Abstract
Auditory forebrain pathways exhibit several morphological and physiological properties that underlie their specific neurobiological roles in auditory processing. Anatomically, such projections can be distinguished by their terminal size, arborization patterns, and postsynaptic dendritic locations. These structural features correlate with several postsynaptic physiological properties, such as EPSP amplitude, short-term plasticity, and postsynaptic receptor types. Altogether, these synaptic properties segregate into two main classes that are associated with either primarily information-bearing (Class 1) or modulatory (Class 2) roles, and have been used to delineate the principle routes of information flow through the auditory midbrain, thalamus, and cortex. Moreover, these synaptic properties engender as yet unexplored issues regarding the neuronal processing of auditory information, such as the convergent integration and long-term plasticity of auditory forebrain inputs.
Keywords: cortex, thalamus, midbrain, auditory cortex, medial geniculate body, inferior colliculus
1. Introduction
Acoustic information from the cochlea is processed successively through brainstem nuclei (Young et al., 1992), the inferior colliculus (Oliver and Huerta, 1992), medial geniculate body (Winer, 1992), and eventually the auditory cortex (Hackett, 2011), and many of the synaptic mechanisms by which this information is transferred, transmitted, and transformed are now being revealed (Atencio et al., 2009; Tan et al., 2007; Zhou et al., 2010). In each of these auditory structures, neurons receive convergent synaptic inputs from several extrinsic and intrinsic sources whose influences on neuronal excitability vary considerably according to neurotransmitter type and postsynaptic mode of action (Lee and Winer, 2010; Winer et al., 1999). For example, neurons in layer 4 of the primary auditory cortex (AI) receive convergent synaptic inputs from thalamic, cortical and brainstem sources that utilize glutamate (Lee and Sherman, 2008), GABA (Yuan et al., 2010), acetylcholine (Weinberger, 2007), dopamine (Bao et al., 2001), and other neurotransmitters. While each of these systems affect the target recipient neurons, most are not principally involved in transmitting auditory information, but instead the bulk of these inputs act to modulate neuronal responsiveness (Gil et al., 1997; Metherate and Hsieh, 2003; Sherman and Guillery, 1998).
Even among glutamatergic inputs, heterogeneous postsynaptic effects and morphologies distinguish many afferent pathways (Bartlett and Smith, 2002; Lee and Sherman, 2008; Llano and Sherman, 2008; Smith et al., 2007). At several major auditory forebrain structures, glutamatergic pathways can be segregated into two main classes: Class 1 (previously called driver) and Class 2 (previously called modulator) (Table 1) (Lee and Sherman, 2010a; Sherman and Guillery, 2006). Class 1 inputs are characterized by properties suited for the reliable and efficient transfer of information across the synapse (Bartlett and Smith, 2002; Lee and Sherman, 2008, 2010b), while those of Class 2 are not primarily information bearing, but instead are best suited to modulate the transmission of Class 1 inputs (Lee and Sherman, 2009c; Reichova and Sherman, 2004; Sherman and Guillery, 2006). And, such a classification has proven useful for delineating the major information-bearing pathways (Lee and Sherman, 2008, 2009c, 2010b; Llano and Sherman, 2008).
Table 1.
Anatomical and physiological features of auditory pathways
| Class 1 | Class 2 | |
|---|---|---|
| Terminal Size1,3,8,9,11,12,13 | Large | Small |
| Axonal diameter1,3,8,9,11,12,13 | Thick | Thin |
| Arborization pattern1,3,8,9,11,12,13 | Dense | Sparse |
| Dendritic location1,3,8,9,11,12,13 | Proximal | Distal |
| Postsynaptic receptors2,4,5,6,7,10,11 | iGluR | iGluR and mGluR |
| EPSP amplitude2,4,5,6,7,10,11 | Large | Small |
| Short-term plasticity2,4,5,6,7,10,11 | Depressing synapse | Facilitating synapse |
Bartlett, E.L., Smith, P.H. 1999. J Neurophysiol 81, 1999-2016.
Bartlett, E.L., Smith, P.H. 2002. Neuroscience 113, 957-974
Huang, C.L., Winer, J.A. 2000. J Comp Neurol 427, 302-331
Lee, C.C., Sherman, S.M. 2008. J Neurophysiol 100, 317-326.
Lee, C.C., Sherman, S.M. 2009a. Cereb Cortex. 19,2281-2289.
Lee, C.C., Sherman, S.M. 2009c. Front Syst Neurosci 3, 3.
Lee, C.C., Sherman, S.M. 2010b. Proc Natl Acad Sci U S A 107, 372-377.
Llano, D.A., Sherman, S.M. 2008. J Comp Neurol 507, 1209-1227.
Ojima, H. 1994. Cereb Cortex 4, 646-663.
Rose, H.J., Metherate, R. 2001. J Neurophysiol 106, 331-340.
Smith, P.H., Bartlett, E.L., Kowalkowski, A. 2007. J Neurophysiol 2007, 681-695
Winer, J.A., Larue, D.T., Huang, C.L. 1999. J Comp Neurol 413, 181-197.
Winer, J.A., Diehl, J.J., Larue, D.T. 2001. J Comp Neurol 430, 27-55.
These recent findings provoke several questions: To what extent do these two afferent types exist in the auditory forebrain? What is their role in auditory information flow? Do differences exist in their long-term plasticity? And, how do neurons integrate these convergent synaptic inputs? Pertinent to these questions, we here review the anatomical and physiological properties of these pathways, their relative distribution in the auditory forebrain, and their relationship to the transmission of auditory information.
2. Properties of Class 1 and 2 pathways
Class 1 and 2 input types have been described broadly in forebrain circuitry and are not limited to auditory pathways (Lee and Sherman, 2008; Llano and Sherman, 2008; MacLean et al., 2006; Reichova and Sherman, 2004), and they seem to represent the vast majority of pathways in the various circuits so far tested. Table 1 summarizes their properties. Class 1 inputs are exemplified by the retinogeniculate afferents (Guillery, 1966; Li et al., 2003; Reichova and Sherman, 2004) and the thalamocortical inputs to layer 4 (Huang and Winer, 2000; Lee and Sherman, 2008), while Class 2 inputs are represented by the layer 6 corticothalamic and certain intracortical inputs (Lee and Sherman, 2009c; Llano and Sherman, 2008; Reichova and Sherman, 2004).
Anatomically, axonal and terminal structure distinguishes the Class 1 and 2 inputs (Table 1, e.g. Fig 2). Class 1 afferents, such as the retinal inputs to the lateral geniculate nucleus (Guillery, 1966), have thick axons, dense arbors (Famiglietti and Peters, 1972; Guillery, 1966; Ralston, 1971), and large terminals that synapse on proximal dendrites (Llano and Sherman, 2008; Winer et al., 1999). By comparison, Class 2 inputs, like the layer 6 corticothalamic projection, have thin axons, sparse arbors, and small terminals ending on distal dendrites (Bartlett and Smith, 1999; Llano and Sherman, 2008; Sherman and Guillery, 2006; Smith et al., 2007).
