Abstract
Rationale
Cocaine and opioids are often co-abused. As yet, however, there is no clear evidence that the drugs interact to make the mixture a more effective reinforcer.
Objective
The present study examined the relative reinforcing potency and maximum effectiveness of the cocaine–opioid combination in monkeys given a choice between cocaine–opioid mixtures and the single-component drugs.
Method
Rhesus monkeys were allowed to choose between injections of cocaine (100 μg/kg/inj) and other doses of cocaine (10–560 μg/kg/inj) or remifentanil (0.03–3.0 μg/kg/inj). A dose-addition model was used to select dose combinations for mixtures of cocaine and remifentanil predicted to be equivalent to 100 μg/kg/inj of cocaine in reinforcing effect if the drugs were additive. The monkeys were then allowed to choose between (a) cocaine and mixtures predicted to be equivalent to 100 μg/kg/inj of cocaine, (b) increasing doses of the mixtures and the single-component drugs, and (c) cocaine or remifentanil at doses that were in the highest safe range.
Results
Generally, monkeys preferred the mixtures over 100 μg/kg/inj of cocaine, evidence for superadditivity. However, preferences for the mixture ceased when relatively high doses of single-component drugs were offered as alternatives. When doses within the mixture were raised and offered with relatively high doses of the single drugs, there was no clear preference for either option. The highest dose of remifentanil was chosen over the highest dose of cocaine by all monkeys.
Conclusion
The current results indicate that cocaine–opioid combinations can be super-additive in terms of potency, but are not, at maximum, more effective than the single-component drugs.
Keywords: Cocaine, Remifentanil, Polydrug abuse, Rhesus monkey, Self-administration
Introduction
Cocaine and opioids are often co-abused, and their concurrent use may increase the likelihood of users developing dependence on one or both drugs. In addition to an increased risk of dependence, the concurrent use of cocaine and opioids is associated with a higher incidence of drug overdose (Coffin et al. 2003; Ochoa et al. 2001), relapse following treatment (Downey et al. 2000; Perez et al. 1997; Williamson et al. 2006), and an increased risk of contracting blood-borne diseases (Grella et al. 1995; Joe and Simpson 1995). These risk factors highlight the need to identify the motivating factors that lead some users to take cocaine and opioids concurrently.
Anecdotal evidence suggests that cocaine and opioids are taken together because the resulting high “feels better” than what is achieved by either drug alone (Kosten et al. 1988). However, empirical efforts aimed at identifying such an effect of the combination have not provided consistent evidence for this interaction. In human subjects, positive subjective ratings (i.e., “rush”, “good effects”, and “liking”) for cocaine were reported to be higher in methadone-maintained subjects than controls not maintained on methadone (Preston et al. 1996). However, Foltin and Fischman (1992) compared the subjective effects of intravenous (i.v.) cocaine, morphine, and combinations of the two, and found that subjects generally rated the positive subjective effects of the combination (i.e., “high” and “drug liking”) similar to the single-component drugs. As such, there was no indication that the concurrent administration of cocaine and an opioid interacted in humans to affect the drugs’ relative subjective effects. It should be noted, though, that the subjective effects of a drug or drug combination are not a direct measure of reinforcing effect (Comer et al. 2008).
For the investigation of reinforcement, drug self-administration studies are typically used. In the study of cocaine–opioid combinations, published research is limited to non-humans and has generally focused on the hypothesis that the drug mixture is a more effective reinforcer (i.e., more reinforcing at maximum) than the individual drugs. Several studies have used progressive-ratio (PR) schedules of reinforcement which allow assessment of both the potency and effectiveness (as measured by breakpoint) of a reinforcer in a single assay (for reviews see Arnold and Roberts 1997; Rowlett 2000; Stafford et al. 1998). Cocaine–opioid combinations have been studied in both rodents (Duvachelle et al. 1998; Ranaldi and Munn 1998; Ward et al. 2005) and non-human primates (Rowlett et al. 2005, 2007; Rowlett and Woolverton 1997; Woolverton et al. 2008). In five of the seven studies cited above, adding an opioid to cocaine (or vice versa) shifted the dose–response function leftward, suggesting that the cocaine–opioid combination was, under some conditions, a more potent reinforcer than either drug alone (Duvachelle et al. 1998; Rowlett et al. 2005, 2007; Rowlett and Woolverton 1997; Woolverton et al. 2008). However, in all cases but one (see Ranaldi and Munn 1998), the asymptotes of the dose–response functions for the combinations were no different from at least one of the component drugs, suggesting that the cocaine–opioid combination was not a more effective reinforcer than either drug alone.
