Abstract
Src-family tyrosine kinases (SFKs) play critical roles in regulating cellular proliferation in epithelial cells, and SFK activity is increased in many human carcinomas. Src-activating and signaling molecule (Srcasm) is a novel SFK substrate that downregulates SFK activityand promotes keratinocyte differentiation. Srcasm has also been to shown to function as an anti-oncogene in the epidermis and its levels are decreased in cutaneous squamous cell carcinoma (SCC). The purpose of this study is to determine if Srcasm levels are decreased in esophageal SCC (ESCC) compared with unremarkable mucosa. Given that Srcasm functions as an anti-onocogene in squamous epithelium, we hypothesized that Srcasm levels should be decreased in esophageal SCCs compared to unremarkable mucosa. To evaluate this hypothesis, we performed protein immunohistochemistry for Srcasm on nine unremarkable esophageal mucosal specimens and twelve esophageal SCCs. Our results show that Srcasm protein staining levels are decreased in esophageal SCC compared to unremarkable mucosa. These data show that the pattern of Srcasm staining inversely correlates with ESCC formation and is consistent with the hypothesis that Srcasm may function as an anti-oncogene in esophageal squamous mucosa.
Keywords: Src-activating and signaling molecule, Src-family tyrosine kinases, esophageal squamous cell carcinoma
INTRODUCTION
Esophageal squamous mucosa is a self-renewing tissue that maintains a stable barrier through controlled regulation of keratinocyte growth and differentiation. The mechanisms that regulate the transition of keratinocytes from proliferative to post-mitotic cells are important for understanding esophageal disorders such as esophageal squamous cell carcinoma (ESCC).
Src-family tyrosine kinases (SFKs) play critical roles in regulating cellular differentiation and proliferation 1. Three SFKs, Src, Fyn and Yes, are ubiquitously expressed in human tissues, including cutaneous and esophageal keratinocytes; whereas others such as Lyn, Lck, Hck are mainly expressed in non-adherent cells of the hematopoietic system 1. Elevated SFK activity has been observed in many kinds of human carcinomas, including colon carcinoma, breast carcinoma, and non-small cell lung cancer 2–5. However, activating mutations in SFKs are rarely found in human carcinomas suggesting that impaired negative regulatory mechanisms likely account for the increased kinase activity 6, 7.
Src-activating and signaling molecule (Srcasm) is a substrate of SFKs that binds to the kinase after phosphorylation and targets it for degradation in the lysosome 8–10. In vitro studies demonstrate that increased Srcasm is phosphorylated downstream of the EGF receptor and SFKs in keratinocytes and modulates the activity of Erk 1/2 to promote differentiation 8. In vivo studies using K14-Fyn transgenic mice, a model of epidermal hyperplasia, demonstrate a thickened, hyperplastic epidermis that correlates with increased Fyn expression 9. Increasing Srcasm levels using a double-transgenic system suppresses Fyn-induced epidermal hyperproliferation, and promotes cellular differentiation. In contrast, increased expression of a dominant-negative mutant form of Srcasm does not correct the hyperproliferative phenotype. More recent studies with K14-Fyn Y528F/K14-Srcasm mice show that raising Srcasm levels can inhibit skin SCC formation through a mechanism that involves p53 and Notch 1 10. In this model, raising Srcasm levels decreases levels of phospho-Erk 1/2, phospho-STAT-3, and phosphor-PDK-1.
Additional data suggesting that Srcasm plays a role in carcinogenesis include decreased protein and transcript levels in human cutaneous SCCs, as determined by immunohistochemistry, qRT-PCR, and Western blotting 8, 10, 11. Recent in vitro studies suggest that Srcasm also may downregulate the EGF receptor through a lysosomal mechanism that requires Grb2 12. Since increased EGF receptor activity is found in SCCs of the GI tract and Srcasm is phosphorylated by EGF in cutaneous keratinocytes, it is reasonable to suggest that Srcasm levels may be decreased in keratinocytic tumors of the GI tract 13, 14.