Fig. 2.
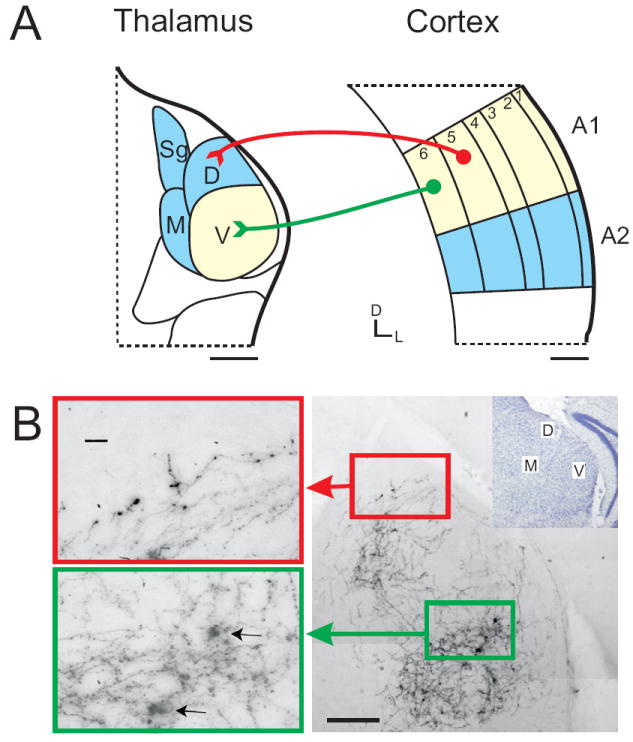
Anatomy of the descending corticothalamic projections, as an example to illustrate the morphology of Class 1 and 2 glutamatergic inputs. (A) Two CT pathways originate from the primary auditory cortex (AI). Class 1 feedforward projections originate from layer 5 of AI and terminate in MGBd, while Class 2 feedback projections originate from layer 6 of AI and terminate in MGBv. Scale bars = 250μm. (B) Class 1 projections (red box) exhibit thick axons with large synaptic terminals compared with Class 2 terminals (green box) which exhibit thin axons and small terminals. Scale bars: left = 20μm, right = 200μm. Portions of the figure adapted from Paxinos and Franklin (2001) and Llano and Sherman (2008). See list for abbreviations.
Physiologically, Class 1 inputs produce large EPSPs, exhibit synaptic depression, and only activate ionotropic glutamate receptors (iGluRs) (Bartlett and Smith, 2002; Li et al., 2003; Reichova and Sherman, 2004) (Fig. 1). In contrast, Class 2 inputs exhibit facilitation of small EPSPs, and activate iGluRs and metabotropic glutamate receptors (mGluRs) (Bartlett and Smith, 2002; Li et al., 2003; Reichova and Sherman, 2004).
Fig. 1.
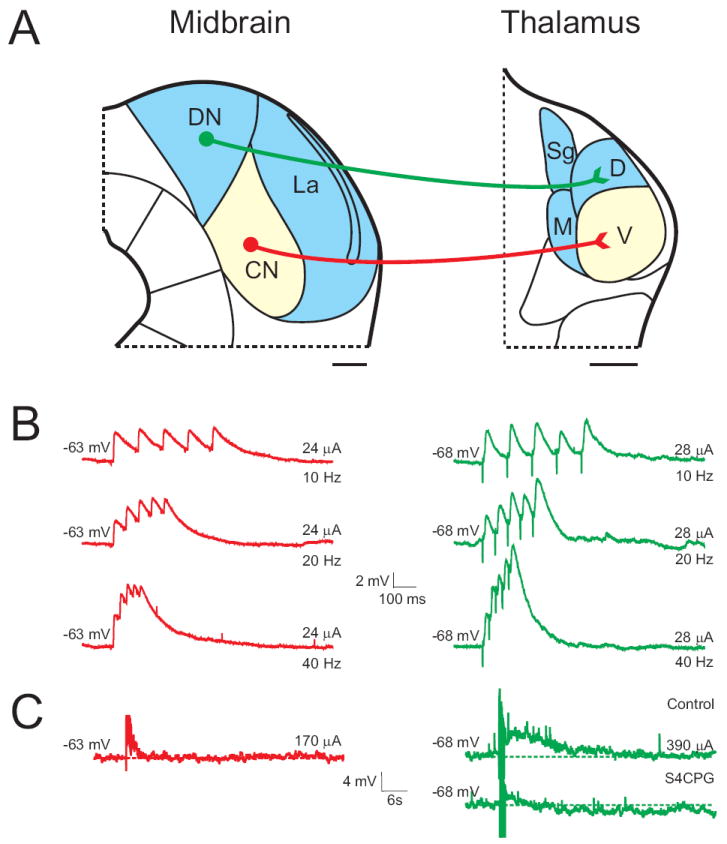
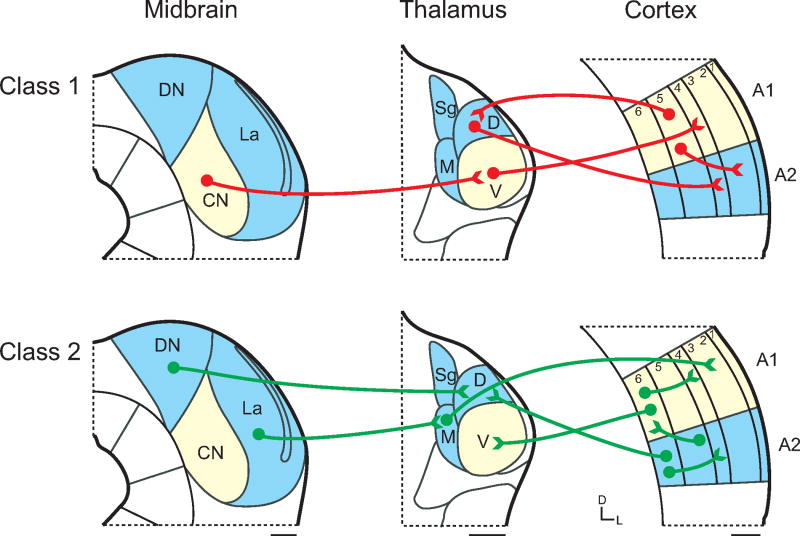
Physiology of the ascending tectothalamic projection, as an example illustrating the synaptic properties of Class 1 and 2 glutamatergic inputs in the auditory forebrain. (A) Schematic illustration of the connectivity between tonotopic (light yellow) and non-tonotopic (ice blue) regions of the inferior colliculus (IC; left) and the medial geniculate body (MGB; right). Class 1 glutamatergic projections (red) originate from the central nucleus of the IC (CN) and terminate in the ventral division (V) of the MGB, while Class 2 projections originate from non-tonotopic shell regions, such as the dorsal (DN) and lateral (La) nuclei of the IC and terminate in the dorsal (D) and medial (M) divisions of the MGB. Scale bars = 250μm. (B) Class 1 postsynaptic responses (red) exhibit large EPSPs that depress in response to paired-pulse stimulation, while Class 2 responses (green) facilitate in response to paired-pulses. (C) After blocking iGluRs and stimulation with a high frequency tetanus, the Class 2 tectothalamic input (green) exhibits a long-lasting depolarization that is blocked by group 1 mGluR antagonists. Portions of the figure adapted from Paxinos and Franklin (2001) and Lee and Sherman (2010). See list for abbreviations.