Other studies have addressed this question using behavioral economics (BE; for a recent review, see Hursh and Silberburg 2008). This approach relates the consumption of a reinforcer to its cost and yields a demand function with an elasticity term that is thought to reflect the effectiveness of the reinforcer (Bickel et al. 1993; Hursh 1984, 1993; Hursh and Silberburg 2008). A key feature of the BE approach is that the elasticity term is independent of dose, and therefore provides a quantification of effectiveness across drugs that may vary in potency by other measures (Hursh and Silberburg 2008). In one study, monkeys were allowed to self-administer smoked cocaine and heroin, alone and combined (Mattox et al. 1997). The combination, although less elastic than heroin, was not different from cocaine alone. In a second study, Winger et al. (2006) reported that the elasticity of demand for combinations of i.v. cocaine and the short-acting mu agonist remifentanil were no different from either drug alone. Taken together, then, the results of both PR and BE studies indicate that the reinforcing effectiveness of cocaine and opioid combinations are no greater than, at the very least, cocaine alone.
Drug vs. drug choice procedures have also been used to investigate the relative effectiveness of drug reinforcers (Johanson and Schuster 1975; Woolverton and Johanson 1984) and this approach has been applied to the cocaine/opioid combination. For example, Wang et al. (2001) reported that, in monkeys, response rates for orally consumed cocaine and methadone, both alone and combined, were comparable when the solutions were offered singly. However, when the combinations and the single-component drugs were offered concurrently, monkeys generally chose the mixtures over the single-component drugs. In a second example, Ward et al. (2005) reported that when rats were allowed to choose between 0.75 mg/kg/inj of cocaine alone or lower doses of cocaine combined with 50.0 μg/kg/inj of heroin, they preferred the combination. These results suggest that drug vs. drug choice procedures may be particularly sensitive to the relative reinforcing effects of drug combinations. However, the choice experiments conducted thus far involved limited dose manipulations and did not allow the distinction between potency and effectiveness as underpinnings of preference.
The purpose of the present experiment was to use a drug vs. drug choice procedure to investigate the relative potency and effectiveness of a cocaine–opioid combination. Initially, rhesus monkeys were allowed to choose in a discrete-trials choice procedure between a fixed, moderate dose of i.v. cocaine (100 μg/kg/inj) and a range of doses of either cocaine (10–560 μg/kg/inj) or remifentanil (0.03–3.0 μg/kg/inj). Isobolograms and a dose-addition model were then used to select dose combinations of cocaine and remifentanil that would be predicted to be chosen as frequently as 100 μg/kg/inj of cocaine if the drugs were additive in reinforcing effect. It was reasoned that, if the drugs were super-additive as reinforcers, combinations would be chosen over 100 μg/kg/inj of cocaine. Next, monkeys were allowed to choose between a preferred mixture and increasing doses of the individual drugs. If the mixture was a more effective reinforcer than the individual drugs, it was reasoned that the mixture would be chosen regardless of the dose of the individual drug. In the next phase, monkeys were allowed to choose between high doses of the individual drugs and increasing doses of the mixture. Again, it was reasoned that, if the cocaine–opioid combination was a more effective reinforcer than either drug alone, there would be combination(s) for which preference would be insurmountable by any dose, however high, of the single-component drugs. Lastly, to determine the relative reinforcing effectiveness of the single drug options, monkeys were allowed to choose between the highest safe doses of cocaine (560 μg/kg/inj) and remifentanil (1.7 μg/kg/inj).
Materials and methods
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (1996).
Animals and apparatus
The subjects were six male rhesus monkeys (Macaca mulatta) weighing between 9.1 and 12.9 kg at the beginning of the study. Five monkeys (DJ9J, DK12, CJ82, R02069 and 20H) had no history of drug self-administration prior to the experiment. One monkey (R02073) had a history of self-administration of cocaine and pentobarbital mixtures under a PR schedule (Woolverton and Wang 2009). All monkeys were provided with sufficient food to maintain stable body weights (200–300 g/day, Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI, USA) and had unlimited access to water. Fresh fruit was provided daily, and a vitamin supplement was given three times a week. Lighting was cycled to maintain 16 h of light and 8 h of dark, with lights on at 0600 hours.
Each monkey was fitted with a mesh jacket (Lomir Biomedical, Malone, NY, USA) that was attached by a tether to the rear wall of the experimental cubicle (1.0 m3, Plaslabs, Lansing, MI, USA). The front door of the cubicle was made of transparent plastic, and the remaining walls were opaque. Two response levers (PRL-001, BRS/LVE, Beltsville, MD, USA) were mounted on the inside of the door. Four jeweled stimulus lights, two red and two white, were mounted above each lever. Drug injections were delivered by two peristaltic infusion pumps, one for each of the two levers (Cole-Parmer, Chicago, IL, USA). A Macintosh computer with custom interface and software controlled all events in an experimental session and recorded data.
Surgery
Each monkey had a double-lumen i.v. catheter implanted according to the following protocol. The monkey was injected with a combination of atropine sulfate (0.04 mg/kg i.m.) and ketamine hydrochloride (10 mg/kg i.m.) followed 20–30 min later by inhaled isoflurane. When anesthesia was adequate, the catheter was surgically implanted into a major vein with the tip terminating near the right atrium. The distal end of the catheter was passed subcutaneously to the mid-scapular region, where it exited the subject’s back. After surgery, the monkey was returned to the experimental cubicle. The catheter was threaded through the tether and out the back of the cubicle where it connected to a double-lumen swivel (Lomir). The individual lumens were then connected to the separate infusion pumps. An antibiotic (Kefzol; Eli Lilly & Company, Indianapolis, Indiana) was administered (22.2 mg/kg i.m.) twice daily for 7 days post-surgery to prevent infection. If a catheter became nonfunctional during the experiment, it was removed, and the monkey was removed from the experiment for a 1–2-week period to allow any infection to clear. After health was verified (i.e., no sign of systemic or local infection), a new catheter was implanted. The catheter was filled between sessions with a solution of 40 units/ml heparin to prevent clotting at the catheter tip.
