Abstract
BACKGROUND
TRALI is the leading cause of transfusion-related deaths. Donor HLA antibodies have been implicated in TRALI cases. Blood centers are implementing TRALI risk reduction strategies based on HLA antibody screening of some subpopulations of ever-pregnant apheresis platelet donors. However, if screening assay cutoffs are too sensitive, donation loss may adversely impact blood availability.
STUDY DESIGN
Pregnancy history and HLA antibody screening and single antigen bead (SAB) data from blood donors in the REDS-II Leukocyte Antibody Prevalence Study (LAPS) were evaluated for correlations between assay screening values, HLA antibody titer, and number of HLA antigen specificities. The probabilities of matching a cognate antigen in a recipient were calculated and examined in association with total number of specificities observed and screening values. The relative impact of imposing various screening assay cutoffs or pregnancy stratification was examined in relation to detection of HLA antibody reactive donations and loss of donors and donations.
RESULTS
We provide evidence that higher HLA Ab screening assay values are associated with maintaining higher screening signals upon dilution and an increased breadth of specificities compared with lower screening values; the latter correlated with an increased risk of a cognate antigen match in potential recipients. Depending upon the TRALI risk reduction strategy used, the potential loss of donations ranged between 0.9 and 6.0%.
CONCLUSION
This analysis should enable blood centers to decide upon a TRALI risk reduction strategy for apheresis platelets that is consistent with how much donation loss the blood center can tolerate.
Keywords: TRALI, Transfusion-Related Acute Lung Injury, HLA antibodies, platelet transfusions
INTRODUCTION
Based on FDA fatality data, Transfusion Related Acute Lung Injury (TRALI) continues to be the leading cause of blood transfusion-related deaths1. Between 2005 and 2009, 48% of all confirmed transfusion related deaths were caused by TRALI: 47% in FY05, 56% in FY06, 65% in FY07, 35% in FY08, and 30% in FY09. In recognition of this, in November 2006, the AABB published an Association Bulletin that recommended that blood collection and transfusion centers implement actions to reduce the risk of TRALI2. This included minimizing the transfusion of plasma-rich components from donors (such as previously pregnant females) likely to be alloimmunized to human leukocyte antigens (HLA), since HLA antibodies in donated blood are thought to contribute to a portion of TRALI cases3–5. However, it should be noted that other factors such as human neutrophil antibodies (HNA)6–8 and non-antibody substances (such as bioactive lipids)9 may also elicit TRALI reactions.
As of 2009, almost all US blood centers have adopted a TRALI risk reduction strategy of using transfusable plasma supplied primarily by male or never pregnant female donors1. With regard to apheresis platelets (and apheresis plasma), many centers have begun to screen some apheresis donors for HLA Ab based on their pregnancy history1; the specific triage strategy used at a given center attempts to balance the potential for TRALI risk reduction against the cost and impact of the strategy on product availability.
The REDS-II Leukocyte Antibody Prevalence Study (LAPS) study was conducted to measure the prevalence of HLA class I and class II and HNA antibodies in donors with or without a history of pregnancy or transfusion10. Results from the REDS-II LAPS study have shown that HLA Ab prevalence was strongly associated with pregnancy history (i.e., increased with each pregnancy up to 4 pregnancies)10,11,11,12. LAPS results also showed HLA Ab prevalence was similar in non-transfused males, transfused males and never pregnant females10. In addition, since there are various HLA Ab assay platforms available, REDS-II has subsequently conducted another study to compare the performance of five different HLA Ab assays on the same sample set13.
A major challenge of screening donors for HLA antibodies is how to decrease the risk of TRALI for recipients while maintaining an adequate supply of plasma-rich blood products such as platelets. For those centers using Luminex testing methodologies, it is possible for the testing laboratory to determine the assay cutoff and consequently several different cutoffs are currently in use throughout the US14. The underlying assumptions for those centers selecting higher assay cutoffs is that blood samples yielding higher values on the screening assay indicate a higher concentration of (i.e. titer)15 and a wider breadth of antibodies (i.e. greater variety of antigen specificities) compared with samples yielding lower values on the screening assay and that this translates into a greater risk of a recipient developing TRALI.
The major aims of this manuscript are to provide evidence supporting the assumptions concerning assay cutoffs and HLA Ab titer and breadth, and then to evaluate a series of potential screening options based on various pregnancy histories and assay cutoffs; the latter assessment was accomplished by projecting apheresis donation loss rates under these various strategies. This analysis should enable blood centers to decide upon a TRALI reduction strategy for apheresis platelets or other blood products that is consistent with how much donation loss the blood center can tolerate.
MATERIALS AND METHODS
Subject and sample selection
LAPS was a prospective cross-sectional six-center study conducted by the Retrovirus Epidemiology Donor Study – II (REDS-II) program of the National Heart, Lung, and Blood Institute. All six REDS-II blood centers participated in the study. These included: American Red Cross New England region (Dedham, MA), American Red Cross Southern Region (Douglasville, GA), BloodCenter of Wisconsin (Milwaukee, WI), Blood Centers of the Pacific (San Francisco, CA), Hoxworth Blood Center/University of Cincinnati Academic Health Center (Cincinnati, OH) and the Institute for Transfusion Medicine (Pittsburgh, PA). The REDS-II Coordinating Center is Westat (Rockville, MD) and the REDS-II central laboratory is Blood Systems Research Institute (San Francisco, CA). LAPS enrollment and study design have been previously described in detail10. Briefly, donors consenting to the study provided a blood sample for HLA Class I and II Ab testing and a detailed history of pregnancy and transfusion. A total of 8171 (6011 females, 2160 males) donors were enrolled. Females and transfused males were intentionally oversampled. The demographics (gender, age, race/ethnicity, and parity) of LAPS donors that had a donation screened for HLA Abs (see below) were: 26% male, 74% female; mean age = 47.1 years (+/− 14.6 years); approximately 90% white, 3% black, 3% hispanic, 2% asian, and less than 2% other race/ethnicity; and 31% with zero pregnancies, 11% with 1 pregnancy, 23% with 2 pregnancies, 18% with 3 pregnancies, and 17% with 4 or more pregnancies.