3. Prevalence of Class 1 and 2 pathways in the auditory system
Pathways throughout the central auditory system exhibit either Class 1 or 2 properties (Bartlett and Smith, 2002; Huang and Winer, 2000; Lee and Sherman, 2008, 2009c, 2010b; Llano and Sherman, 2008; Winer et al., 1999). Although both classes are found across different stages of the auditory pathway, their distribution varies, as discussed below.
3.1. Tectothalamic pathways
The inferior colliculus (IC) is the source of glutamatergic and GABAergic inputs to the medial geniculate body (MGB) (Peruzzi et al., 1997; Winer et al., 1996). These are topographically organized and originate from lemniscal and non-lemniscal IC subdivisions (Lee and Sherman, 2010b). Among the tectal projections, those from the central nucleus of the inferior colliculus (ICc) terminate primarily in the ventral division of the MGB (MGBv), while non-lemniscal projections from the dorsal (ICd), lateral (ICl), and caudal cortices of the IC (ICca) terminate respectively in the dorsal (MGBd) and medial (MGBm) divisions of the MGB (Malmierca et al., 2008; Romand and Ehret, 1990; Wenstrup, 2005; Winer, 2005).
However, the synaptic properties of lemniscal and non-lemniscal glutamatergic inputs differ (Fig. 1). ICc projections exhibit Class 1 properties, such as large terminations and depressing synapses with only iGluR activation (Table 1; Fig. 1B: red) (Bartlett and Smith, 1999, 2002; Lee and Sherman, 2010b). In contrast, projections from ICd and ICl exhibit Class 2 properties, such as small terminal arbors, facilitating synapses and recruitment of mGluRs (Fig. 1B: green) (Bartlett and Smith, 2002; Lee and Sherman, 2010b; Smith et al., 2007). Thus, both Class 1 and 2 inputs are found in the auditory tectothalamic system and distinguish the lemniscal and non-lemniscal pathways.
3.2. Thalamocortical pathways
Two major projection systems from the medial geniculate body terminate in the auditory cortex (Huang and Winer, 2000; Jones, 2009). One terminates in layer 4 with some branches to layer 6, as exemplified by the MGBv and MGBd projections to the primary (AI) and secondary (AII) auditory areas (Kaas and Hackett, 2000; Lee and Winer, 2008a; Llano and Sherman, 2008; Theyel et al., 2010). The other terminates most densely in layers 2/3, as seen primarily with projections from the MGBm to AI and AII (Huang and Winer, 2000; Jones, 2009).
These thalamocortical afferents differ in their synaptic properties. The projections to layer 4 of AI and AII, respectively, demonstrate Class 1 properties: dense arborizations (Huang and Winer, 2000; Llano and Sherman, 2008), large EPSPs that depress and activate only iGluRs (Lee and Sherman, 2008; Rose and Metherate, 2001). By comparison, the projections to layers 2/3 display primarily Class 2 properties, although a minority (~20%) have Class 1 or mixed properties (Viaene et al., 2011).
3.3. Corticothalamic pathways
The MGB itself receives descending corticothalamic (CT) projections from layers 5 and 6 of the auditory cortex (Llano and Sherman, 2008; Ojima, 1994; Winer et al., 2001; Winer et al., 1999). The layer 6 CT projection feeds back to the same nucleus from which it receives its main core TC input, e.g., the layer 6 AI to MGBv projection (Fig. 2A). In contrast, layer 5 sends a non-reciprocal feedforward CT projection to the MGB, e.g., the layer 5 AI to MGBd projection (Fig. 2A). Thus both MGBv and MGBd receive feedback layer 6 projections (from AI and AII, respectively), and MGBd but not MGBv receives CT inputs from layer 5 (Llano and Sherman, 2008; Winer et al., 2001). This non-reciprocal layer 5 CT projection displays Class 1 characteristics, i.e., thick axons and large terminations in the MGBd (Fig. 2B: red box) with triadic structures in the cat1 (Llano and Sherman, 2008; Ojima and Murakami, 2010; Winer et al., 2001), distinguishing it from the feedback layer 6 CT projection exhibiting Class 2 properties, i.e. thin axons and small terminations in MGBv (Fig 2B: green box) and facilitating EPSPs (Bartlett and Smith, 2002; Cappe et al., 2009; Llano and Sherman, 2008; Ojima, 1994; Winer et al., 2001). By extrapolation from studies in the visual and somatosensory systems, because similar experiments have not been fully carried out in the auditory system, we expect that the layer 5 projection should evoke large, depressing EPSPs with no mGluR component (Li et al., 2003; Reichova and Sherman, 2004).
3.4. Local intracortical pathways
Within each auditory area, inter- and intra-laminar projections connect neurons within and across cortical columns (Lee and Sherman, 2009b; Matsubara and Phillips, 1988; Read et al., 2001; Yuan et al., 2010). Glutamatergic pyramidal cells predominate across all layers in the auditory cortex, including layer 4, where they assume the role of the spiny stellate cells in the visual cortex (Smith and Populin, 2001). GABAergic interneurons provide approximately 20% of the intracortical inputs (Yuan et al., 2010). Cortical neurons interconnect prolifically (Binzegger et al., 2004; Lee and Winer, 2008c, 2010), with glutamatergic projections extending across wider cortical territories than do GABAergic inputs (Barbour and Callaway, 2008; Lee and Sherman, 2009b; Yuan et al., 2010).
Interestingly, Class 1 and 2 synaptic properties distinguish some of the intracortical glutamatergic projections (Lee and Sherman, 2008, 2009b, c). Among these, the layer 6 projection to layer 4 is notable for its anatomical robustness, providing approximately 30% of synaptic inputs (Binzegger et al., 2004), and exhibiting Class 2 synaptic properties, i.e. paired-pulse facilitation and recruitment of Groups I and II mGluRs (Fig.3) (Lee and Sherman, 2008, 2009a, c). This contrasts with the glutamatergic projections from layer 4 to layer 3 and those from layer 3 to layer 5, which instead exhibit Class 1 synaptic properties (Lee and Sherman, 2009b). However, the copious interconnectivity of the intracortical projection system still leaves many intrinsic inputs as yet uncharacterized.