Procedure
Experimental sessions began at 12:00 P.M. each day, 7 days per week, and were signaled by illumination of the white lever lights. Each session consisted of forced-choice (sampling) and free-choice trials, the number of which varied across phases of the experiment (see Fig. 1). During the forced-choice trials, only one lever was active per trial, signaled by the illumination of the corresponding set of white lever lights. The active lever was alternated for each forced-choice trial to ensure that the subject had exposure to the contingencies programmed for each lever before being allowed to choose between them. During free-choice trials, both sets of white lever lights were illuminated and both levers were active. For all trials, a single lever press (FR 1) extinguished the white lights above that lever and illuminated the red lights during a 10-s injection of the drug or drug mixture associated with that lever. All trials were separated by a 30-min-intertrial interval during which the lever lights were extinguished, and responses on either lever had no programmed consequence.
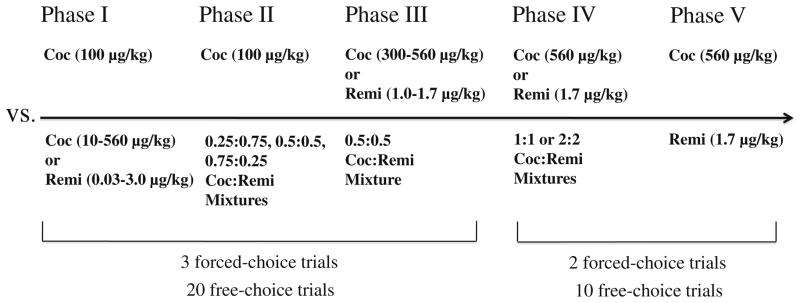
Fig. 1.
Choice options for each phase of the study. Cocaine, Coc; remifentanil, Remi
The experiment was divided into five phases (see Fig. 1). Experimental sessions consisted of three forced-choice and twenty free-choice trials during phases I–III and, because of the high doses studied, two forced-choice and ten free-choice trials during phases IV and V.
Phase I
The purpose of phase I was to determine doses of cocaine and remifentanil that, when combined, would be predicted to maintain 50% choice levels relative to a fixed dose of cocaine (100 μg/kg/inj) if the drugs were additive. To do this, monkeys were allowed to choose between 100 μg/kg/inj of cocaine (fixed-dose condition) on one lever or one of a range of doses of either cocaine (10–560 μg/kg/inj) or remifentanil (0.03–3.0 μg/kg/inj; variable-dose condition) on the other lever. A dose–response function was completed for one drug before testing began with the other drug. The order in which cocaine and remifentanil were presented was counterbalanced between monkeys, and doses within each drug condition were studied in an irregular order both within and between monkeys (i.e., the doses were not presented in ascending or descending order). A given choice condition was in effect until choice was stable, defined as: (1) the number of choices of the variable-dose condition on free-choice trials was within 10% of the running three-session mean for three consecutive sessions; and (2) there were no upward or downward trends over the three sessions. Once stability was achieved, the injections associated with the two levers were reversed, and stable preference was re-determined.
Phase II
The purpose of phase II was to study choice between the fixed dose of cocaine (100 μg/kg/inj) and mixtures of cocaine and remifentanil that were predicted by dose addition to be equivalent in reinforcing effect (i.e., chosen approximately equally) to the fixed dose of cocaine if the two drugs were additive. Four monkeys (CJ82, DK12, R02069, and R02073) were allowed to choose between cocaine (100 μg/kg/inj) and three cocaine–remifentanil mixtures with different dose ratios of the component drugs. To make the mixtures, ED50 values were calculated for each monkey from the dose–response functions using regression analysis (Graphpad Prism 4.0) and were used to construct an isobologram. The equation for the line of additivity was used to determine dose combinations that would be predicted to be chosen as frequently as 100 μg/kg/inj of cocaine if the component drugs were additive in reinforcing effect (see Tallarida 2000). Combinations were chosen in three mixture ratios that were determined in each monkey: 0.25:0.75, 0.5:0.5, and 0.75:0.25 cocaine:remifentanil, where the ratios denote a fraction of each drug’s respective ED50. These proportions were selected to include a range of ratios where drug interactions have been shown to vary (Tallarida 2000; Woolverton 1987). The order in which the mixtures were tested was counterbalanced across monkeys. All other procedural aspects of phase II were identical to those used in phase I.