Given these data, we hypothesized that Srcasm levels may be decreased in ESCC compared to unremarkable mucosa. To evaluate this hypothesis, Srcasm immunohistochemical staining levels were assessed in formalin-fixed esophageal SCCs and mucosal specimens. The data show that Srcasm levels are decreased in ESCC compared to esophageal mucosa (UNR). This pattern of Srcasm staining suggests that it may function as an anti-oncogene in esophageal SCCs.
MATERIALS AND METHODS
Immunohistochemistry
This study utilized formalin-fixed, paraffin embedded surgical specimens from the archives of the Department of Thoracic Surgery of the University of Zhengzhou in Zhengzhou, Henan, China. These specimens were de-identified. Twelve cases of esophageal SCC and nine specimens of unremarkable esophageal mucosa were evaluated. For all cases, recut slides were stained with hematoxylin/eosin and examined to confirm the diagnoses. Rabbit anti-Srcasm antibody was used in this study 11. In brief, sample slides were heated, deparaffinized, rehydrated, and rinsed in distilled water. Antigen retrieval was performed by incubating the slides in 10mM citrate buffer (pH6.0) at 85°C for 20 minutes, and then allowing them to cool to room temperature, followed by washing in distilled water. Endogenous peroxidase activity was blocked in 3% H2O2 for 10 minutes, then the slides were washed three times in PBST. The tissue sections were blocked at room temperature for 1 hour with 10% normal goat serum, and then incubated with a 1:50 dilution of affinity purified rabbit anti-Srcasm antibody over night at 4°C. Affinity purified biotinylated goat anti rabbit polyclonal immunoglobulin (BD Biosciences Pharmingen, San Diego, CA, USA) was applied to the tissue sections at a 1:100 dilution for 30 minutes. Prediluted streptavidin-horseradish peroxidase (BD Biosciences Pharmingen San Diego, CA, USA) was applied to the sections and incubated for 40 minutes, and histochemical development was performed using a liquid 3,3′-Diaminobenzidine tetrahydrochloride substrate kit (Invitrogen San Francisco, CA, USA). The slides were subsequently counterstained in hematoxylin, dehydrated, and cleared. Purified rabbit IgG was used as a negative control (Invitrogen San Francisco, CA, USA). No significant staining was seen in the negative controls.
Analysis of staining intensity
All slides were evaluated independently by two individuals (YQ and JS) for staining intensity and the extent of staining in esophageal squamous cell carcinoma (ESCC) and unremarkable esophageal mucosa (UNR). The intensity of staining was graded as follows: none = 1, weak = 2, moderate = 3, and strong = 4. The extent of staining as a percent of positively staining cells was assessed as follows: 1–25% =1, 26–50% = 2, 51–75% = 3, 76–100% =4. For each specimen a staining index was determined by multiplying the intensity factor by the extent factor. The maximum staining index would be 16. For lesions with two different staining patterns, for example weak staining in 50% of cells and moderate staining in 50% of cells the index would be 2 × 2+3 × 2 =10.
Statistical analysis
The staining values were analyzed to determine the mean and standard deviation; a student’s T-test was performed to determine the statistical significance of differences in the staining values in the UNR and ESCC samples. Ap value < 0.05 was considered statistically significantly.
RESULTS
Atotal of nine cases of unremarkable esophageal mucosa (UNR) and twelve cases of ESCC were examined. No significant staining was seen with control antibody (Figs. 1C and D). Immunohistochemical staining for Srcasm in unremarkable mucosa demonstrated staining in the low and mid levels of the spinous layer. The staining signal appeared stronger in the suprabasilar levels compared to the basal layer as would be expected for a protein associated with differentiation (Figs. 1E and F). Minimal staining was seen in the lamina propria and muscularis muscosa.
Figure 1. Immunohistochemical staining for Src-activating and signaling molecule (Srcasm) in unremarkable esophageal mucosa.
