Abstract
Purpose
Congenital cataracts are a clinically and genetically heterogeneous lens disorder. The purpose of this study was to identify the genetic mutation and the molecular phenotype responsible for the presence of autosomal dominant congenital nuclear cataract disease in a Chinese family.
Methods
Patients were given physical examinations and their blood samples were collected for DNA extraction. Genotyping was performed by microsatellite markers and logarithm-of-odds (LOD) scores were calculated using the LINKAGE programs. Mutation detection was performed by direct sequencing.
Results
Linkage to the major intrinsic protein (MIP) locus was identified. Sequencing MIP revealed an A→G transition at nucleotide position c.530, which caused a conservative substitution of Tyr to Cys at codon 177 (P.Y177C). The Y177C mutation is located in the fifth transmembrane sequence. This mutation was identified in all affected individuals but is not found in any of the 100 control chromosomes.
Conclusions
Our results identify that the c.530 (A→G) mutation in MIP is responsible for the Chinese pedigree. Our results further identify that the mutation in MIP is responsible for congenital cataract. The mutation found in our study broadens the spectrum of MIP mutations.
Introduction
Congenital cataracts are common and cause approximately one-third of infant blindness; they occur in approximately 1–6 out of every 10,000 live births. One quarter of congenital cataract disease is hereditary [1-4].
Congenital cataracts are a clinically and genetically heterogeneous lens disorder. Cataracts that are phenotypically identical can result from mutations at different genetic loci and can have different inheritance patterns. Conversely, cataracts with dissimilar phenotypes may result from mutations in a single gene or gene family. It is believed that the type of genetic mutation is related to the morphology of the cataract [5]. To date, about 40 genetic loci have been linked to congenital cataracts and 26 genes have been cloned and sequenced, including crystallins, connexins, heat shock transcription factor-4, aquaporin-0, and beaded filament structural protein-2 [5].
Recently, we found a four-generation pedigree from northeast China with congenital nuclear cataract. Mutation screening in the major intrinsic protein gene (MIP) identified an A→G transition at nucleotide position c.530. This nucleotide change resulted in the substitution of highly-conserved Tyr by Cys at codon 177 (P.Y177C).
Methods
Patients and clinical data
The five-generation family enrolled in this study was found in a Northeastern province of China. Clinical examination, peripheral blood collection and DNA extraction were performed in the Department of Ophthalmology, Peking Union Medical College Hospital, Beijing, China. The study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board and Ethics Committee of Peking city; informed consent was obtained from all participants.
Recruited family members included 7 confirmed patients with congenital nuclear cataract. Blood was collected from 6 patients and 5 unaffected family members. Clinical data for these 11 subjects was ascertained by detailed ocular examinations.
Genotyping and linkage analysis
DNA was isolated from the blood samples of the 11 individuals and fluorescently-labeled microsatellite markers were used for linkage analysis of 26 candidate gene regions. Two-point linkage analysis was performed with MLINK from the LINKAGE program package.
Mutation analysis
All coding exons of MIP were amplified by polymerase chain reaction (PCR) using a set of four pairs of primers [6], shown in Table 1. The PCR products were sequenced on an ABI3730 Automated Sequencer (PE Biosystems, Foster City, CA).
Table 1. Primers used for PCR amplification of MIP.
| Exon | primer (5′-3′) | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| MIP-1F |
GACTGTCCACCCAGACAAGG |
|
|
| MIP-1R |
TCAGGGAGTCAGGGCAATAG |
492 |
58 |
| MIP-2F |
TGAAGGAGCACTGTTAGGAGATG |
|
|
| MIP-2R |
AGAGGGATAGGGCAGAGTTGATT |
500 |
58 |
| MIP-3F |
CCAGACAGGGCATCAGT |
|
|
| MIP-3R |
TGGTACAGCAGCCAACAC |
373 |
58 |
| MIP-4F |
AAGGTGTGGGATAAAGGAGT |
|
|
| MIP-4R | TTCTTCATCTAGGGGCTGGC | 429 | 58 |
Cited from reference [6].