Phase III
The purpose of phase III was to determine if choice could be shifted away from a preferred mixture by offering higher doses of cocaine and remifentanil as alternatives. To do this, the four monkeys from phase II were allowed to choose between the 0.5:0.5 mixture and two high doses of cocaine (300 and 560 μg/kg/inj) or remifentanil (1.0 and 1.7 μg/kg/inj). One monkey (R02069) running on 3.0 μg/kg/inj of remifentanil died unexpectedly during this phase. As a precaution, the maximum dose of remifentanil was set at 1.7 μg/kg/inj for the remainder of the study. During phase III, the order in which the single-component drugs were presented and the doses within each drug condition were counterbalanced across monkeys. All other procedural aspects of phase III were identical to phases I and II.
Phase IV
The purpose of phase IV was to determine if raising the doses of the component drugs within the mixture could make preference for the drug combination “insurmountable” when high doses of the component drugs were offered as alternatives. For the higher dose mixtures, the doses of cocaine and remifentanil in the 0.5:0.5 mixture were doubled and quadrupled to produce more concentrated mixtures of 1:1 and 2:2 cocaine/remifentanil. Thus, the 1:1 mixture combined doses of cocaine and remifentanil that were equal to their respective ED50 values, and the 2:2 mixture was twice that dose for each drug. During phase IV, four monkeys (CJ82, DJ9J, DK12, and 20 H) were allowed to choose between 1:1 or 2:2 mixtures of cocaine/remifentanil and high doses of cocaine (560 μg/kg/inj) or remifentanil (1.7 μg/kg/inj) alone. In addition, the monkeys were allowed to choose between the 1:1 or 2:2 mixtures and 100 μg/kg/inj of cocaine to ensure that these mixtures were preferred to a lower dose of cocaine. The high doses for the single-component drugs were chosen because they were maximally reinforcing in the previous phases of the experiment and were at the high end of the safe range for i.v. self-administration. As an added precaution, the number of forced-choice and free-choice trials was shortened from three and twenty to two and ten, respectively, and the stability criterion was changed to ±20% (two injections) of the running three-session mean for three consecutive sessions. The order in which the mixtures and the single-component drugs were presented was counterbalanced across monkeys. All other procedural aspects of phase IV were identical to the previous phases.
Phase V
The purpose of phase V was to examine choice between the high, maximally reinforcing doses of the single-component drugs used in phase IV. In phase V, the same four monkeys used in phase IV were allowed to choose between cocaine (560 μg/kg/inj) or remifentanil (1.7 μg/kg/inj). All other procedural aspects of phase V were identical to phase IV.
Data analysis
Choice data were analyzed over the three stable sessions of a condition and calculated as a percentage of trials. This percentage was calculated for the original lever:injection pairing and its reversal, and the two values were averaged. In phase I, the dependent measure was percent choice of the drug, cocaine or remifentanil, which was the variable–dose condition. A minimum of three doses were tested that covered the range between ≤20% and ≥80% choice. If there was no intermediate choice between 20% and 80% at adjacent doses for one of the conditions, four doses were tested, two ≤20% and two ≥80%. One exception was subject R02073, who pulled one catheter and had another removed due to infection. To increase the chances of completing the study with this subject, two data points were determined for cocaine and remifentanil choice, one ≤20% and one ≥80%, and the outer adjacent points were extrapolated for the ED50 calculations. In phases II–IV, the dependent measure was percent choice of the mixture relative to the single drug condition. In phase V, the dependent measure was percent choice of remifentanil (1.7 μg/kg/inj) relative to the cocaine alternative (560 μg/kg/inj). For all choice conditions, preference was defined as ≥80% choice for a reinforcer option.
Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA), and remifentanil hydrochloride was purchased commercially. Final solutions were prepared using 0.9% saline. Doses were expressed as the salt forms of the drugs.
Results
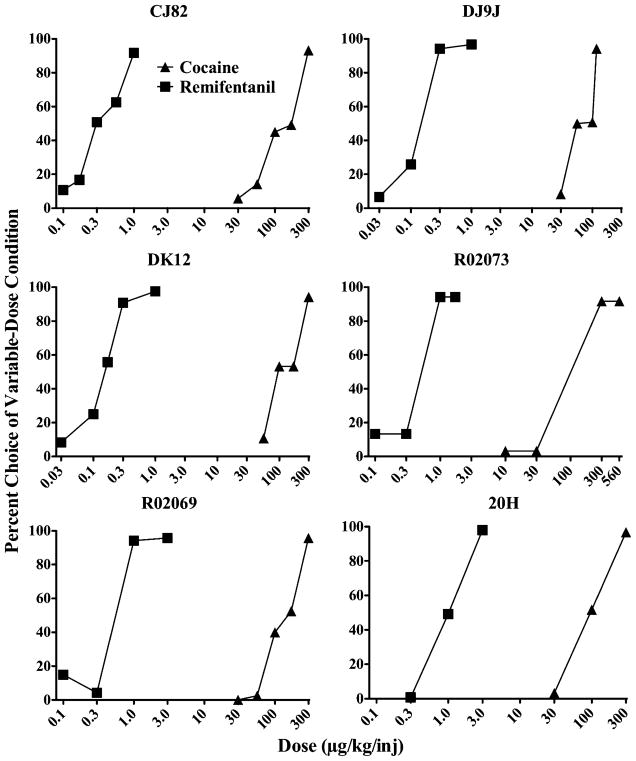
For all conditions, the time required to reach stability was approximately five to ten sessions, and with reversals a single-choice condition took approximately ten to twenty sessions to complete. During phase I, choice for cocaine or remifentanil relative to 100 μg/kg/inj cocaine increased as a function of dose (Fig. 2). ED50 values for cocaine and remifentanil in individual subjects ranged between 98.0–138.9 and 0.15–1.01 μg/kg, respectively (see Table 1). On average, the potency of remifentanil as a reinforcer was approximately 250 times greater than cocaine.