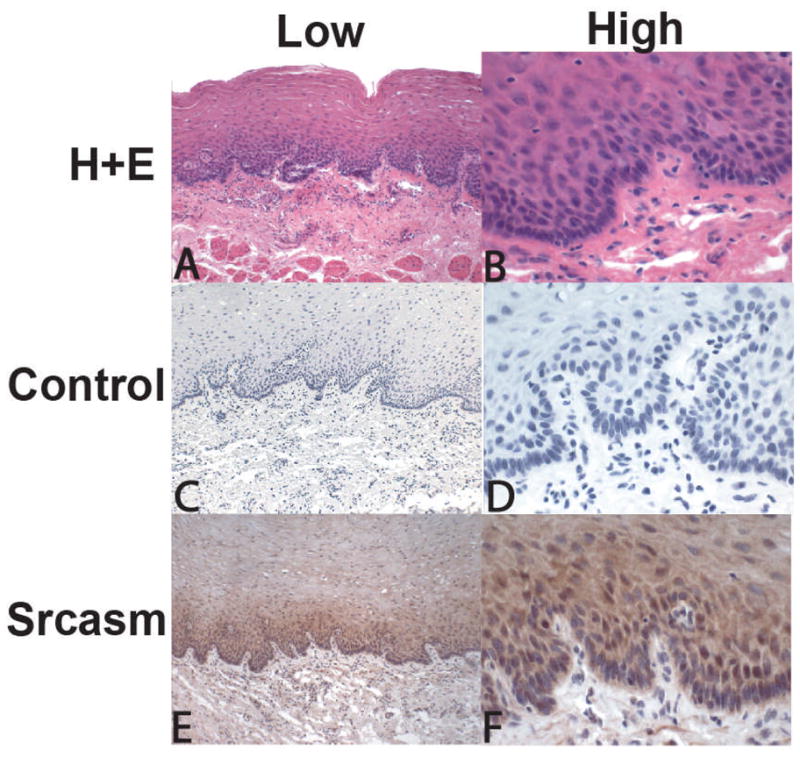
A and B) H+E staining of mucosa showing low and high magnification. C and D) Negative control slides using purified rabbit IgG as the primary antibodyshowed no staining. E and F) Srcasm antibody demonstrates strong staining of mucosal epithelium in the lower levels above the basal cell layer. N=9
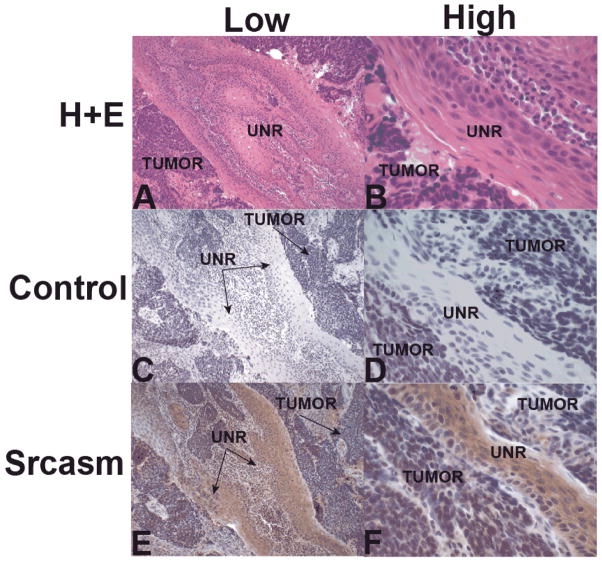
Immunohistochemical staining for Srcasm in ESCC lesions demonstrated weak Srcasm staining in tumor cells and stronger Srcasm staining in adjacent unremarkable mucosa, in the subset of samples that contained portions of mucosa (Figs. 2E and F).
Figure 2. Immunohistochemical staining for Src-activating and signaling molecule (Srcasm) in esophageal squamous cell carcinoma (ESCC).
