Abstract
Introduction
Pediatric acute liver failure (ALF) is often accompanied by hepatic encephalopathy, cerebral edema and raised intracranial pressure (ICP). Elevated ICP can be managed more effectively with intracranial monitoring, but ALF-associated coagulopathy is often considered a contraindication for invasive monitoring due to risk for intracranial bleeding. We reviewed our experience with use of early ICP monitoring in ALF in children listed for liver transplantation.
Methods
Retrospective review of all intubated pediatric ALF patients with Grade 3 and Grade 4 encephalopathy requiring intracranial pressure monitoring and evaluated for potential liver transplant were identified from an institutional liver transplant patient database from 1999 to 2009.
Result
14 patients were identified that met inclusion criteria. Age ranged from 7 months to 20 yrs. Diagnoses of ALF were infectious (3), drug induced (7), autoimmune hepatitis (2) and indeterminate (2). Grade 3 and 4 encephalopathy was seen in 10 (71%) and 4 (29%) patients respectively. CT scans prior to ICP monitor placement showed cerebral edema in 5 (35.7%) patients. Prior to ICP monitor placement, fresh frozen plasma, Vitamin K and activated recombinant factor VIIa were given to all 14 patients with significant improvement in coagulopathy (p<.04). Initial ICP ranged from 5 – 50 cmH2O; ICP was significantly higher in patients with cerebral edema by CT (p<.05). 11/14 (78%) patients received hypertonic saline and 3 (22%) received mannitol for elevated ICP. 8 of 14 (56%) monitored patients were managed to liver transplant with 100% surviving neurologically intact. 4/14 (28%) patients had spontaneous recovery without liver transplant. 2 of 14 (14%) patients died due to multiple organ failure prior to transplant. One patient had a small 9mm intracranial hemorrhage but survived after receiving a liver transplant. No patient developed intracranial infection.
Conclusion
In our series of patients, ICP monitoring had a low complication rate and was associated with a high survival rate despite severe hepatic encephalopathy and cerebral edema in the setting of pediatric ALF. In our experience, monitoring of ICP allowed interventions to treat increased ICP and provided additional information regarding central nervous system injury prior to liver transplant. Further study is warranted to confirm if monitoring allows more directed ICP therapy and improves survival in pediatric ALF.
Keywords: Intracranial monitoring, cerebral edema, acute liver failure, children
Introduction
Pediatric acute liver failure (ALF) is a dramatic clinical syndrome in which children with no known evidence of chronic liver disease rapidly lose hepatic function, develop hepatic-based coagulopathy (defined as prothrombin time [PT] > 15 seconds or INR > 1.5 not corrected by Vitamin K in the presence of hepatic encephalopathy [HE] or PT ≥ 20 seconds or INR ≥ 2 regardless of the presence or absence of clinical HE), and become critically ill within days. 1
In contrast to adults, the early stages of hepatic encephalopathy are difficult to assess in children, and ALF-related cerebral edema may not be apparent until terminal stages of ALF in infants.2,3 Children who develop ALF are at high risk for death unless liver transplantation can be provided in a timely fashion.4,5,6 Cerebral edema leading to intracranial hypertension (ICH) is reported in up to 80% of children with severe ALF and hepatic encephalopathy, and complications related to cerebral edema constitute the leading cause of death in ALF.7,8 The impact of severity of intracranial hypertension in ALF-induced cerebral edema has not been well studied. However, in certain settings, directed treatment of increased ICH is typically performed based on direct monitoring of intracranial pressure (ICP), with evidence of improved outcomes9,10
While potentially providing useful information, placement of ICP monitors in ALF patients can bring potential risk due to the uniform presence of severe coagulopathy in these patients and the potential for bleeding.11 In one adult study, the incidence of intracranial bleeding was reported to be almost 20% in patients with ALF receiving ICP monitor placement.12 Given the lack of reported experience and study, indications for the use and placement of ICP monitors in pediatric ALF remain an area of ongoing debate. At our center, we have utilized ICP monitors in pediatric ALF patients with hepatic encephalopathy awaiting liver transplant to determine the degree of intracranial hypertension and to guide the management of elevated ICP. We reviewed our experience with intracranial monitoring and treatment in children in this setting. We hypothesized that ICP monitoring could be performed with minimal complications, would demonstrate increased ICP in ALF patients, and would be useful to guide directed therapy for increased ICP.
Materials and Methods
We performed a retrospective review of all pediatric ALF patients less than or equal to 18 years of age with hepatic encephalopathy receiving an ICP monitor in the intensive care unit at our quaternary care children’s medical center, from January 1999 through January 2009. The pediatric intensive care unit at Children’s Healthcare of Atlanta at Egleston is a 30 bed multidisciplinary unit with average 1600 annual admissions. The liver transplant program performs transplants on an average of 22 patients per year and recently reported a 10-year experience of 33 liver transplants for ALF13. ALF was based on criteria from the Pediatric Acute Liver Failure study group (PALFSG) 1 and defined as acute onset of liver injury in children without history or evidence of chronic liver disease, with liver-associated coagulopathy in the presence or absence of encephalopathy. Inclusion criteria for this study included all intubated ALF patients with Grade 3 and Grade 4 encephalopathy receiving intracranial pressure (ICP) monitoring and evaluated for potential liver transplant. Patients were identified from the institutional prospective liver transplant clinical care database. This study was approved by the Children’s Healthcare of Atlanta institutional review board with a waiver of informed consent.