Fig. 2.
Dose–response functions for cocaine and remifentanil choice in each of six monkeys when 100 μg/kg/inj of cocaine was the alternative. Each data point is the average of three stable sessions with lever reversals, for a total of six sessions. Numbers on the abscissa represent drug dose (μg/kg/inj) of cocaine or remifentanil. Numbers on the ordinate represent percent choice for various doses of cocaine and remifentanil when 100 μg/kg/inj of cocaine was the alternative
Table 1.
ED50 values for cocaine and remifentanil in each of six monkeys and averaged across monkeys
| Subject | ED50 (μg/kg)
|
Cocaine:remifentanil mixture ratio (μg/kg)
|
|||||
|---|---|---|---|---|---|---|---|
| Cocaine | Remifentanil | 0.25:0.75 | 0.5:0.5 | 0.75:0.25 | 1:1 | 2:2 | |
| CJ82 | 131.6 | 0.35 | 33.0:0.26 | 65.8:0.18 | 98.7:0.08 | 131.6:0.35 | 263.2:0.70 |
| DK12 | 120.4 | 0.15 | 30.1:0.11 | 60.2:0.08 | 90.3:0.04 | 120.4:0.15 | 240.8:0.30 |
| R02069 | 138.9 | 0.57 | 34.7:0.43 | 69.5:0.29 | 104.2:0.14 | NR | NR |
| R02073 | 114.8 | 0.50 | 28.7:0.38 | 57.4:0.25 | 86.1:0.13 | NR | NR |
| DJ9J | 100.2 | 0.15 | NR | NR | NR | 100.2:0.15 | 200.4:0.30 |
| 20 H | 98.0 | 1.01 | NR | NR | NR | 98.0:1.01 | 196.0:2.02 |
| Mean (SEM) | 117.3 (6.5) | 0.46 (0.32) | |||||
Numbers in the mixture ratios are fractions of each monkey’s respective ED50 for cocaine and remifentanil
Numbers in parentheses represent SEM
NR, not run
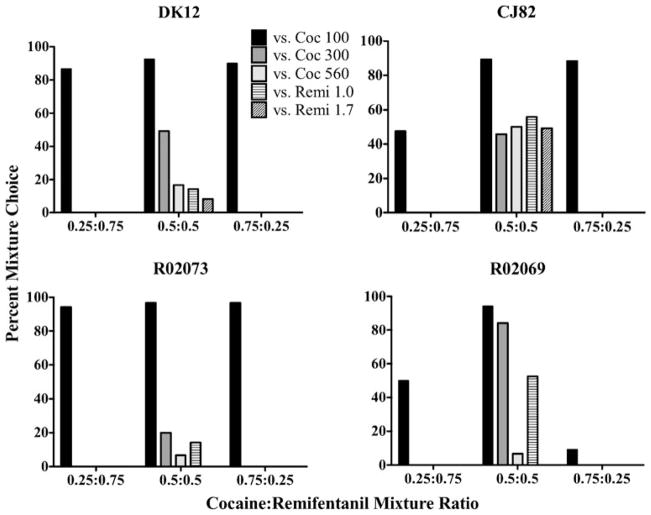
During phase II, all four monkeys preferred the 0.5:0.5 cocaine/remifentanil mixture over 100 μg/kg/inj of cocaine (Fig. 3; Table 1). Three of the monkeys (DK12, CJ82, and R02073) preferred the 0.75:0.25 mixture over 100 μg/kg/inj of cocaine and two monkeys (DK12 and R02073) preferred the 0.25:0.75 mixture over 100 μg/kg/inj of cocaine. Raising the dose of the cocaine alternative (phase III) shifted preference to the single drug alternative in three of the four monkeys in a dose-related manner (DK12, R02073, and R02069; Fig. 3). In the fourth monkey (CJ82), raising the dose of the cocaine alternative to 560 μg/kg/inj shifted preference away from the 0.5:0.5 mixture but did not result in a preference for the single-drug option. When 1.0 μg/kg/inj of remifentanil was offered as a single-drug alternative to the 0.5:0.5 mixture, choice was shifted to the single-drug alternative in two of the monkeys (DK12 and R02073). In the other two monkeys (CJ82 and R02069), choice was shifted away from the mixture but did not result in a preference for the single-drug option. When offered a higher dose of remifentanil (1.7 μg/kg/inj) as an alternative to the 0.5:0.5 mixture, CJ82 continued to show no preference for either option. Unfortunately, R02069 died before 1.7 μg/kg/inj of remifentanil could be tested.