HLA Class I and Class II Ab screening
HLA Ab test results were obtained using the One Lambda (Canoga Park, CA) LabScreen mixed Luminex assay (lot number 13) on plasma samples from 7,841 blood donors10. Samples were from individual donations from each of the 7,841 donors; please note that the term donation refers to the number of donation procedures, not the number of split apheresis products. The assay signal outputs (i.e. normalized background [NBG] ratios from the highest one of eight multi-antigen beads) from 1,138 never-transfused males (used as non-alloexposed population) were log transformed and the mean plus 3, 4, and 5 standard deviations (SD) were calculated separately for HLA class I (CL-I) and class II (CL-II). The log transformed mean plus 3SD, 4SD, or 5SD potential cutoff values were linearized into the following values: 3SD: CL-I= 10.8; CL-II = 6.9. 4SD: CL-I= 25.4; CL-II = 13.8. 5SD: CL-I= 59.3; CL-II = 27.5. The LAPS study investigators decided upon the 3SD values for defining HLA positivity, based on approximately 1% of non-transfused males being reactive for HLA antibodies at that cutoff10.
HLA Ab titration studies
A total of 96 plasma samples were selected from different donations and were prepared at varying dilutions. Test samples were diluted with plasma from a single male donor with low levels of signal on all beads of the HLA Ab screening assay.
Using a set of lower NBG values previously suggested by the manufacturer, thirty-two samples were selected from donations that were designated as positive for Class I only (CL-I NBG >2.2 and CL-II NBG <2.2), 32 from donations that were designated as positive for Class II only (CL-II NBG >2.2 and CL-I NBG <2.2), and 32 from donations that were designated as positive for Class I and Class II (both CL-I and CL-II NBG >2.2). Within each of those three groupings, roughly equal numbers of samples were further selected based on two further parameters: HLA Ab screening NBG values and the number of Ab specificities found in the HLA single antigen bead (SAB) test. Screening assay categories were: Low, which included donations with screening NBG values greater than 2.2 but less than or equal to 3SD (CL-I= 10.8; CL-II = 6.9); Medium, which included donations with screening NBG values greater than 3SD but less than or equal to 4SD (CL-I= 25.4; CL-II = 13.8); High, which included donations with screening NBG values greater than 4SD but less than or equal to 5SD (CL-I= 59.3; CL-II = 27.5); and Very High, which included donations with screening NBG values greater than 5SD. As previously reported, single antigen assay categories were based on the number of reactive SAB (1, 2 to 3, 4 or more) identified by the One Lambda LS1A04 or LS2A01 SAB assay using the following cutoffs for determining reactivity: CL-I Median Fluorescence Intensity (MFI) > 2500; Cl-II MFI > 150011.
Donation Loss
The projected plateletpheresis donation losses were calculated for testing under different pregnancy triage criteria and antibody cutoffs. LAPS HLA Ab screening results were weighted for 2007 REDS-II donation data, which included 37% apheresis donations from females, 66% of whom had a history of prior pregnancy (12% one pregnancy, 23% two pregnancies, 16% three pregnancies, 15% four or more pregnancies).
Calculated Panel Reactive Antibody (CPRA) calculation
The “CPRA calculator (available” at http://www.unos.org/resources) was used to calculate the probability that a randomly selected recipient would have a cognate antigen matching the specific HLA antibodies in the donor’s plasma. Specific HLA antibodies in the donor’s blood were determined using the definition of MFI >2500 for CL-I SABs or MFI>1500 for CL-II SABs11. For this calculation, only donations that had a screening NBG value greater than the 3SD cutoff were included, as this was the criterion previously used for the initial LAPS analysis.
Statistical Analysis
SAS (v 9.1.3 (2004) SAS Institute Inc., Cary, NC) was used to calculate titration curve slopes and the Kendall Tau correlation statistics.
RESULTS
Rates of HLA Ab positivity are higher in ever pregnant females at three HLA Ab screening assay cutoff thresholds
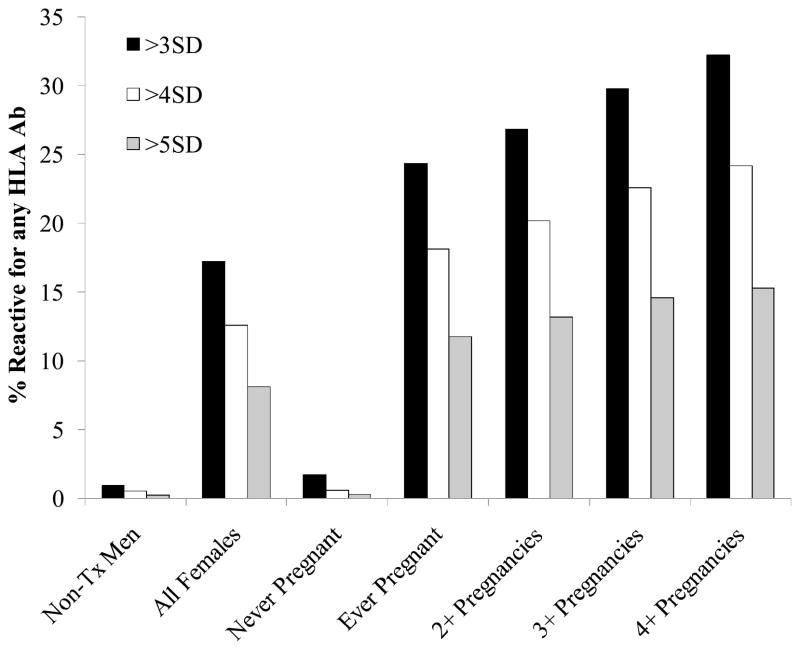
The positivity rates for HLA antibodies in the LAPS donors were determined using the >3SD, >4SD, and >5SD HLA Ab assay screening cutoff values (Fig. 1). As previously reported10 HLA Ab positive rates (determined using the 3SD cutoff for the screening assay) increased with each pregnancy. In the current analysis, when the higher cutoff values of 4SD and 5SD were applied, fewer donors were designated as positive, but the observed trend for rates of positivity to increase with an increasing number of pregnancies remained. Using the >3SD cutoff, 24% of all ever pregnant females, 27% of females with 2 or more pregnancies, 30% of females with 3 or more pregnancies, and 32% of females with 4 or more pregnancies were found to have HLA antibodies. Using the >4SD cutoff, between 18% (ever pregnant females) and 24% (females with 4 or more pregnancies) had HLA antibodies. Using the >5SD cutoff, the range was between 12% (ever pregnant females) and 15% (females with 4 or more pregnancies) with HLA antibodies.