Fig. 3.
Summary of the major Class 1 (red) and Class 2 (green) glutamatergic inputs in the auditory forebrain. Class 1 projections (red) establish a novel route for the interareal transfer of information between AI and AII via a corticothalmocortical route. Class 2 projections (green) are not likely the primary conduits for information in the auditory system, but rather act to modulate that information. Scale bars = 250μm. Portions of the figure adapted from Paxinos and Franklin (2001). See list for abbreviations.
3.5. Corticocortical pathways between areas
Auditory cortical areas are linked by an expansive corticocortical (CC) network (Hackett, 2011; Lee and Winer, 2008b, c; Shi and Cassell, 1997), which contributes nearly half of the extrinsic input to a cortical area (Lee and Winer, 2010). The laminar sources of these CC projections differ according to areal origins, with supragranular, infragranular, and bilaminar origins often defining hierarchical relationships among auditory areas (Hackett, 2011; Lee and Winer, 2010; Rouiller et al., 1991) whose number varies on a species-specific basis (Bizley et al., 2005; Budinger et al., 2000; Fitzpatrick et al., 1998; Stiebler et al., 1997).
Among conserved areas, AI and AII are reciprocally connected by pyramidal neurons in layers 2-6 (Winer, 1992). Befitting their abundant connections, the organization of CC synaptic properties between AI and AII is the most complex, varying according to laminar origins and terminations. In general, neurons in layers 5b receive Class 1 synaptic inputs, layers 5a and 6 receive Class 2 inputs, and layers 2-4 receive a combination of Class 1 and 2 inputs (Covic et al., 2009). Interestingly, the origins of these projections are generally mixed across each layer, i.e. layers 2-6 are the sources of both Class 1 and 2 projections (Covic et al., 2009). Such microtopographic distributions further enhance and complicate CC synaptic organization (Lee and Winer, 2008c, 2010).
4. Functional implications
4.1. Role in information processing
The distinct properties of Class 1 and 2 pathways suggest different roles for each in the processing of auditory information. Class 1 inputs, with their large terminal morphologies, postsynaptic positioning close to the cell body, and high probability of release (associated with paired-pulse depression) (Gil et al., 1999), should be proficient as information-bearing inputs (Sherman and Guillery, 1998). Moreover, a key feature of Class 1 inputs, paired-pulse depression, appears to act as a gain control mechanism during high levels of activity (Abbott et al., 1997), a useful property for an information channel. By contrast, the characteristics of Class 2 inputs suggest a vastly different role, one as a modulator of information flow (Lee and Sherman, 2009c, 2010a; Sherman and Guillery, 2006). In particular, Class 2 synaptic activation of mGluRs results in protracted changes in membrane potential, which, in addition to providing efficient control of various time- and voltage-dependent ionic conductances (reviewed in Sherman and Guillery, 2006), often significantly outlasts activity in the afferent inputs (Lee and Sherman, 2009a, c); this is useful for modulation but would serve to distort information flow. Thus, these features, when added to their smaller terminal morphology, postsynaptic positioning far from the cell body, and lower release probability, lead us to conclude that Class 2 inputs likely subserve a modulatory role (Gil et al., 1999; Sherman and Guillery, 2006; Stratford et al., 1996).
However, it should be noted that this does not imply that Class 2 inputs convey zero information. Instead, we suggest that Class 2 inputs operate like other classic modulatory inputs, such as cholinergic or serotonergic inputs. The point is that, while all of these inputs convey some information, a distinction should be made among glutamatergic pathways between those that are primarily information-bearing (Class 1) and those that are primarily modulatory (Class 2).
4.2. Parceling of thalamic nuclei
MGB nuclei, like other sensory thalamic nuclei, can be divided into two groups based on the source of their Class 1 inputs (Reichova and Sherman, 2004; Sherman and Guillery, 2006). In this scheme, the MGBv is classified as a first order (FO) nucleus, since it receives Class 1 synaptic input from ascending tectothalamic streams that originate at the sensory periphery (Fig.3) (Lee and Sherman, 2010b). In this way, the MGBv is analogous to both the lateral geniculate nucleus, which receives Class 1 input from the retina, and the ventroposterior nucleus, which receives Class 1 input from the medial lemniscal system (Sherman and Guillery, 2006). In contrast, the MGBd is classified as a higher order (HO) nucleus, since its Class 1 input originates from layer 5 of the auditory cortex (Fig. 2), similar to the LP-pulvinar complex (vision) and posterior medial nucleus (somatosensory), which also receive Class 1 inputs from layer 5 of cortex (Li et al., 2003; Llano and Sherman, 2008; Reichova and Sherman, 2004; Sherman and Guillery, 2006).
Class 1 inputs to thalamic relay cells represent the major source of information to be relayed, e.g. the retinal input to the lateral geniculate nucleus. FO relays, like the MGBv, then, represent the first relay to cortex of a particular type of input (e.g., auditory or visual), whereas HO relays, because they receive Class 1 input from cortex, serve as a relay station of information already in cortex between cortical areas (Sherman and Guillery, 2002, 2006). This is in addition to any direct CC projections, and it is curious that many, and perhaps all, areas of cortex appear to have parallel direct (CC) and indirect (cortico-thalamo-cortical) pathways. The possible difference in information content of these parallel pathways between cortical areas has been discussed elsewhere (Sherman and Guillery, 2006; Guillery and Sherman, 2011).
4.3. Information-bearing routes
Given the putative information-bearing function of Class 1 inputs and their prevalence in the central auditory system, a model of information flow from the inferior colliculus to the auditory cortex arises naturally (Fig. 3: red) (Lee and Sherman, 2010a). In this model, auditory information from the cochlea reaches the ICc and is sent to the MGBv and then to AI (Bartlett and Smith, 2002; Lee and Sherman, 2008, 2010b). From here, layer 5 of AI transmits information to MGBd, which then advances it to AII (Fig. 3: red) (Lee and Sherman, 2008; Llano and Sherman, 2008). As shown in Fig. 3, this cortico-thalamo-cortical pathway parallels a direct CC pathway. However, the resultant transthalamic route for information flow between AI and AII via MGBd contrasts with the canonical models for intraareal communication mediated strictly by corticocortical connections (Felleman and Van Essen, 1991; Rouiller et al., 1991).