Fig. 3.
Percent choice in four monkeys for three mixture ratios of cocaine/remifentanil predicted to be equivalent in reinforcing effect to 100 μg/kg/inj of cocaine if the drugs were additive. The alternatives to the mixtures were cocaine at 100 μg/kg/inj (Coc 100; black bars), 300 μg/kg/inj (Coc 300; dark gray bars) or 560 μg/kg/inj (Coc 560; light gray bars), and remifentanil at 1.0 μg/kg/inj (Remi 1.0; horizontally hatched bars) or 1.7 μg/kg/inj (Remi 1.7; diagonally hatched bars). Missing bars indicate conditions that were not run, not zero percent choice
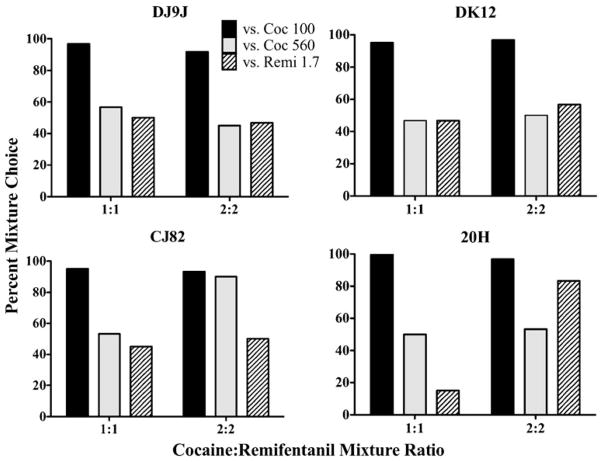
When mixture doses were increased (phase IV), all monkeys preferred the 1:1 and 2:2 cocaine/remifentanil mixtures to 100 μg/kg/inj of cocaine (Fig. 4). When the dose of the cocaine alternative was 560 μg/kg/inj, preference shifted away from the 1:1 mixture in all four monkeys and from the 2:2 mixture in three monkeys (DJ9J, DK12, and 20 H), but did not result in a preference for the cocaine alternative. The fourth monkey (CJ82) preferred the 2:2 mixture to the 560 μg/kg/inj dose of cocaine. When the single drug alternative to the mixtures was 1.7 μg/kg/inj of remifentanil, three monkeys (DJ9J, DK12, and CJ82) showed no preference for either mixture option or remifentanil alone. The fourth monkey (20 H) preferred 1.7 μg/kg/inj of remifentanil to the 1:1 mixture, but preferred the 2:2 mixture to 1.7 μg/kg/inj of remifentanil. It should be noted that in this monkey the dose of remifentanil in the 2:2 mixture exceeded the 1.7 μg/kg/inj remifentanil alternative (see Table 1). As such, the preference for the 2:2 mixture may have been due to the monkey’s preference for the higher dose of remifentanil rather than a preference for the mixture.
Fig. 4.
Percent choice in four monkeys for 1:1 and 2:2 mixtures of cocaine/remifentanil when cocaine was offered as an alternative at 100 μg/kg/inj (Coc 100; black bars) or 560 μg/kg/inj (Coc 560; gray bars) and remifentanil at 1.7 μg/kg/inj (Remi 1.7; diagonally hatched bars)
During phase V, all four monkeys preferred 1.7 μg/kg/inj of remifentanil to 560 μg/kg/inj of cocaine. Remifentanil choice ranged between 90% and 100% of the trials (data not shown).
Discussion
Consistent with previous reports (Koffarnus and Woods 2008; Wade-Galuska et al. 2007; Winger et al. 2006; Woolverton et al. 2008), cocaine and remifentanil, both alone and combined, functioned as positive reinforcers in monkeys. Choice for both drugs was dose-dependent, with remifentanil being approximately 250 times more potent than cocaine. Remi-fentanil’s relative potency was greater than what has been previously reported in a PR study comparing the reinforcing effects of these drugs (Woolverton et al. 2008). In that report, remifentanil was approximately 150 times more potent than cocaine. The difference in remifentanil:cocaine potency ratios between the PR results in Woolverton et al. (2008) and the choice results in the current report may be due to procedural differences. Indeed, although both assays are used to measure reinforcement, they may not render the same relative potencies for drugs because it is not certain that they are indexing a unitary aspect of reinforcement (Ward et al. 2005). In any case, remifentanil is consistently more potent than cocaine as a reinforcer.
In phase II, when monkeys were allowed to choose between cocaine alone and combinations predicted to be equivalent to that dose of cocaine if the drugs were additive (e.g., see Tallarida 2000; Woolverton 1987), the monkeys preferred combinations of cocaine and remifentanil in nine out of twelve cases. Moreover, mixture preference was always observed when the proportion of the two drugs was 0.5:0.5 of the ED50s. Taken together with the results from the PR tests cited above, the current data are a strong indication that the cocaine–opioid combination is, under some conditions, a more potent reinforcer than cocaine alone.