Figure 1. HLA Ab screen reactive rates in LAPS donations at >3SD, >4SD, or >5SD cutoffs by pregnancy.
The percent reactive donations at the various HLA Ab screen assay cutoffs was determined. 3SD cutoffs = 10.8 for Class I, 6.9 for Class II. 4SD cutoffs = 25.4 for Class I, 13.8 for Class II. 5SD cutoffs = 59.3 for Class I, 27.5 for Class II. Denominators used in the calculations of reactivity rates were: n=1138 non-transfused males, 5834 all females, 1816 never pregnant females, 3992 females with 1 or more pregnancies, 3358 females with 2 or more pregnancies, 2051 females with 3 or more pregnancies, and 993 females with 4 or more pregnancies.
Screening assay NBG values decreased in a linear fashion in titration studies
In order to examine the assumption that higher values on the screening assay indicate a higher titer of HLA antibodies, we conducted a titration study to determine the relationship between initial NBG values and the signal decrease in response to sample dilution. Plasma samples exhibiting a wide range of screening assay NBG values and representing a range of HLA SAB specificities were assayed. Slopes were calculated for each sample and were correlated with NBG values on corresponding undiluted samples. Titration plots (Fig. 2) show the log NBG ratio has a linear relationship with the log dilution within the range of NBG ratios between 2.2 and 100 (for Class I and Class II). The average Class I slope is −0.555 on the log scale (Standard Error (se)=0.011, p<0.0001), indicating that a 10-fold dilution results in a 3.59-fold (linear scale) reduction in the NBG ratio. The average Class II slope is −0.675 on the log scale (se=0.014, p<0.0001), indicating a 10-fold dilution results in a 4.73-fold reduction in the NBG ratio.
Figure 2. HLA Ab screening NBG values at various dilutions.
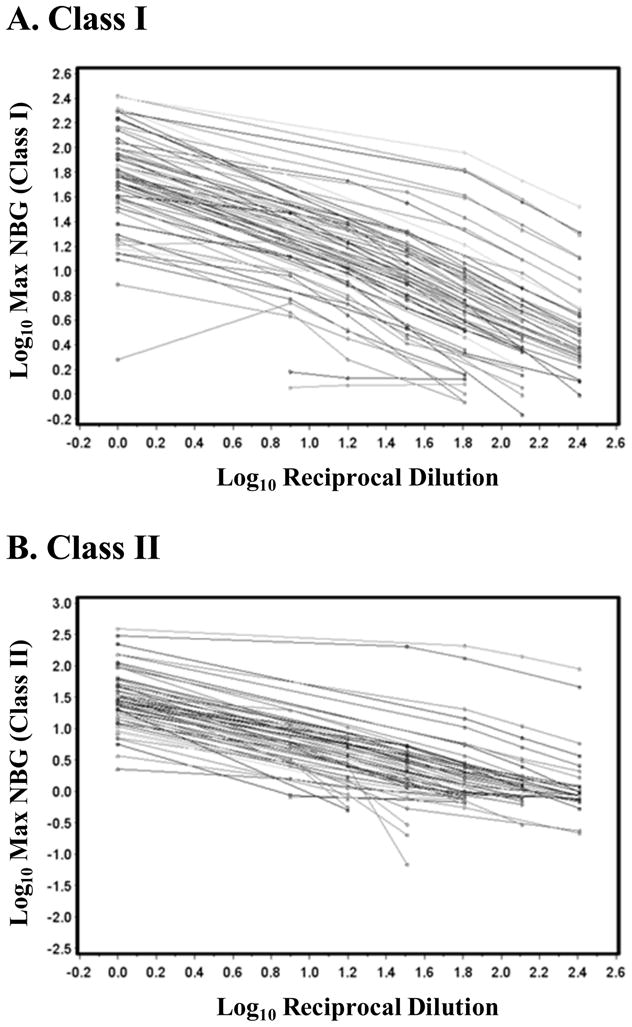
A) Class I HLA Ab results; B) Class II HLA Ab results. The lines connect the results for each individual sample at various dilutions.