A and B) H+E staining of ESCC showing low and high magnification of tumor and unremarkable mucosa (UNR). C and D) Negative control slides using purified rabbit IgG as the primary antibody showed no staining of mucosa or tumor. E and F) Srcasm antibody demonstrates strong staining of mucosa and weak staining of ESCC. N=12
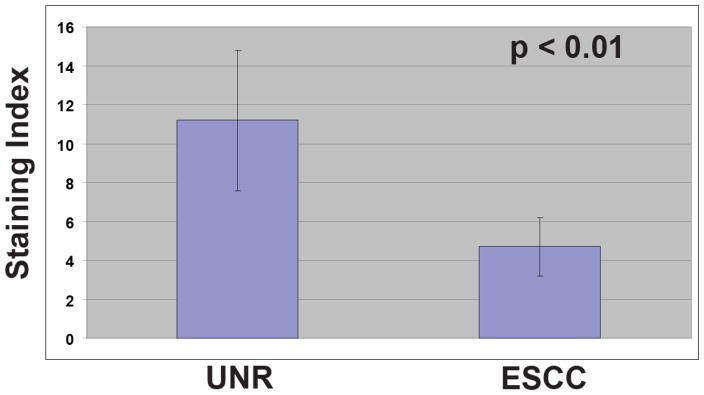
To further characterize the inverse relationship in Srcasm staining between the UNR and the ESCC samples, a semiquantitative analysis of staining was performed. The Srcasm staining index of ESCCs was decreased significantly compared to UNR samples (11.2 +/− 3.6 vs 4.7 +/− 1.5) (Table 1). This observed difference in staining was statistical significantly (p < 0.01).
Table 1. Srcasm staining indices for UNR and ESCC.
The staining indices for Srcasm in unremarkable esophageal epithelium (UNR, 11.2 +/− 3.6) and esophageal squamous cell carcinoma (ESCC, 4.7 +/− 1.5) are shown. Standard deviations are indicated.
|
DISCUSSION
To characterize the levels of Srcasm in human esophageal mucosa and ESCC, immunohistochemical analysis was performed. The data show that the Srcasm staining is significantly stronger in esophageal mucosa than in ESCCs (Figs. 1 and 2). Decreased Srcasm staining was seen in all the SCC samples suggesting that decreased Srcasm levels are a common molecular feature of ESCC. Such data implicate decreased Srcasm levels in the pathogenesis of ESCC, which is similar to data seen in cutaneous SCCs 8, 10. The data presented suggest that decreased Srcasm levels may be a distinguishing factor between unremarkable esophageal mucosa and ESCC.
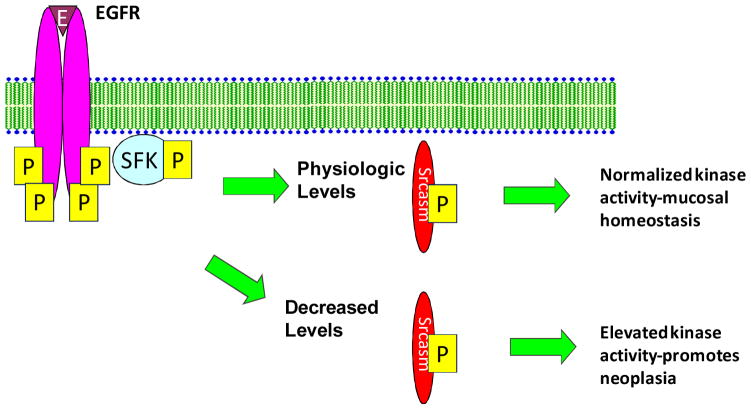
Previous studies have shown that EGFR is upregulated in ESCC 15, 16. Our prior work has shown that SFKs are activated downstream of EGF and TGF-α in human cutaneous keratinocytes, and that this activation leads to phosphorylation of Srcasm and promotes kinase downregulation through a lysosomal dependent mechanism 8. Recent in vitro studies show that Srcasm plays a role in targeting activated EGF receptor for endosomal degradation 12. The observation of decreased Srcasm levels in ESCC provides a plausible mechanism for increasing EGFR and SFK activity in these tumors (Fig. 3).