In previous studies that used dose-addition analysis to investigate the relative reinforcing effects of cocaine–opioid combinations, the most reinforcing mixtures were the ones with higher cocaine to opioid ratios (Negus 2005; Woolverton et al. 2008). In the present study, the mixture with a higher proportion of cocaine was preferred by three of four monkeys. The consistent observation of super-additivity at the 50% effect mixture (i.e., the 0.5:0.5 mixture) implies that the combination of a moderate dose of cocaine with an equi-effective dose of remifentanil is a more potent reinforcer than double the dose of cocaine alone. However, this finding does not necessarily lead to the conclusion that the mixture is a more effective reinforcer than either drug alone. Hypothetically, if drug A were a more effective reinforcer than drug B, it would seem to follow that at some dose of drug A it would be impossible to restore choice of drug B by increasing the dose of B. In fact, in the present study, when higher doses of cocaine or remifentanil alone were offered as alternatives to the combination (phase III), choice shifted away from the 0.5:0.5 combination and towards the single drugs. When doses of cocaine and remifentanil in the mixture were then increased by two- and fourfold and offered concurrently with high doses of the single-component drugs (phase IV), choice was generally equal for the two options, suggesting that cocaine and remifentanil, whether alone or combined, were similar in effectiveness. This is consistent with Winger et al. (2006), who reported that cocaine and remifentanil, both alone and combined, were comparable in reinforcing effectiveness when tested in a BE procedure. It should be noted that in phase IV, the doses of cocaine and remifentanil in the mixtures were lower than the single-drug alternatives. As such, there remains the possibility that raising the doses in the mixture beyond the highest tested (2:2) could have resulted in a mixture that was preferred to all doses of the single-component drugs. However, it is unlikely that raising the mixture doses further would have resulted in a preference for the mixture because the choice results appeared to be at an asymptote at the 1:1 and the 2:2 mixtures.
The observation that choice was approximately equal for the combinations and the highest doses of the single-component drugs implies that all of the reinforcer options during this phase were comparable in reinforcing effectiveness and that the monkeys were indifferent to the options. It should be noted that these indifference points are the consequence of monkeys failing to switch levers when the reinforcer/lever pairings were reversed, i.e., a position preference for one or the other lever. The result is a common outcome under concurrent FR schedules of drug choice (Johanson and Schuster 1975; Woolverton et al. 2007). Interestingly, when the monkeys were allowed to choose between the highest safe doses of cocaine (560 μg/kg/inj) and remifentanil (1.7 μg/kg/inj) in phase V, all of the monkeys chose remifentanil. This outcome would not have been predicted based on the results of phase IV if choice were transitive, i.e., if A is equal to B and B is equal to C, then A should equal C. In phase IV, the 1:1 and 2:2 mixtures were chosen with similar frequency to 560 μg/kg/inj of cocaine and 1.7 μg/kg/inj of remifentanil, which suggested that these doses of cocaine and remifentanil were comparable in reinforcing effect. However, all monkeys preferred remifentanil to cocaine in phase V. This result suggests that rank-ordering drugs as reinforcers assuming transitivity of choice may be unreliable.
The fact that remifentanil was preferred to cocaine in phase V contradicts the results of a previous report that compared cocaine and remifentanil in monkeys responding under a PR schedule (Woolverton et al. 2008). In that report, maximum responding maintained by cocaine was higher than for remifentanil, suggesting that cocaine was the more effective reinforcer. The PR dose–response function for remifentanil reached an asymptote between 0.4 and 0.8 μg/kg/inj, suggesting that these doses of remifentanil were maximally reinforcing. Cocaine produced breakpoints that were higher than the maximum achieved by remifentanil and did so at a dose as low as 200 μg/kg/inj, suggesting that cocaine was more reinforcing at this dose than the maximum for remifentanil. It is unlikely that these differences in breakpoints were particular to remifentanil because other studies comparing cocaine and heroin under a PR schedule have also found cocaine to be the more effective reinforcer in both monkeys (Rowlett and Woolverton 1997) and in rats (Duvachelle et al. 1998). Taken together with previous choice assessments (Johanson and Schuster 1975; Wang et al. 2001; Ward et al. 2005), the current results are a strong indication that PR and choice procedures do not always provide identical estimates of relative reinforcing effectiveness.