Higher HLA Ab screening values correlate with a broader array of HLA antigen specificities
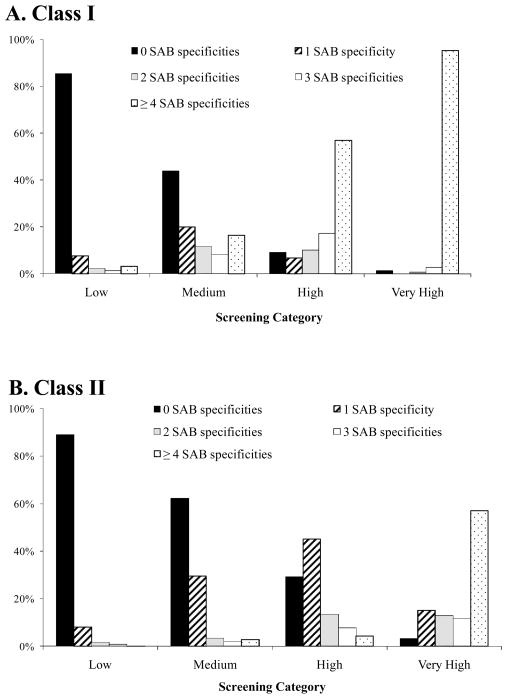
Higher values on the screening assay may also indicate an increased breadth of HLA Ab specificities (i.e., antibodies to a larger number of HLA antigens). In order to examine this, the LAPS samples were categorized according to both the HLA Ab screening values (Low, Medium, High, Very High – see Methods section) and SAB assay results. The number of HLA antigens was based on the number of reactive SABs (0, 1, 2, 3, 4 or >4).
Compared with samples that yielded lower signals, samples with higher signals on the Luminex-based screening assay were associated with a broader array of specificities (Fig. 3). The majority (85.5% CL-I; 89.0% CL-II) of samples in the lowest screening assay reactivity category had 0 SABs. The proportion of samples with 0 reactive SABs decreased as the screening values increased: 43.8% CL-I and 62.3% CL-II of samples in the medium screen category, 9.1% CL-I and 29.3% CL-II of samples in the high screen category, and 1.4% CL-I and 3.2% CL-II of samples in the very high screen category. The opposite trend was observed for those with larger numbers of reactive SABs. Kendall’s tau-b statistics showed highly significant correlations (p<0.001) amongst the screening categories and single antigen reactivity categories for both CL-I and CL-II antibodies.
Figure 3. Number of SAB serological specificities in donors who screened positive with different screen cutoffs.
Class I data (A) and Class II data (B) were categorized by screening result and SAB reactivity. See text for more information regarding these categories.
Samples with a broader array of specificities or higher HLA Ab screening values have higher probability of matching a cognate antigen in a recipient
The specific concern about samples with an increasing number of HLA Ab single antigen specificities is that there may be a greater potential for plasma from such individuals to be transfused to recipients with at least one cognate antigen. To evaluate this, we estimated the percentage of recipients that could be expected to have cognate antigen using the Calculated Panel Reactive Antibody (CPRA) calculator. Donations with a larger number of specificities tended to have a greater probability of matching cognate antigens in randomly selected potential recipients, as is seen in Table 1, where the average CPRA values are shown to increase with increasing numbers of reactive SABs. Donations that screened positive for CL-I HLA antibodies at the >3SD cutoff (LAPS definition of screen positive) yielded an average CPRA value of 16.0 when only one reactive CL-I SAB was detected, 20.8 with two CL-I SABs, 28.1 with three CL-I SABs, 34.8 with four CL-I SABs, and 54.8 with more than four CL-I SABs. A similar trend was observed for CL-II, where donations that screened positive for CL-II HLA antibodies at the >3SD cutoff yielded an average CPRA value of 23.5 when only one reactive CL-II SAB was detected, 38.5 with two CL-II SABs, 48.4 with three CL-II SABs, 55.0 with four SABs, and 76.3 with more than four CL-II SABs. Average CPRA values over 50 indicate that more than 50% of potential recipients are expected to have cognate antigen(s). This level was reached with donations that screened positive at the >3SD cutoff and had more than four CL-I SABs (average CPRA = 54.8) or had four CL-II SABs (average CPRA = 55.0).
Table 1.
Probability of Ab Matching Cognate Antigen in Recipients
| HLA antibody class | Screening Cutoff selected for determining a positive result | Average CPRAa: Percentage of Recipients Expected to Have Cognate Antigen(s) SAB Category (# of Reactive SABs) |
|||||
|---|---|---|---|---|---|---|---|
| 1 SAB b | 2 SAB b | 3 SAB b | 4 SAB b | >4 SAB b | Total | ||
| Class I | >3 SD | 16.0 | 20.8 | 28.1 | 34.8 | 54.8 | 38.6 |
| >4 SD | 12.2 | 19.4 | 30.2 | 35.7 | 56.2 | 45.7 | |
| >5 SD | N/A | 31.0 | 28.5 | 27.0 | 67.9 | 63.9 | |
| Class II | >3 SD | 23.5 | 38.5 | 48.4 | 55.0 | 76.3 | 47.0 |
| >4 SD | 23.5 | 38.4 | 48.1 | 54.5 | 76.1 | 49.0 | |
| >5 SD | 22.8 | 37.5 | 47.5 | 54.8 | 76.2 | 53.9 | |
CPRA calculator (http://www.unos.org/resources ) was used to calculate the probability of an antibody-antigen match for recipients of each donation that had one or more reactive beads on the SAB assay. Donations with specificities not on the calculator (e.g. Cw3) were excluded from this analysis.
Reactive SABs were defined as those have MFI > 2500 for Class I beads or MFI > 1500 for Class II beads (Endres 2010).
The probability of potential recipients to have cognate antigen(s) also increased with increasing NBG cutoff levels, regardless of the number of SABs. The overall CPRA for CL-I at the >3SD cutoff level was 38.6%. This increased to 45.7% at the >4SD cutoff level and as high as 63.9% at the >5SD cutoff level. A similar trend was observed for CLI-II, although not as marked as with CL-I. The overall CPRA for CLI-II was 47.0% at the >3SD level, 49.0% at the >4SD level, and 53.9% at the >5SD level.