Furthermore, this model proposes that Class 2 projections largely modulate the flow of information in recipient neurons (Fig 3: green) (Lee and Sherman, 2010a). Thus, the ascending pathway from the ICd may not behave as a parallel route for auditory information reaching the MGBd (Hu, 2003; Syka et al., 2000) but instead act to modulate transmission of the layer 5 input to MGBd. Similarly, the TC projections from MGB to layers 2/3 as well as the layer 6 projections both to layer 4 and back to the MGB, in this model, act as modulators of information flow (Lee and Sherman, 2009b; Llano and Sherman, 2008; Viaene et al., 2011), which extends previous models of lemniscal and non-lemniscal parallel pathways for ascending auditory information (Calford and Aitkin, 1983; Lorente de Nó, 1938), and posits a role for the non-specific thalamocortical projection system(Jones, 2007). Finally, the direct CC pathway also has modulatory components (Covic et al., 2009).
5. Unresolved features
5.1. Ubiquity
The relative pervasiveness of Class 1 and 2 properties in these central auditory projections generates an obvious question: Are such features ubiquitous throughout the auditory system? Conceivably, such classifications could be extended and tentatively applied to very early stages of the auditory pathway (Gulley et al., 1978; Hoffpauir et al., 2006; Rowland et al., 2000). For example, in the cochlear nucleus at the bulb of held, bushy cells receive large terminations from type I spiral ganglion cells (Gulley et al., 1978), whose synapses have high release probabilities (Isaacson and Walmsley, 1995). And, these Class 1-like features are even more prominent in the medial nucleus of the trapezoid body at the calyx of held (Hoffpauir et al., 2006; Müller et al., 2010; Rowland et al., 2000), one of the largest synaptic terminals in the brain. Such properties are practically de rigueur for these synapses to faithfully transmit information, particularly at the calyx of held, where accurate timing is critical for sound localization (Kopp-Scheinpflug et al., 2003; Mc Laughlin et al., 2008).
More questionable, though, is the extension to those pathways whose properties are as yet uncharacterized, e.g., very local, intrinsic cortical (Barbour and Callaway, 2008; Tan and Wehr, 2009) or commissural (Chadderton et al., 2009; Lee and Winer, 2008b). Perhaps, among the most intriguing are the numerous corticofugal projections (Winer, 2006), whose targets include the IC (Winer et al., 1998), pons (Schuller et al., 1991), striatum (Beneyto and Prieto, 2001), amygdala (Romanski and LeDoux, 1993), and as remote even as the cochlear nucleus (Schofield and Coomes, 2005). The remarkable breadth of these descending cortical projections affect not only direct auditory processing streams, but also motor and limbic processes (Winer, 2006). Thus, whether such projections have the properties and attendant functional significance of Class 1 and 2 inputs, or properties quite different involving other classes, would have profound consequences for the cortical control of auditory processing through these regions.
5.2. Convergent integration
While the characterization of these pathways highlights their potential functional roles, a broader perspective recognizes naturally that they are not isolated and that their combined effects largely govern neuronal activity. Indeed, multiple synaptic sources converge, combine and integrate temporally and spatially to generate a neuronal output (Mel, 1993; Polsky et al., 2004; Trevelyan and Jack, 2002). Neurons in layer 4, for example, receive convergent Class 1 and 2 inputs from thalamic (Lee and Sherman, 2008), intrinsic cortical (Lee and Sherman, 2009c) and corticocortical (Covic et al., 2009) sources. And, the MGB receives convergent inputs from the cortex (Llano and Sherman, 2008) and IC (Lee and Sherman, 2010b). Simple passive biophysical models predict that these various synaptic inputs should summate linearly (Polsky et al., 2004; Trevelyan and Jack, 2002). Yet, the Class 2 synaptic activation of mGluRs, with their longer time-scale and downstream modifications to time- and voltage gated channels (Francesconi and Duvoisin, 2000; Stefani et al., 1996), may introduce unexpected non-linearities (Wyart et al., 2005). This broader issue is further complicated by the effects of convergent inhibitory (Yuan et al., 2010) and neuromodulatory (Varela and Sherman, 2007, 2009) projections, whose effects on the integration of Class 1 and 2 inputs are also as yet unknown and remain to be investigated.
5.3. Divergent projections
Axonal branching is prevalent throughout the auditory system, from the projections of type I spiral ganglion cells to the cochlear nucleus (Fekete et al., 1984) to the thalamocortical (Kishan et al., 2008; Lee and Winer, 2008a) and corticothalamic (Kimura et al., 2005; Ojima, 1994) pathways. At their simplest, terminal arborizations allow one axon to form multiple terminal boutons, as in the olivocochlear collaterals to the interstitial nucleus of the vestibular nerve root (Brown et al., 1988). At the other extreme, axons branch to targets millimeters apart, as with some thalamocortical fibers (Cetas et al., 1999; Hashikawa et al., 1995; Huang and Winer, 2000). These divergent projections may create feedback–gain loops (Ye et al., 2000), establish lateral inhibition (de la Rocha et al., 2008; Tan and Wehr, 2009), propagate similar computational processes to remote locations (Guillery and Sherman, 2011; Kuwabara et al., 1991), or synchronize temporal aspects of neural discharge (Ojima et al., 1991).
Such branching poses particular issues with regards to Class 1 and 2 synaptic properties. Do all neuronal branches have similar properties? For instance, layer 5 inputs to MGBd exhibit Class 1 properties, but the layer 5 axons branch to innervate multiple extrathalamic targets as well (Bajo et al., 2007; Ojima, 1994; Winer, 2006). Do the other targets of these axons respond to inputs with Class 1, or other, properties? If not, what does this imply for the function of separate branches? In the one case, layer 6 neurons send branched projections to the MGB and layer 4 of the cortex (Thomson, 2010), which both exhibit Class 2 synaptic properties (Bartlett and Smith, 2002; Lee and Sherman, 2009c; Llano and Sherman, 2008). The shared attributes of these separate branches suggests that layer 6 neurons modulate both the thalamic relay neuron and its layer 4 target (Lee and Sherman, 2009c; Thomson, 2010).
5.4. Long-term plasticity
The ability of auditory neurons to plastically reorganize their receptive field properties is somewhat contradictory from an information processing standpoint (Kilgard and Merzenich, 1998; Weinberger, 2007). On the one hand, auditory information is encoded with high-fidelity, yet on the other hand, it is malleable to enable learning (Bakin and Weinberger, 1990). This large-scale reorganization of physiological parameters is enabled by plastic changes occurring at the synapse. While long-term potentiation (LTP) and depression (LTD) of the synapse in response to tetanic stimulation protocols has been actively studied for several decades (Malenka and Bear, 2004), more recent investigations of spike-timing dependent plasticity (STDP) have defined the critical windows for initiating plasticity at the synaptic level (Dan and Poo, 2004).