Results
Clinical findings
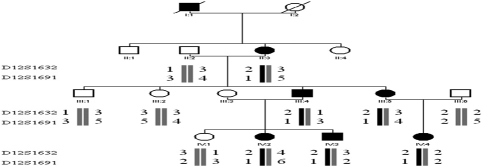
We identified a four-generation family with seven conformed individuals affected with congenital cataract (Figure 1). Blood was collected from six patients and five other family members. None of the participating patients have any other clinically-related ophthalmic syndromes.
Figure 1.
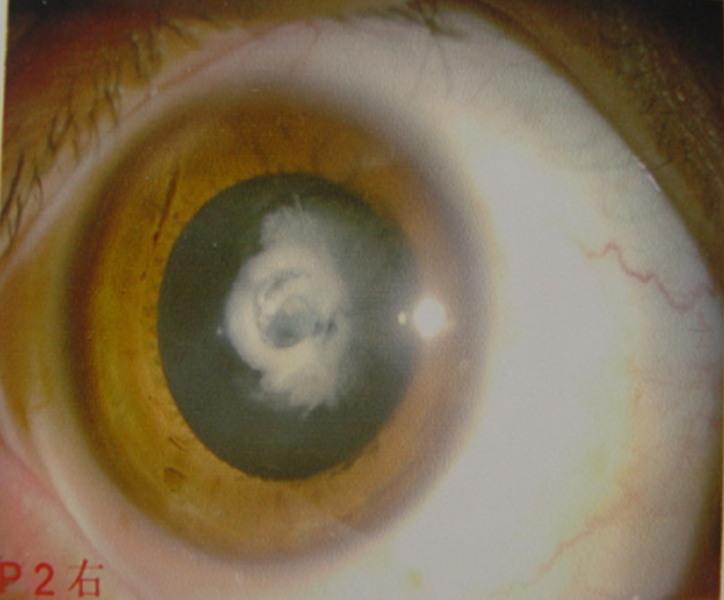
Slit lamp photograph showing nuclear cataract of patient IV:3 from Figure 2.
Linkage and haplotype analysis
We obtained positive logarithm-of-odds (LOD) scores with markers at 12q13 (Table 2). A maximum positive LOD score of 2.11 at θ=0.00 was obtained with marker D12S1632. The haplotype showed complete cosegregation in all six of the affected individuals analyzed (Figure 2).
Table 2. Result of linkage analysis.
|
Marker |
LOD scores at θ= |
|||||
|---|---|---|---|---|---|---|
| 0.00 | 0.01 | 0.10 | 0.20 | 0.30 | 0.40 | |
| D12S1632 |
2.11 |
2.07 |
1.74 |
1.33 |
0.88 |
0.41 |
| D12S1691 | 2.11 | 2.07 | 1.74 | 1.33 | 0.88 | 0.41 |
Figure 2.
Pedigree and haplotype of the family. A four-generation pedigree with eleven available members is shown. Two markers (D12S1632 and D12S1691) close to MIP were used. The disease haplotype (represented by the black bar) cosegregated with all affected members but was not shared with any of the unaffected members.
Mutation analysis
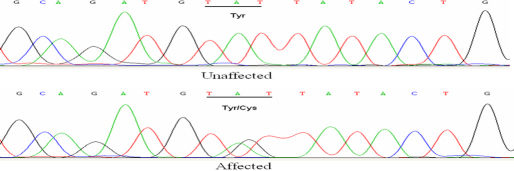
By directly sequencing the coding region of MIP, a novel heterozygous A→G transition at nucleotide position 530 (c. 530 A→G) was detected in this family (Figure 3). This transition leads to the replacement of a highly-conserved Tyr to Cys at codon 177 (P.Y177C). Analysis of all other members of this family showed cosegregation of this change with the disease phenotype only in the affected individuals. This nucleotide substitution was not observed in any of the 100 control chromosomes from individuals with the same ethnic background.
Figure 3.
DNA sequences of MIP in unaffected and affected individuals. A heterozygous change A>G at codon 177 (UAU-UGU) resulted in the substitution of Tyr by Cys (P.Y177C) in the affected individuals.