Relative impact of imposing higher screening assay cutoffs or pregnancy stratification on detection of HLA Ab reactive donations was assessed
As a potential TRALI reduction strategy, many blood centers are testing ever pregnant females or females in certain pregnancy groupings for HLA antibodies. We therefore examined the impact of testing females with increasing numbers of previous pregnancies on detection of HLA Ab reactive donations at >3, >4 and >5 SD cutoffs. The >3SD cutoff was selected for our definition of HLA Ab reactivity in this analysis since that was the cutoff employed in the primary REDS-II LAPS study manuscript10. By applying the criteria of testing all females with any history of pregnancy for HLA antibodies, such as is done by 40% of the blood centers polled in the recent AABB survey14, 97% of all LAPS donations that were reactive above the 3SD cutoff would have been detected (Table 2). If we instead applied a criterion of testing only females with a history of four or more pregnancies, only 31% of all LAPS donations that were reactive above the 3SD cutoff would have been detected. In a more stringent scenario that involves testing only females with a history of four or more pregnancies and also applying the highest cutoff presented in this analysis (>5SD cutoff), only 15% of all LAPS donations that were reactive (above the 3SD cutoff) would have been detected. Thus in this last scenario (>5SD cutoff), approximately 85% of donations that would be considered to have HLA antibodies (i.e. reactive above the 3SD cutoff) would have been missed.
Table 2.
Detection of HLA Ab screen reactive donorsa if HLA Ab testing were restricted to females based on various pregnancy histories.
| Screening Cutoff | Total # of LAPS Donors Positive for Any HLA Ab | All Females | Ever Pregnant | 2+ Pregnancies | 3+ Pregnancies | 4+ Pregnancies |
|---|---|---|---|---|---|---|
| n | n (%) of all screen positive LAPS Donors with any HLA antibodies using a 3SD cutoffb2 | |||||
| >3SD | 1033 | 1006 (97.4%) | 973 (94.2%) | 902 (87.3%) | 611 (59.1%) | 320 (31.0%) |
| >4SD | 748 | 735 (71.2%) | 723 (70.0%) | 677 (65.5%) | 463 (44.8%) | 240 (23.2%) |
| >5SD | 482 | 476 (46.1%) | 469 (45.4%) | 442 (42.8%) | 299 (28.9%) | 152 (14.7%) |
HLA Ab screen reactivity was defined as having NBG values greater than the 3SD cutoffs on the Luminex LabScreen Mixed assay.
Denominator = Total # of LAPS donors screen positive for any HLA Ab using 3SD cutoffs as the definition of screen positive (n=1033). Samples were from individual donations from unique donors.
Since we showed samples with a higher number of reactive SABs are more likely to demonstrate cognate antigen recognition (CPRA) and may present a greater risk to recipients, we examined the potential impact of applying various screening cutoffs on detection of donations with higher numbers of reactive SABs. By testing all females or just ever pregnant females and applying the >3SD cutoff, between 98 and 99.6% of all screen positive LAPS donations with a large number (four or more) of CL-I or CL-II SABs were detected (Table 3). The detection rate of screen positive LAPS donations with four or more CL-I or CL-II SABs dropped to 15–29% when only females with 4 or more pregnancies and the highest cutoff analyzed (>5SD) was used.
Table 3.
HLA Ab screened positive samples with 4 or more reactive SABs, by pregnancy
| Screening Cutoff | Total # of LAPS Donors with 4 or more reactive SABs | All Females | Ever Pregnant | 2+ Pregnancies | 3+ Pregnancies | 4+ Pregnancies | |
|---|---|---|---|---|---|---|---|
| N | n (%) of all screen positive LAPS donors using a 3SD cutoff with 4 or more reactive SABs1 | ||||||
| >3SD | Class I | 300 (100%) | 298 (99.3%) | 296 (98.7%) | 279 (93.0%) | 182 (60.7%) | 94 (31.3%) |
| Class II | 241 (100%) | 240 (99.6%) | 236 (97.9%) | 220 (91.3%) | 145 (60.2%) | 72 (29.9%) | |
| >4SD | Class I | 257 (85.7%) | 255 (85.0%) | 253 (84.3% | 237 (79.0%) | 155 (51.7%) | 80 (26.7%) |
| Class II | 237 (98.3%) | 236 (97.9%) | 232 (96.3%) | 216 (89.6%) | 143 (59.3%) | 71 (29.5%) | |
| >5SD | Class I | 138 (46.0%) | 137 (45.7%) | 135 (45.0%) | 129 (43.0%) | 87 (29.0%) | 46 (15.3%) |
| Class II | 231 (95.9%) | 230 (95.4%) | 226 (93.8%) | 212 (88.0%) | 139 (57.7%) | 69 (28.6%) | |
Denominator = total number of screen positive (at 3SD) LAPS Donors with 4 or more reactive SABs (n=300 for Class I; n= 241 for Class II).
Projections of potential donation loss vary depending upon screening assay cutoffs and pregnancy restrictions
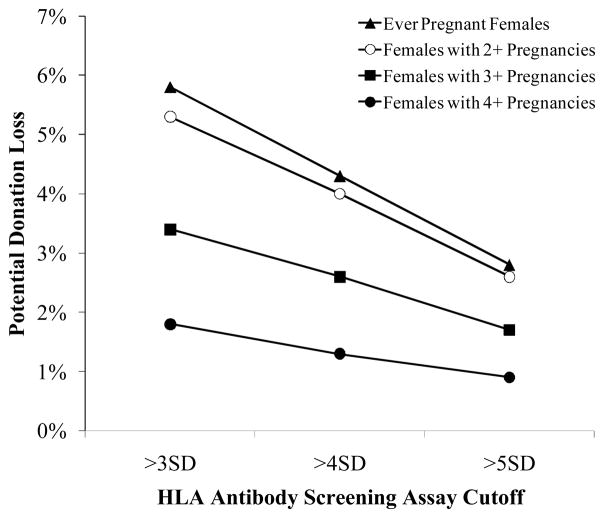
In order for blood collection and transfusion centers to make a decision regarding whether to consider blood donation restrictions based on pregnancy as well as HLA Ab reactivity levels, it is critical to be knowledgeable about the potential trade-off between apheresis donation loss and HLA Ab detection associated with imposing various criteria for screening donations. By restricting plasma and/or platelet donations from females that have any history of a pregnancy and have HLA Ab reactivity above the lowest threshold (>3SD), blood centers with a population similar to REDS-II centers (an average of 37% female apheresis platelet donors, with variation across centers ranging from 35% to 41%16) can expect to lose approximately 6% of their current apheresis donations (Fig. 4); if newly recruited apheresis donors have similar proportions of females with similar parity histories to those represented in the REDS-II blood centers, then similar donation loss would be expected on an ongoing basis. As the cutoffs and pregnancy restrictions get more stringent, the donation loss decreases. Donation loss ranged from 0.9% (using the most stringent criteria presented: four or more pregnancies and >5SD cutoff) to 5.8% (using one of the most liberal criteria presented: one or more pregnancies and >3SD cutoff).