An open question is whether such plasticity is equally expressed at all synapses in the auditory system. For example, should the Class 1 thalamocortical input to layer 4 of auditory cortex be more or less plastic than the Class 2 intrinsic cortical input from layer 6? Or, are they similar? Although reorganization of physiological map parameters has been observed at multiple levels of the auditory pathway (Edeline and Weinberger, 1991; Ma and Suga, 2009; Weinberger et al., 1984; Zhang and Suga, 2000), the literature remains unresolved on the issue of the relative plasticity of central auditory synapses (Dan and Poo, 2004; Malenka and Bear, 2004). The demonstration of STDP is scattered across numerous systems and preparations, which differ in methodology and measures (Bell et al., 1997; Boettiger and Doupe, 2001; Debanne et al., 1998; Magee and Johnston, 1997; Markram et al., 1997). Interestingly, Group 1 mGluRs (Lüscher and Huber, 2010) and dendritic synapse location (Froemke et al., 2010) are likely factors mediating such plasticity, suggesting that plastic differences indeed should exist among Class 1 and 2 pathways. With regard to the auditory system, there has not been a systematic study of the changes in synaptic strength from STDP or neuromodulator activation across multiple levels of the central auditory pathway. Thus, it remains to be resolved whether the Class 1 and 2 auditory pathways exhibit different capacities for plastic reorganization.
6. Conclusions
The diverse morphological and physiological properties of Class 1 and 2 pathways underscore their functional importance in the transmission and modulation of information through the central auditory system. These differences also should make clear that treating these various inputs as functionally homogeneous is counterproductive. Further distinctions among these pathways may emerge, such as with receptor subunit composition (Hermida et al., 2010; Hunter et al., 1993; Petralia et al., 2000), vesicular glutamate transporters (Altschuler et al., 2008; Zhou et al., 2007), and their interactions with inhibitory pathways (Ito et al., 2009; Yuan et al., 2010). The current classification of two types, with Class 1 suggesting the subset of inputs involved primarily in information transfer, has revealed unexpected routes for auditory information flow and adds insight to the higher order computational processes occurring in the mammalian brain, perhaps ones fundamental to the organization of such neuronal circuits across systems and species, but whose ontogeny and pervasiveness remain to be discovered.
Acknowledgments
Supported by: National Institutes of Health grants F32NS054478 (CCL) and R01DC008794 (SMS)
Abbreviations
- AC
Auditory cortex
- AI
Primary auditory cortex
- AII
Second auditory area
- CN
Central nucleus of the inferior colliculus
- D
Dorsal division of the medial geniculate body, or dorsal
- DN
Dorsal nucleus of the inferior colliculus
- IC
Inferior colliculus
- La
Lateral nucleus of the inferior colliculus
- M
Medial division of the medial geniculate body, or medial
- MGB
Medial geniculate body
- Sg
Suprageniculate nucleus
- V
Ventral division of the medial geniculate body, or ventral
Footnotes
Triadic arrangements are special synaptic structures associated with Class 1 input. A prominent example is the retinal input to the LGN (reviewed in Sherman and Guillery, 2006). However, a key element to these arrangements is a terminal from an interneuronal dendrite. Since interneurons are absent from the thalamus of rats and mice outside of the LGN (Arcelli et al., 1997), triads are not found in the thalamus of these rodent species outside of the LGN.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Tong L, Holt AG, Oliver DL. Immunolocalization of vesicular glutamate transporters 1 and 2 in the rat inferior colliculus. Neuroscience. 2008;154:226–232. doi: 10.1016/j.neuroscience.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull. 1997;42:27–37. doi: 10.1016/s0361-9230(96)00107-4. [DOI] [PubMed] [Google Scholar]
- Atencio CA, Sharpee TO, Schreiner CE. Hierarchical computation in the canonical auditory cortical circuit. Proc Natl Acad Sci U S A. 2009;106:21894–21899. doi: 10.1073/pnas.0908383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 2007;17:475–491. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Barbour DL, Callaway EM. Excitatory local connections of superficial neurons in rat auditory cortex. J Neurosci. 2008;28:11174–11185. doi: 10.1523/JNEUROSCI.2093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. J Neurophysiol. 1999;81:1999–2016. doi: 10.1152/jn.1999.81.5.1999. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience. 2002;113:957–974. doi: 10.1016/s0306-4522(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Prieto JJ. Connections of the auditory cortex with the claustrum and endopiriform nucleus in the cat. Brain Res Bull. 2001;54:485–498. doi: 10.1016/s0361-9230(00)00454-8. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Doupe AJ. Developmentally restricted synaptic plasticity in a songbird nucleus required for song learning. Neuron. 2001;31:809–818. doi: 10.1016/s0896-6273(01)00403-2. [DOI] [PubMed] [Google Scholar]
- Brown MC, Liberman MC, Benson TE, Ryugo DK. Brainstem branches from olivocochlear axons in cats and rodents. J Comp Neurol. 1988;278:591–603. doi: 10.1002/cne.902780410. [DOI] [PubMed] [Google Scholar]
- Budinger E, Heil P, Scheich H. Functional organization of auditory cortex in the Mongolian gerbil (Meriones unguiculatus). IV. Connections with anatomically characterized subcortical structures. Eur J Neurosci. 2000;12:2452–2474. doi: 10.1046/j.1460-9568.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- Calford MB, Aitkin LM. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983:2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappe C, Morel A, Barone P, Rouiller EM. The thalamocortical projection systems in primate: an anatomical support for multisensory and sensorimotor interplay. Cereb Cortex. 2009;19:2025–2037. doi: 10.1093/cercor/bhn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetas JS, de Venecia RK, McMullen NT. Thalamocortical afferents of Lorente de Nó: medial geniculate axons that project to primary auditory cortex have collateral branches to layer I. Brain Res. 1999;830:203–208. doi: 10.1016/s0006-8993(99)01355-4. [DOI] [PubMed] [Google Scholar]
- Chadderton P, Agapiou JP, McAlpine D, Margrie TW. The synaptic representation of sound source location in auditory cortex. J Neurosci. 2009;29:14127–14135. doi: 10.1523/JNEUROSCI.2061-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covic EN, Farmer L, Petrof I, Sherman SM. Functional characterization of directly projecting feed-back auditory corticocortical connections. Proc Soc Neurosci. 2009;35:452.3. [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- de la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. J Neurosci. 2008;28:9151–9163. doi: 10.1523/JNEUROSCI.1789-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Subcortical adaptive filtering in the auditory system: associative receptive field plasticity in the dorsal medial geniculate body. Behav Neurosci. 1991;105:154–175. doi: 10.1037//0735-7044.105.1.154. [DOI] [PubMed] [Google Scholar]