Figure 3. Srcasm may negatively regulate tyrosine kinase signaling in human esophageal squamous cell carcinoma.
Srcasm is a component of the EGFR and SFK signaling pathway, and act as a negative regulator of both molecules 9, 12. Our data show that Srcasm protein levels are decreased in esophageal SCC compared to esophageal mucosa. We hypothesize that decreased Srcasm levels promote increased EGFR and SFK activity.
Srcasm functioning as an anti-oncogene and its levels being inversely related to SFK activity in human carcinoma were first described in cutaneous SCC and related precursor lesions 17. These studies showed that actinic keratoses, cutaneous squamous cell carcinoma in situ (SCISs), and cutaneous squamous cell carcinomas demonstrated elevated levels of activated SFKs compared to unremarkable skin. Subsequent studies have shown that Srcasm levels are decreased in these same lesions using immunohistochemistry, qRT-PCR, and western blotting 8, 10. Together, these studies show that there is an inverse relationship between SFK activity and Srcasm levels in cutaneous SCCs, which should be further investigated in ESCCs and related precursor lesions.
Recent data show that Src kinases can negatively regulate Notch 1 in cutaneous squamous cell carcinoma 10. Previous studies have shown that Notch 1 protein appears to promote differentiation and is highly expressed in the basal layer of the murine esophagus and human epidermis 18, 19. As in cutaneous SCCs, ESCCs have lower levels of Notch1 compared to adjacent unremarkable epithelium 20. Therefore, there appears to be downregulation of both Notch 1 and Srcasm in SCCs of the skin and esophagus.
The data presented parallel studies in the skin and confirm that Srcasm levels are decreased in ESCC compared to esophageal mucosa. The results of this study suggest that Srcasm levels in ESCC and esophageal unremarkable epithelium are likely inversely related to EGFR protein levels 21, 22. This conclusion would be consistent with data from human cutaneous keratinocytes showing that Srcasm acts as a negative regulator of tyrosine kinase signaling downstream of EGFR and SFK signaling 8.
These data provide support for the hypothesis that Srcasm may function as an anti-oncogene in ESCC by negatively regulating EGFR levels and EGFR-dependent activation of SFKs 8, 9.
Acknowledgments
We thank Dr. John Stanley for advice and support. This work was supported by the Department of Dermatology, University of Pennsylvania MedicalSchool, and in part by grant AR-51380 (NIAMS) to J.T.S. The authors have no financial conflicts of interest relevant to this work.
References
- 1.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annual Review of Cell & Developmental Biology. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 2.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–51. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 3.Cook RM, Miller YE, Bunn PA., Jr Small cell lung cancer: etiology, biology, clinical features, staging, and treatment. Current Problems in Cancer. 1993;17:69–141. doi: 10.1016/0147-0272(93)90010-y. [DOI] [PubMed] [Google Scholar]
- 4.Iravani S, Mao W, Fu L, Karl R, Yeatman T, Jove R, et al. Elevated c-Src protein expression is an early event in colonic neoplasia. Laboratory Investigation. 1998;78:365–71. [PubMed] [Google Scholar]
- 5.Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, et al. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer. 2006;95:1410–4. doi: 10.1038/sj.bjc.6603444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang NM, Yeh KT, Tsai CH, Chen SJ, Chang JG. No evidence of correlation between mutation at codon 531 of src and the risk of colon cancer in Chinese. Cancer Letters. 2000;150:201–4. doi: 10.1016/s0304-3835(99)00398-5. [DOI] [PubMed] [Google Scholar]