Figure 4. HLA Ab screening reactivity rates and projected donation loss under various potential TRALI reduction strategies.
The potential loss of donations at the various cutoffs if blood centers implemented a policy of testing ever pregnant females (triangles), females with 2 or more pregnancies (open circles), 3 more pregnancies (boxes), or 4 more pregnancies (closed circles).
DISCUSSION
TRALI risk-reduction strategies have been widely implemented by blood centers and often involve testing female apheresis donors, or certain subsets of female apheresis donors based on assessing pregnancy history, for the presence of HLA Ab, which is thought to be a major cause of TRALI7,8,17. Blood centers performing HLA Ab testing by Luminex technology have implemented a variety of cutoff levels for HLA Ab assays11. Our study provides data impacting the operational issue of how to potentially reduce the risk of TRALI by screening for HLA antibodies while maintaining an adequate supply of blood products. This analysis is not intended to recommend specific cutoffs. Rather, the data presented here should enable blood centers to set their own parity and assay cutoffs based on how much donation loss they can tolerate. Selecting a cutoff requires one to balance enhanced safety with potential donation loss. Ideally, clinical data would be evaluated in a prospective study to determine the levels (e.g., end-point dilution titers, NBG ratios, Optical Densities (OD)) and breadth of HLA reactivities in plasma from HLA-Ab-reactive donations implicated in TRALI cases to support establishing definitive cutoffs to achieve optimal safety. Although no such prospective data exist, support for the premise of our analysis is provided by a recent Japanese retrospective study of recipients with transfusion reactions.18 This study showed that donor HLA antibody strength as determined by both ELISA and flow cytometry single antigen bead assays, when combined with reactivity to the recipient’s cognate antigen, correlated with the occurrence of TRALI.
Our analyses demonstrate that samples yielding higher signals on a Luminex based screening assay are associated with a broader array of antigen specificities, compared with samples that yielded lower signals. Donations with a larger number of Ab specificities tended to have a greater probability of finding cognate antigens in randomly selected potential recipients; this was seen by the average CPRA values increasing as the NBG ratios increased (overall) and also as the number of SABs increased (Table 1). Since the majority of donations in the very high screen category (>5SD) had large numbers of SABs (Fig. 3), this suggests that donations with very high HLA NBG ratio include those donations that present the greatest risk to potential recipients. Consequently, establishing higher HLA Ab screening assay cutoffs may result in removing most of the donations that are associated with the high risk for TRALI, while minimizing donor/product loss.
In examining the relationship between assay signal strength and Ab titer, titration plots showed a linear relationship between the log dilution and the log NBG ratio values within the NBG ratio range of 2.2 and 100. These data support previous findings obtained in the organ transplant setting by Mizutani et al15 showing that HLA Ab titer was directly associated with values from Luminex assays. This titration work provides data to allow one to estimate how diluting (reducing) the plasma in blood components for transfusion can impact HLA antibody screening values and consequently the levels of HLA Abs infused into patients. For example, a 3-fold dilution resulted in a 1.84-fold reduction in the CL-I and 2.10-fold reduction in the CL-II NBG ratios. A 3-fold reduction of plasma in an apheresis platelet component may be achieved by use of platelet additive solutions. Although platelet additive solutions are used in some European countries, there are no available hemovigilance data to indicate whether their use reduces the incidence of TRALI.19,20
Imposing a strategy of screening all females and applying higher cutoffs would impact detection of donations with large numbers of CL-I specificities more so than donations with large numbers of CL-II specificities. For example, 98% and 95% of those with 4 or more CL-II SABs were detected at the >4SD and >5SD cutoffs, respectively (Table 4). However, moving from the >4SD cutoff to the >5SD cutoff had a bigger impact on CL-I SAB detection. At the >4SD cutoff, 85.0% of those with 4 or more CL-I SABs were detected; this detection rate dropped to less than 50% of those with 4 or more CL-I SABs when the >5SD screening cutoff was applied. The pattern described here for all females was similar in an analysis that was restricted to ever pregnant females (not shown).
Imposing a TRALI risk reduction strategy of screening females by pregnancy also would reduce detection of donations with reactivity to large numbers of SABs. The biggest impact on detection of donations with reactivity to four or more SABs was observed between 2 or more pregnancies and 3 or more pregnancies, where detection dropped by approximately one third (average reduction of 28%; range of 14% decrease for CL-I at >5SD cutoff to 32% decrease for CL-I at >3SD cutoff; Table 3). Moving from the 3 or more pregnancies to 4 or more pregnancies categories also resulted in a large decrease in detection of donations with large numbers of SABs, with detection rates declining between 1.9 and 2.0-fold. When both extreme TRALI reduction strategies were combined, detection of donations with large numbers of reactive SABs was further reduced; i.e. at the highest cutoff presented (>5SD) and largest pregnancy restriction (4 or more pregnancies), only 15% of all donations with 4 or more CL-I SABs and 29% of all donations with 4 or more CL-II SABs were detected.