- Famiglietti EVJ, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972;144:285–334. doi: 10.1002/cne.901440304. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Rouiller EM, Liberman MC, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats. J Comp Neurol. 1984;229:432–450. doi: 10.1002/cne.902290311. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Olsen JF, Suga N. Connections among functional areas in the mustached bat auditory cortex. J Comp Neurol. 1998;391:366–396. [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci U S A. 2000;97:6185–6190. doi: 10.1073/pnas.97.11.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Letzkus JJ, Kampa B, Hang GB, Stuart G. Dendritic synapse location and neocortical spike-timing-dependent plasticity. Front Syna Neurosci. 2010;2:1–14. doi: 10.3389/fnsyn.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortcal synaptic connections: quanta, innervation, and reliability. Neuron. 1999;23:385–397. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Guillery RW. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966;128:21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Branched thalamic afferents: What are the messages that they relay to the cortex? Brain Res Rev. 2011;66:205–219. doi: 10.1016/j.brainresrev.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley RL, Landis DM, Reese TS. Internal organization of membranes at end bulbs of Held in the anteroventral cochlear nucleus. J Comp Neurol. 1978;180:707–741. doi: 10.1002/cne.901800405. [DOI] [PubMed] [Google Scholar]
- Hackett TA. Information flow in the auditory cortical network. Hear Res. 2011;1:133–146. doi: 10.1016/j.heares.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa T, Molinari M, Rausell E, Jones EG. Patchy and laminar terminations of medial geniculate axons in monkey auditory cortex. J Comp Neurol. 1995;362:195–208. doi: 10.1002/cne.903620204. [DOI] [PubMed] [Google Scholar]
- Hermida D, Mateos JM, Elezgarai I, Puente N, Bilbao A, Bueno-López JL, Streit P, Grandes P. Spatial compartmentalization of AMPA glutamate receptor subunits at the calyx of Held synapse. J Comp Neurol. 2010;518:163–174. doi: 10.1002/cne.22189. [DOI] [PubMed] [Google Scholar]
- Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA. Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. J Neurosci. 2006;26:5511–5523. doi: 10.1523/JNEUROSCI.5525-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res. 2003;153:543–549. doi: 10.1007/s00221-003-1611-5. [DOI] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol. 2000;427:302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hunter C, Petralia RS, Vu T, Wenthold RJ. Expression of AMPA-selective glutamate receptor subunits in morphologically defined neurons of the mammalian cochlear nucleus. J Neurosci. 1993;13:1932–1946. doi: 10.1523/JNEUROSCI.13-05-01932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol. 1995;73:964–973. doi: 10.1152/jn.1995.73.3.964. [DOI] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. J Neurosci. 2009;29:13860–13869. doi: 10.1523/JNEUROSCI.3454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kimura A, Donishi T, Okamoto K, Tamai Y. Topography of projections from the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular nucleus in the rat. Neuroscience. 2005;135:1325–1342. doi: 10.1016/j.neuroscience.2005.06.089. [DOI] [PubMed] [Google Scholar]
- Kishan AU, Lee CC, Winer JA. Branched projections in the auditory thalamocortical and corticocortical systems. Neuroscience Neuroscience. 2008;154:283–293. doi: 10.1016/j.neuroscience.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dörrscheidt GJ, Rübsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J Assoc Res Otolaryngol. 2003;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol. 1991;314:684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical intputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009a;19:2281–2289. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Intrinsic cortical circuits of the primary and secondary areas of the mouse auditory cortex. Proc Soc Neurosci. 2009b;35:452.2. [Google Scholar]
- Lee CC, Sherman SM. Modulator property of the intrinsic cortical projections from layer 6 to layer 4. Front Syst Neurosci. 2009c;3:3. doi: 10.3389/neuro.06.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Front Neurosci. 2010a;4:79–86. doi: 10.3389/neuro.01.014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Topography and physiology of ascending streams in the auditory tectothalamic pathway. Proc Natl Acad Sci U S A. 2010b;107:372–377. doi: 10.1073/pnas.0907873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: I. Thalamocortical system. J Comp Neurol. 2008a;507:1879–1900. doi: 10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: II. Commissural system. J Comp Neurol. 2008b;507:1901–1919. doi: 10.1002/cne.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol. 2008c;507:1920–1943. doi: 10.1002/cne.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Convergence of thalamic and cortical pathways in cat auditory cortex. Hear Res. 2010 doi: 10.1016/j.heares.2010.05.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol. 2003;90:3429–3440. doi: 10.1152/jn.00456.2003. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for non-reciprocal organization of the mouse auditory thalamocortical-corticothalamic projections systems. J Comp Neurol. 2008;507:1209–1227. doi: 10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Architectonics and structure of the cerebral cortex. In: Fulton JF, editor. Physiology of the Nervous System. London, Oxford: 1938. pp. 291–237. [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Specific and nonspecific plasticity of the primary auditory cortex elicited by thalamic auditory neurons. J Neurosci. 2009;29:4888–4896. doi: 10.1523/JNEUROSCI.0167-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Fenstermaker V, Watson BO, Yuste R. A visual thalamocortical slice. Nat Methods. 2006;3:129–134. doi: 10.1038/nmeth849. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embrarassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Izquierdo MA, Cristaudo S, Hernández O, Pérez-González D, Covey E, Oliver DL. A discontinuous tonotopic organization in the inferior colliculus of the rat. J Neurosci. 2008;28:4767–4776. doi: 10.1523/JNEUROSCI.0238-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Matsubara JA, Phillips DP. Intracortical connections and their physiological correlates in the primary auditory cortex (AI) of the cat. J Comp Neurol. 1988;268:38–48. doi: 10.1002/cne.902680105. [DOI] [PubMed] [Google Scholar]
- Mc Laughlin M, Van der Heijden M, Joris PX. How secure is in vivo synaptic transmission at the calyx of Held? J Neurosci. 2008;28:10206–10219. doi: 10.1523/JNEUROSCI.2735-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mel BW. Synaptic integration in an excitable dendritic tree. J Neurophysiol. 1993;70:1086–1101. doi: 10.1152/jn.1993.70.3.1086. [DOI] [PubMed] [Google Scholar]
- Metherate R, Hsieh CY. Regulation of glutamate synapses by nicotinic acetylcholine receptors in auditory cortex. Neurobiol Learn Mem. 2003;80:285–290. doi: 10.1016/s1074-7427(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Müller M, Goutman JD, Kochubey O, Schneggenburger R. Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity. J Neurosci. 2010;30:2007–2016. doi: 10.1523/JNEUROSCI.4378-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Ojima H, Honda CN, Jones EG. Patterns of axon collateralization of identified supragranular pyramidal neurons in the cat auditory cortex. Cereb Cortex. 1991;1:80–94. doi: 10.1093/cercor/1.1.80. [DOI] [PubMed] [Google Scholar]
