/gquery. [2011]. www.ncbi.nlm.nih.gov/sites/gquery
- 38.Katzung B.G., editor. Lange Basic and Clinical Pharmacology. 10th. San Francisco, CA: McGraw Hill Medical; 2007. [Google Scholar]
- 39.Wilson J.D., editor; Foster D.W., editor; Kronenberg H.M., editor; Larsen P.R., editor. Williams Textbook of Endocrinology. 9th. Toronto, Ontario, Canada: W.B. Saunders; 1998. [Google Scholar]
- 40.Osborne C.K. Zhao H. Fuqua S.A. Selective estrogen receptor modulators: structure, function, and clinical use. J. Clin. Oncol. 2000;18:3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 41.Manni A., editor; Verderame M., editor. Selective Estrogen Receptor Modulators: Research and Clinical Applications. 1st. Totowa, NJ: Humana Press; 2002. [Google Scholar]
- 42.Rudnicka A.R. Birch J. Diabetic Eye Disease. 1st. Oxford, England: Butterworth/Heinemann; 2000. [Google Scholar]
- 43.Harmey J., editor. VEGF and Cancer. Georgetown, TX: Landes Bioscience; 2004. [Google Scholar]
- 44.Garner A. Histopathology of diabetic retinopathy in man. Eye. 1993;7(Pt 2):250–253. doi: 10.1038/eye.1993.58. [DOI] [PubMed] [Google Scholar]