By applying more stringent TRALI risk-reduction strategies, such as screening females with four or more pregnancies and/or higher HLA Ab screening assay reactivity thresholds, centers can expect to lose a smaller number of donations. While the most stringent criteria decreased the loss of donations to less than 1%, using those criteria also caused the percentage of HLA Ab reactive donations detected to decrease by more than 6.5-fold compared with a strategy of screening ever pregnant females and implementing the lowest cutoff (>3SD) presented (Table 2). Additionally, using the most stringent criteria decreased the detection rate for donations with high numbers of CL-I and CL-II SABs. Therefore, imposing higher screening assay cutoffs and pregnancy criteria may allow loss of fewer donations, but in doing so, one needs to carefully consider whether the potential reduction in detection of HLA Ab reactive donations, particularly those with higher titers and larger numbers of SAB specificities, is acceptable.
Combining different HLA Ab assay cutoff levels with strategies of triaging female donors to be tested based on the number of reported pregnancies results in wide variations in the number of tests performed and the number of HLA Ab reactive donors detected. Although statistical approaches such as receiver operator curves (ROC) could be applied to generating a HLA Ab assay NBG cutoff that would be predicted to be “most effective”, the “optimal” NBG cutoff value with the desired sensitivity for detecting HLA antibodies that cause TRALI remains unknown. Hashimoto, et. al.18 used the approach of ROC analysis and determined a range of potential optimal cutoffs (2.0 to 6.5) for an ELISA HLA Ab assay; those results were based on testing a small number of donations that went to recipients diagnosed with TRALI (n=32) or other nonhemolytic transfusion reactions (n=36). However, currently, there is no reliable clinical case information to be able to make this assessment for the assays used in our study. In the absence of such data, a local hemovigilance program that monitors the occurrence of TRALI cases in hospitals served by the blood center, when feasible, could assist the center in assessing the effectiveness of their intervention strategy.
This study provides blood centers information to help formulate informed decisions on implementation of pregnancy and assay cut-offs as a potential means to minimize the risk of TRALI while maintaining an adequate platelet supply. Several blood centers began their risk reduction programs using high cutoff values (such as >5SD) as well as a high number of pregnancies (such as 4+ pregnancies) in order to minimize donor and donation loss14. However, as these centers successfully compensate for the loss of plateletpheresis donors and platelet inventories become stable after the introduction of a given screening strategy, they may wish to identify a larger number of HLA antibody donors (thereby theoretically increasing their TRALI risk reduction) by lowering either the pregnancy cutoff or the assay cutoff values. Our data show that the strategy of decreasing the number of pregnancies that trigger testing and retaining a high NBG cutoff threshold, would be the a more effective way of detecting donations associated with higher titers and broader specificities than would the alternative approach of decreasing the assay cutoff and retaining the current pregnancy triage criteria.
There are some limitations to the estimates of donation loss presented in this manuscript. First, in projecting donation loss, we assumed a donor and donation gender and parity distribution that is comparable to the REDS-II centers16. Second, the donation loss estimates presented here may be overestimating the rate of component loss since it is likely that females make fewer annual apheresis donations than do males and are also less likely to have their plateletpheresis split into two or three transfusable apheresis units. Thirdly, these estimates of donation loss from current blood donors do not reflect the loss of a similar percentage of donations from newly recruited donors if centers continue to recruit female apheresis donors. If newly recruited parous female donors were to be recruited in a similar proportion, then similar rates of future donation loss would be expected.
It is possible that potential TRALI risk for recipients may be more accurately linked to the average overall PRA of the transfused component than to the number of SAB specificities. The average calculated PRA for different groups of donors, grouped by NBG ratio and by number of SAB specificities is shown in Table 1. The overall calculated PRA for donors classified as HLA antibody positive at each screening cutoff level can be assessed by multiplying the proportion of donors with the designated number of SAB specificities at each cutoff (derived from Figure 3) by the PRA for that number of SAB specificities (derived from Table 1). Figure 3 shows that the largest proportion of donors with high numbers of SAB specificities were found in the group that was reactive at >4 SD for Class I (High or Very High in Figure 3) and >5 for Class II (Very High).. Table 1 shows that this group of donors also had the highest average PRAs calculated. When combined, these two observations clearly demonstrate that the largest proportion of risk for potential cognate antigen-antibody interactions in recipients is found in donors that are screened as positive at the higher cutoff levels.
A limitation of the information in Table 1 is that samples demonstrating antibodies against HLA-C and HLA-DP specificities (approximately 30% for the group with >4SAB but <10% for the other groups) were excluded because those specificities could not be entered into the CPRA calculation. These donors would be expected to have higher CPRA values on average because the majority of them also had antibody specificities against HLA-A, B, DR and/or DQ specificities. Donors with HLA-C and DP specificities were predominantly within the groups with >4 SAB specificities at the higher cutoff values. It should also be pointed out that a fraction (approximately 12.5%) of donors that screened above NBG 2.2 demonstrated antibodies against both Class I and Class II specificities, which would also raise the CPRA above the levels shown in Table 1. These were also predominantly within the >4 SAB groups at higher cutoff values. Finally, the PRAs included in the Table are calculated values rather than values obtained from in – vitro PRA assays. Although these limitations suggest that the number of SAB specificities may not be a direct surrogate for measured PRA, our analysis nevertheless establishes a correlation between number of SAB specificities and the likelihood of a cognate antigen-antibody match.
The three screening cutoffs examined in this analysis are not meant to be definitive numbers to be used by centers using Luminex technology. Rather, they should be viewed as illustrative to help describe the impact of implementing moderate, high, or very high cutoffs for HLA Ab screening assays. Different HLA Ab screening assays will require different cutoffs to yield comparable performance13, in terms of detection of HLA Ab positive donations and donor loss, to those reported here. Additionally, sample type (plasma or serum)21 and lot-to-lot variation can alter assay signal strength. Furthermore, the results presented here are based on the REDS-II LAPS cohort. While LAPS included donors from across the country, an individual blood center’s own donor population may vary from the demographics of LAPS donors10. Centers are therefore encouraged to determine their own cutoffs using their own population of non-alloexposed donors and their chosen HLA Ab assay.