- Ojima H, Murakami K. Triadic synaptic interactions of large corticothalamic terminals in non-lemniscal thalamic nuclei of the cat auditory system. Hear Res. 2010 doi: 10.1016/j.heares.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Huerta MF. Inferior and superior colliculi. In: Webster DB, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research, volume 1, The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 168–221. [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. Academic Press; New York: 2001. [Google Scholar]
- Peruzzi D, Bartlett EL, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Rubio ME, Wang YX, Wenthold RJ. Differential distribution of glutamate receptors in the cochlear nuclei. 2000;147:56–69. doi: 10.1016/s0378-5955(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Polsky A, Mel BW, Schiller J. Computational subunits in thin dendrites of pyramidal cells. Nat Neurosci. 2004;7:621–627. doi: 10.1038/nn1253. [DOI] [PubMed] [Google Scholar]
- Ralston HJ., III Evidence for presynaptic dendrites and a proposal for their mode of action. Nature. 1971;230:585–587. doi: 10.1038/230585a0. [DOI] [PubMed] [Google Scholar]
- Read HL, Winer JA, Schreiner CE. Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci U S A. 2001;98:8042–8047. doi: 10.1073/pnas.131591898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Brain Res Dev Brain Res. 1990;54:221–234. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2001;106:331–340. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Simm GM, Villa AEP, de Ribaupierre Y, de Ribaupierre F. Auditory corticocortical interconnections in the cat: evidence for parallel and hierarchical arrangement of the auditory cortical areas. Exp Brain Res. 1991;86:483–505. doi: 10.1007/BF00230523. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Coomes DL. Auditory cortical projections to the cochlear nucleus in guinea pigs. Hear Res. 2005;199:89–102. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Schuller G, Covey E, Casseday JH. Auditory pontine grey: connections and response properties in the horseshoe bat. Eur J Neurosci. 1991;3:648–662. doi: 10.1111/j.1460-9568.1991.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Phil Trans R Soc Lond B. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus and its role in cortical function. 2. MIT Press; London: 2006. [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid projections of the rat temporal cortex. J Comp Neurol. 1997;382:153–175. [PubMed] [Google Scholar]
- Smith PH, Bartlett EL, Kowalkowski A. Cortical and collicular inputs to cells in the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. J Neurophysiol. 2007;2007:681–695. doi: 10.1152/jn.00235.2007. [DOI] [PubMed] [Google Scholar]
- Smith PH, Populin LC. Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. J Comp Neurol. 2001;436:508–519. doi: 10.1002/cne.1084. [DOI] [PubMed] [Google Scholar]
- Stefani A, Pisani A, Mercuri NB, Calabresi P. The modulation of calcium currents by the activation of mGluRs. Functional implications. Mol Neurobiol. 1996;13:81–95. doi: 10.1007/BF02740753. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol A. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KAC, Bannister NJ, Jack JJB. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelár J, Kvasnák E, Astl J. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Exp Brain Res. 2000;133:254–266. doi: 10.1007/s002210000426. [DOI] [PubMed] [Google Scholar]
- Tan AY, Atencio CA, Polley DB, Merzenich MM, Schreiner CE. Unbalanced synaptic inhibition can create intensity-tuned auditory cortex neurons. Neuroscience. 2007;146:449–462. doi: 10.1016/j.neuroscience.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Tan AY, Wehr M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience. 2009;163:1302–1315. doi: 10.1016/j.neuroscience.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Lee CC, Sherman SM. Specific and nonspecific thalamocortical connectivity in the auditory and somatosensory thalamocortical slices. Neuroreport. 2010;21:861–864. doi: 10.1097/WNR.0b013e32833d7cec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010;4:13. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Jack J. Detailed passive cable models of layer 2/3 pyramidal cells in rat visual cortex at different temperatures. J Physiol. 2002;539(Pt.2):623–636. doi: 10.1113/jphysiol.2001.013291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to muscarinic activation between first and higher order thalamic relays. J Neurophysiol. 2007;98:3538–3547. doi: 10.1152/jn.00578.2007. [DOI] [PubMed] [Google Scholar]
- Varela C, Sherman SM. Differences in response to serotonergic activation between first and higher order thalamic nuclei. Cereb Cortex. 2009;19:1776–1786. doi: 10.1093/cercor/bhn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices. J Neurophysiol. 2011;105:279–292. doi: 10.1152/jn.00747.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Hopkins W, Diamond DM. Physiological plasticity of single neurons in auditory cortex of the cat during acquisition of the pupillaiy conditioned response: I. Primary field (AI) Behav Neurosci. 1984;98:171–188. doi: 10.1037//0735-7044.98.2.171. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ. The tectothalamic system. In: Winer JA, Schreiner CE, editors. The inferior colliculus. Springer; New York: 2005. pp. 200–230. [Google Scholar]
- Winer JA. The functional architecture of the medial geniculate body and the primary auditory cortex. In: Webster DB, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research, volume 1, The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 222–409. [Google Scholar]
- Winer JA. The central auditory system: a functional analysis. In: Winer JA, Schreriner CE, editors. The Inferior Colliculus. Springer-Verlag; New York: 2005. pp. 1–68. [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res. 2006;212:1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol. 2001;430:27–55. [PubMed] [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol. 1998;400:147–174. [PubMed] [Google Scholar]
- Winer JA, Larue DT, Huang CL. Two systems of giant axons terminals in the cat medial geniculate body: convergence of cortical and GABAergic inputs. J Comp Neurol. 1999;413:181–197. [PubMed] [Google Scholar]
- Winer JA, Saint-Marie RL, Larue DT, Oliver DL. GABAergic feedforward projections from the inferior colliculus to the medial geniculate body. Proc Natl Acad Sci U S A. 1996;93:8005–8010. doi: 10.1073/pnas.93.15.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart C, Cocco S, Bourdieu L, Léger JF, Herr C, Chatenay D. Dynamics of excitatory synaptic components in sustained firing at low rates. J Neurophysiol. 2005;93:3370–3380. doi: 10.1152/jn.00530.2004. [DOI] [PubMed] [Google Scholar]
- Ye Y, Machado DG, Kim DO. Projection of the marginal shell of the anteroventral cochlear nucleus to olivocochlear neurons in the cat. J Comp Neurol. 2000;420:127–138. [PubMed] [Google Scholar]
- Young ED, Spirou GA, Rice JJ, Voigt HF. Neural organization and responses to complex stimuli in the dorsal cochlear nucleus. Philos Trans R Soc Lond B Biol Sci. 1992;29:407–413. doi: 10.1098/rstb.1992.0076. [DOI] [PubMed] [Google Scholar]
- Yuan K, Fink KL, Winer JA, Schreiner CE. Local connection patterns of parvalbumin-positive inhibitory interneurons in rat primary auditory cortex. Hear Res 2010. 2010 doi: 10.1016/j.heares.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bats. J Neurophysiol. 2000;84:325–333. doi: 10.1152/jn.2000.84.1.325. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liu BH, Wu GK, Kim YJ, Xiao Z, Tao HW, Zhang LI. Preceding inhibition silences layer 6 neurons in auditory cortex. Neuron. 2010;65:706–717. doi: 10.1016/j.neuron.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]