Discussion
Aquaporins are a large family. Since Agre demonstrated the water channel AQP1 in 1992, 13 isoforms have been found in mammals, including AQP0 to AQP12 [7]. Aquaporin expression is found throughout the body but is tissue-specific. Malfunctions of aquaporins are associated with diseases.
The MIP gene, also known as MIP26 or the AQP0 gene, encodes a 263-amino acid peptide. MIP is inserted in the plasma membrane with six transmembrane bilayer-spanning domains. Two NPA box domains form a water pore channel spanning the lipid bilayer. MIP exists as a tetramer in the plasma membrane [8,9].
Up to now, seven mutations have been reported, including c. 401A→G, c. 413C→G, c. 698G→A, c. 97C→T, c. 319G→A, IVS3–1 G→A, and 3223delG [6,8,10-13]. These mutations resulted in different phenotypes. The protein-functions study found that the mutated proteins affected water-permeability properties and trafficking [14,15].
The Y177C mutation is located in the fifth transmembrane. The substitution of Tyr by Cys would create an extra -SH that may result in incorrect disulfide bonds and influence the formation of a correct structure. The mutated protein may changes the water pore channel function, possibly through affecting water-permeability properties or trafficking. Further study of the protein function is needed to provide insights into the molecular mechanism of the mutation identified in this study.
In conclusion, we found a novel heterozygous Y177C mutation in MIP of an autosomal dominant congenital nuclear cataract family. These findings expand the mutation spectrum of MIP.
References
- 1.Rahi JS, Dezateux C, British Congenital Cataract Interest Group Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci. 2001;42:1444–8. [PubMed] [Google Scholar]
- 2.Wirth MG, Russell-Eggitt IM, Craig JE, Elder JE, Mackey DA. Aetiology of congenital and paediatric cataract in an Australian population. Br J Ophthalmol. 2002;86:782–6. doi: 10.1136/bjo.86.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert SR, Drack AV. Infantile cataracts. Surv Ophthalmol. 1996;40:427–58. doi: 10.1016/s0039-6257(96)82011-x. [DOI] [PubMed] [Google Scholar]
- 4.Rahi JS, Dezateux C. Congenital and infantile cataract in the United Kingdom: underlying or associated factors. Invest Ophthalmol Vis Sci. 2000;41:2108–14. [PubMed] [Google Scholar]
- 5.Hejtmancik JF. Cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19:134–49. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J, Jin C, Wang W, Tang X, Shentu X, Wu R, Wang Y, Xia K, Yao K. Identification of a novel splice-site mutation in MIP in a Chinese congenital cataract family. Mol Vis. 2009;15:38–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–7. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 8.Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant 'polymorphic' and lamellar cataracts linked to 12q. Nat Genet. 2000;25:15–7. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- 9.Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–54. [PubMed] [Google Scholar]
- 10.Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, Berry R, Sparkes RS, Jonsen MD, Isenberg SJ, Bateman JB. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am J Ophthalmol. 2006;141:761–3. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu F, Zhai H, Li D, Zhao L, Li C, Huang S, Ma X. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol Vis. 2007;13:1651–6. [PubMed] [Google Scholar]
- 12.Lin H, Hejtmancik JF, Qi Y. A substitution of arginine to lysine at the COOH-terminus of MIP caused a different binocular phenotype in a congenital cataract family. Mol Vis. 2007;13:1822–7. [PubMed] [Google Scholar]
- 13.Wang W, Jiang J, Zhu Y, Li J, Jin C, Shentu X, Yao K. A novel mutation in the major intrinsic protein (MIP) associated with autosomal dominant congenital cataracts in a Chinese family. Mol Vis. 2010;16:534–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Francis P, Chung JJ, Yasui M, Berry V, Moore A, Wyatt MK, Wistow G, Bhattacharya SS, Agre P. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000;9:2329–34. doi: 10.1093/oxfordjournals.hmg.a018925. [DOI] [PubMed] [Google Scholar]
- 15.Varadaraj K, Kumari SS, Patil R, Wax MB, Mathias RT. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp Eye Res. 2008;87:9–21. doi: 10.1016/j.exer.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]