Over the last couple of decades, a number of studies, including the current one, have tried to empirically verify the report that cocaine and opioids “feel better” when taken together than when taken alone (see Leri et al. 2003). Generally, these studies were designed to investigate the relative reinforcing effects of cocaine and opioids, both alone and combined, with the rationale that an enhanced reinforcing effect of the combination motivates users to take the combination. For the most part, accumulating evidence has indicated that while the combination may be a unique subjective stimulus (Foltin and Fischman 1992) and a more potent reinforcer than either drug alone, it is not, at maximum, a more effective reinforcer. The current data extend these findings to a drug vs. drug choice procedure, and further suggest that the preferences for cocaine–opioid combinations demonstrated in previous reports (Wang et al. 2001; Ward et al. 2005) were likely due to the combinations’ greater relative potency, not effectiveness.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant R01 DA-019471 to W.L.W. We gratefully acknowledge Steven Ross for his technical assistance.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Kevin B. Freeman, Email: kfreeman@psychiatry.umsmed.edu, Division of Neurobiology and Behavior Research, Department of Psychiatry and Human Behavior, The University of Mississippi Medical Center, Jackson, MS 39216, USA
William L. Woolverton, Division of Neurobiology and Behavior Research, Department of Psychiatry and Human Behavior, The University of Mississippi Medical Center, Jackson, MS 39216, USA
References
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend. 1993;33:173–192. doi: 10.1016/0376-8716(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Galea S, Ahem J, Leon AC, Vlahov D, Tardiff K. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–1998. Addiction. 2003;98:739–747. doi: 10.1046/j.1360-0443.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent polydrug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992;261:623–632. [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE. Cocaine and crack use and HIV risk behaviors among high-risk methadone maintenance clients. Drug Alcohol Depend. 1995;37:15–21. doi: 10.1016/0376-8716(94)01059-t. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. J Exp Anal Behav. 1984;42:435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 1993;33:165–172. doi: 10.1016/0376-8716(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberburg A. Economic demand and essential value. Psych Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Joe GW, Simpson DD. HIV risks, gender, and cocaine use among opiate users. Drug Alcohol Depend. 1995;37:23–28. doi: 10.1016/0376-8716(94)01030-o. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH. Quantification of drug choice with the generalized matching law in rhesus monkeys. J Exp Anal Behav. 2008;89:209–224. doi: 10.1901/jeab.2008.89-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Antecedents and consequences of cocaine abuse among opioid addicts. A 2.5-year follow-up. J Nerv Ment Dis. 1988;176:176–181. doi: 10.1097/00005053-198803000-00006. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Thompson SS, Carroll ME. Smoked heroin and cocaine base (speedball) combinations in rhesus monkeys. Exp Clin Psychopharmcol. 1997;5:113–118. doi: 10.1037//1064-1297.5.2.113. [DOI] [PubMed] [Google Scholar]
- Negus S. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharm. 2005;180:115–124. doi: 10.1007/s00213-004-2133-y. [DOI] [PubMed] [Google Scholar]
- Ochoa KC, Hahn JA, Seal KH, Moss AR. Overdosing among young injection drug users in San Francisco. Addict Behav. 2001;26:453–460. doi: 10.1016/s0306-4603(00)00115-5. [DOI] [PubMed] [Google Scholar]
- Perez de los Cobos J, Turjols J, Ribalta E, Casas M. Cocaine use immediately prior to entry in an inpatient heroin detoxification unit as a predictor of discharges against medical advice. Am J Drug Alcohol Abuse. 1997;23:267–279. doi: 10.3109/00952999709040946. [DOI] [PubMed] [Google Scholar]
- Preston KL, Sullivan JT, Strain EC, Bigelow GE. Enhancement of cocaine’s abuse liability in methadone maintenance patients. Psychopharm. 1996;123:15–25. doi: 10.1007/BF02246276. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Munn E. Polydrug self-administration in rats: cocaine-heroin is more rewarding than cocaine-alone. Neuro Report. 1998;9:2463–2466. doi: 10.1097/00001756-199808030-00007. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharm. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharm. 1997;133:363–371. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Rodefer JS, Spealman RD. Self-administration of cocaine-opioid combinations by rhesus monkeys: evaluation of the role of μ receptor efficacy using labor supply analysis. J Pharmacol Exp Ther. 2005;312:1289–1287. doi: 10.1124/jpet.104.076646. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Yao WD, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:1135–1143. doi: 10.1124/jpet.107.120766. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharm. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman & Hall/CRC; Boca Raton: 2000. [Google Scholar]
- Wade-Galuska T, Winger G, Woods JH. A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharm. 2007;194:563–572. doi: 10.1007/s00213-007-0858-0. [DOI] [PubMed] [Google Scholar]
- Wang NS, Brown VL, Grabowski J, Meisch RA. Reinforcement by orally delivered methadone, cocaine, and methadone-cocaine combinations in rhesus monkeys: are the combinations better reinforcers? Psychopharm. 2001;156:63–72. doi: 10.1007/s002130100731. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Morgan D, Roberts DCS. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuro-psychopharm. 2005;30:286–295. doi: 10.1038/sj.npp.1300560. [DOI] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teeson M. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend. 2006;81:293–300. doi: 10.1016/j.drugalcdep.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH. Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther. 2006;318:223–229. doi: 10.1124/jpet.105.100461. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Analysis of drug interactions in behavioral pharmacology. In: Thompson T, Dews PB, Barrett JE, editors. Neurobehavioral Pharmacology. Lawrence Erlbaum Associates; Hillsdale, NJ: 1987. pp. 275–302. [Google Scholar]
- Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d, l-cathinone. J Exp Anal Behav. 1984;31:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Self-administration of cocaine-pentobarbital mixtures by rhesus monkeys. Drug Alcohol Depend. 2009;100:272–276. doi: 10.1016/j.drugalcdep.2008.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Tallarida R. Self-administration of cocaine-remifentanil mixtures by monkeys: an isobolographic analysis. Psychopharm. 2008;198:387–394. doi: 10.1007/s00213-008-1152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]