- 7.Daigo Y, Furukawa Y, Kawasoe T, Ishiguro H, Fujita M, Sugai S, et al. Absence of genetic alteration at codon 531 of the human c-src gene in 479 advanced colorectal cancers from Japanese and Caucasian patients. Cancer Research. 1999;59:4222–4. [PubMed] [Google Scholar]
- 8.Li W, Marshall C, Mei L, Dzubow L, Schmults C, Dans M, et al. Srcasm Modulates EGF and Src-kinase Signaling in Keratinocytes. J Biol Chem. 2005;280:6036–46. doi: 10.1074/jbc.M406546200. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Marshall C, Mei L, Gelfand J, Seykora JT. Srcasm Corrects Fyn-induced Epidermal Hyperplasia by Kinase Down-regulation. J Biol Chem. 2007;282:1161–9. doi: 10.1074/jbc.M606583200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Li W, Marshall C, Griffin T, Hanson M, Hick R, et al. Srcasm inhibits Fyn-induced cutaneous carcinogenesis with modulation of Notch1 and p53. Cancer Res. 2009;69:9439–47. doi: 10.1158/0008-5472.CAN-09-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meulener MC, Ayli EE, Elenitsas R, Seykora JT. Decreased Srcasm expression in hyperproliferative cutaneous lesions. J Cutan Pathol. 2009;36:291–5. doi: 10.1111/j.1600-0560.2008.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu NS, Loo LS, Loh E, Seet LF, Hong W. Participation of Tom1L1 in EGF-stimulated endocytosis of EGF receptor. Embo J. 2009;28:3485–99. doi: 10.1038/emboj.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merlino GT, Xu YH, Richert N, Clark AJ, Ishii S, Banks-Schlegel S, et al. Elevated epidermal growth factor receptor gene copy number and expression in a squamous carcinoma cell line. Journal of Clinical Investigation. 1985;75:1077–9. doi: 10.1172/JCI111770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton P, Richards S, Reeves J, Nikolic M, Edington K, Clark L, et al. Epidermal growth factor receptor expression by human squamous cell carcinomas of the head and neck, cell lines and xenografts. British Journal of Cancer. 1994;70:427–33. doi: 10.1038/bjc.1994.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukaida H, Yamamoto T, Hirai T, Toi M, Nakamura T, Wada T, et al. Expression of human epidermal growth factor and its receptor in esophageal cancer. Jpn J Surg. 1990;20:275–82. doi: 10.1007/BF02470661. [DOI] [PubMed] [Google Scholar]
- 16.Ozawa S, Ueda M, Ando N, Abe O, Shimizu N. High incidence of EGF receptor hyperproduction in esophageal squamous-cell carcinomas. Int J Cancer. 1987;39:333–7. doi: 10.1002/ijc.2910390311. [DOI] [PubMed] [Google Scholar]
- 17.Ayli EE, Li W, Brown TT, Witkiewicz A, Elenitsas R, Seykora JT. Activation of Src-family tyrosine kinases in hyperproliferative epidermal disorders. J Cutan Pathol. 2008;35:273–7. doi: 10.1111/j.1600-0560.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem. 2004;52:509–16. doi: 10.1177/002215540405200409. [DOI] [PubMed] [Google Scholar]
- 19.Thelu J, Rossio P, Favier B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2002;2:7. doi: 10.1186/1471-5945-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leethanakul C, Patel V, Gillespie J, Pallente M, Ensley JF, Koontongkaew S, et al. Distinct pattern of expression of differentiation and growth-related genes in squamous cell carcinomas of the head and neck revealed by the use of laser capture microdissection and cDNA arrays. Oncogene. 2000;19:3220–4. doi: 10.1038/sj.onc.1203703. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Wu WK, Yu L, Li ZJ, Sung JJ, Zhang ST, et al. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of beta-adrenoceptors. J Pharmacol Exp Ther. 2008;326:69–75. doi: 10.1124/jpet.107.134528. [DOI] [PubMed] [Google Scholar]
- 22.Wei Q, Chen L, Sheng L, Nordgren H, Wester K, Carlsson J. EGFR, HER2 and HER3 expression in esophageal primary tumours and corresponding metastases. Int J Oncol. 2007;31:493–9. [PubMed] [Google Scholar]