Acknowledgments
This work was supported by NHLBI contracts N01-HB-47174, -47175 and -57181.
We thank Denese Jones for meticulously calculating several hundred CPRA values. The authors thank the staff at all six participating blood centers. Without their help, this study would not have been possible.
Appendix
The Retrovirus Epidemiology Donor Study - II (REDS-II Study Group) is the responsibility of the following persons:
Blood Centers
American Red Cross Blood Services, New England Region
R. Cable, J. Rios and R. Benjamin
American Red Cross Blood Services, Southern Region/Department of Pathology and Laboratory Medicine, Emory University School of Medicine
J.D. Roback
Hoxworth Blood Center, University of Cincinnati Academic Health Center
R.A. Sacher, S.L. Wilkinson and P.M. Carey
Blood Centers of the Pacific, University of California San Francisco, Blood Systems Research Institute
E.L. Murphy, B. Custer and N. Hirschler
The Institute for Transfusion Medicine
D. Triulzi, R. Kakaiya and J. Kiss
Blood Center of Wisconsin
J. Gottschall and A. Mast
Coordinating Center: Westat, Inc
J. Schulman and M. King
National Heart, Lung, and Blood Institute, NIH
G.J. Nemo
Central Laboratory: Blood Systems Research Institute
M.P. Busch and P. Norris
Footnotes
The authors report no conflict of interest.
Reference List
- 1.FDA US Food and Drug Administration, US Department of Health and Human Services. Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2009. 2009. Dec, [Google Scholar]
- 2.Strong DM, Lipton KS. AABB Association Bulletin #06–07. 2006. Nov 3, [Google Scholar]
- 3.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985 Nov;25(6):573–7. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 4.Middelburg RA, van SD, Briet E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: a systematic review. Transfusion. 2008 Oct;48(10):2167–76. doi: 10.1111/j.1537-2995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 5.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007 Mar;136(6):788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 6.Nordhagen R, Conradi M, Dromtorp SM. Pulmonary reaction associated with transfusion of plasma containing anti-5b. Vox Sang. 1986;51(2):102–7. doi: 10.1111/j.1423-0410.1986.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 7.Middelburg RA, van SD, Briet E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: a systematic review. Transfusion. 2008 Oct;48(10):2167–76. doi: 10.1111/j.1537-2995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 8.Popovsky MA, Moore SB. Autologous transfusion in Jehovah’s Witnesses. Transfusion. 1985 Sep;25(5):444. doi: 10.1046/j.1537-2995.1985.25586020125.x. [DOI] [PubMed] [Google Scholar]
- 9.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005 Mar 15;105(6):2266–73. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 10.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009 Sep;49(9):1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endres RO, Kleinman SH, Carrick DM, Steele WR, Wright DJ, Norris PJ, Triulzi D, Kakaiya R, Busch MP. Identification of specificities of antibodies against human leukocyte antigens in blood donors. Transfusion. 2010 Feb 11; doi: 10.1111/j.1537-2995.2010.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakaiya RM, Triulzi DJ, Wright DJ, Steele WR, Kleinman SH, Busch MP, Norris PJ, Hillyer CD, Gottschall JL, Rios JA, et al. Prevalence of HLA antibodies in remotely transfused or alloexposed volunteer blood donors. Transfusion. 2010 Jan 8; doi: 10.1111/j.1537-2995.2009.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrick DM, Johnson B, Kleinman SH, Vorhaben R, Change S, Lee JH, Pandey S, Roback J, Sun Y, Busch MP, et al. Agreement amongst HLA Antibody Detection Assays is Higher in Ever Pregnant Donors and Improved Using a Consensus Cutoff. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinman S, Grossman B, Kopko P. A national survey of transfusion-related acute lung injury risk reduction policies for platelets and plasma in the United States. Transfusion. 2010 Apr 27; doi: 10.1111/j.1537-2995.2010.02659.x. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani K, Terasaki P, Hamdani E, Esquenazi V, Rosen A, Miller J, Ozawa M. The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant. 2007 Apr;7(4):1027–31. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 16.Rios JA, Schlumpf KS, Kakaiya R, Triulzi D, Hillyer CD, Kleinman S, Busch MP, Gottschall JL, Carey P, Nemo G. Blood donations from previously transfused or pregnant donors: a multi-center study to determine the frequency of allo-exposure. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007 Mar;136(6):788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S, Nakajima F, Kamada H, Kawamura K, Satake M, Tadokoro K, Okazaki H. Relationship of donor HLA antibody strength to the development of transfusion-related acute lung injury. Transfusion. 2010 Jul 27; doi: 10.1111/j.1537-2995.2010.02779.x. [DOI] [PubMed] [Google Scholar]
- 19.Triulzi DJ. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009 Mar;108(3):770–6. doi: 10.1213/ane.0b013e31819029b2. [DOI] [PubMed] [Google Scholar]
- 20.Andreu G, Vasse J, Herve F, Tardivel R, Semana G. Introduction of platelet additive solutions in transfusion practice. Advantages, disadvantages and benefit for patients. Transfus Clin Biol. 2007 May;14(1):100–6. doi: 10.1016/j.tracli.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Norris PJ, Lee JH, Carrick DM, Gottschall JL, Lebedeva M, de Castro BR, Kleinman SH, Busch MP. Long-term in vitro reactivity for human leukocyte antigen antibodies and comparison of detection using serum versus plasma. Transfusion. 2009 Feb;49(2):243–51. doi: 10.1111/j.1537-2995.2008.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]