Patient charts were reviewed for 1) demographic characteristics, 2) degree of hepatic encephalopathy based on a standard classification system, 3) severity of overall organ failure as calculated by the pediatric logistic organ dysfunction (PELOD) score,14 4) radiographic imaging, 5) indications for ICP monitoring, 6) preparation of patient prior to ICP monitor placement, 7) complications attributable to ICP placement and monitoring, 8) treatment measures for ICP, 9) subsequent response to treatment and 10) patient outcome. Encephalopathy was graded based on standard criteria adapted to infants/children: Grade I-characterized by confusion and mood changes; Grade II-patient is drowsy or shows inappropriate behavior; Grade III-patient is stuporous but obeys simple commands or is sleepy but arousable; Grade IVa-patient is comatose but arousable with painful stimuli; Grade IVb-patient is in deep coma and not arousable with any stimuli.15,16
Statistical analysis was performed (SPSS 16 for Windows, Chicago IL). Descriptive findings were reported as medians with ranges. The Mann-Whitney U test was used to compare parameters before and after ICP placement. A p value < 0.05 was considered statistically significant. Pearson correlation was used to evaluate the association of treatment interventions and changes in ICP.
General Patient Management Approaches
Placement of ICP monitors was performed in ALF patients for general indications of Grade III or IV encephalopathy who required intubation and mechanical ventilation. Decision for placement was based on consensus decision of the management team, including intensivists, hepatologist, and transplant surgeon. ICP monitors (Camino; Integra Life Sciences Corporation, Plainsboro, NJ) were placed intraparenchymally by a pediatric neurosurgeon utilizing standard placement techniques following evaluation of coagulation state and coagulation factor replacement as needed. It is standard practice in our PICU to use intravenous phytonadione, fresh frozen plasma and activated recombinant factor seven within 30 minutes prior to ICP monitor placement. All patients received ICP monitoring but no cerebrospinal fluid drainage. All patients had basic measures performed to minimize the risk of increased ICP, including elevation of head of bed to 30 degrees, neutral neck positioning, minimizing painful stimuli (including avoidance of routine suctioning when on ventilator) and acceptance of an end tidal carbon dioxide measure between 32–38mmHg. Pentobarbital coma (with continuous EEG monitoring) was used if ICP was refractory to hypertonic saline, mannitol, sedation and neuromuscular blockade. Goal body temperatures were maintained between 35.5–37° C. General approaches to appropriate cerebral perfusion pressure (CPP-calculated as mean arterial pressure – intracranial pressure) management included use of intravenous vasopressors to maintain a CPP of greater than 50 to 60 mm Hg. 17 CPP between 40 to 50mm Hg was accepted in infants and small children. ICP monitoring was discontinued if ICP remained less than 10mm Hg for at least 24 hours without any significant therapeutic intervention for increased ICP.
Results
Patient Characteristics
From 1999–2009, 201 patients received liver transplants at our institution. Of these, 33 received transplants for ALF. Nineteen ALF patients without significant encephalopathy did not receive ICP monitoring. Fourteen of 33 patients with ALF received ICP monitoring in the PICU. The demographic and clinical characteristics of these patients are described in Table 1. Seven of 14 (50%) patients had ALF due to acetaminophen ingestion, 2(14%) had autoimmune hepatitis, 3 (21%) had a viral infectious etiology and in the remaining 2(14%) the etiology was undetermined. All 14 patients had severe hepatic encephalopathy: 10 (71%) with grade III encephalopathy, and 4 (29%) with grade IV encephalopathy. All patients were intubated at the time of ICP monitor placement and had computed tomography (CT) of the brain done prior to ICP monitor placement. All patients received fentanyl infusions for analgesia, and intermittent low dose benzodiazepine (0.05 mg/kg/dose) for anxiolysis. All 14 patients demonstrated significant coagulation abnormalities prior to monitor placement. All 14 patients received intravenous phytadione (vitamin K), fresh frozen plasma and activated recombinant factor 7 (dose 90 to 120 mcg/kg) to correct international normalized ratio (INR) to less than or equal to1.5 within thirty minutes prior to ICP monitor placement. Two patients (14.3%) received plasmapheresis for partial correction of coagulopathy. With therapy, coagulation abnormalities were significantly improved (Table 2). Five of 14 (35.7%) patients had cerebral edema reported by computerized tomography. Seven of 14 (50.1%) patients had a normal head CT prior to ICP monitor placement. Of the remaining two patients one had a small subdural hematoma noted immediately after ICP monitor placement. Opening ICP in this patient was 20mmHg. The other patient had chronic extra-axial fluid collections with enlarged ventricles by CT; opening ICP was 6mmHg.
Table 1.
Clinical characteristics of pediatric patients with acute liver failure and encephalopathy receiving intracranial pressure monitor placement
| N | Values | Percentage | |
|---|---|---|---|
| Median Age in months (range) | 14 | 131(7–240) | - |
| Sex | 14 | Male 6 | 47 |
| Female 8 | 53 | ||
| Median pediatric logistic organ dysfunction (PELOD) score (range) | 14 (12–23) | ||
| Ethnicity: | 14 | ||
| Caucasian | 3 | 21.4 | |
| African American | 9 | 64.3 | |
| Hispanic | 1 | 7.1 | |
| other | 1 | 7.1 | |
| Grades of encephalopathy at presentation | |||
| Grade III | 4 | 4 | 29 |
| Grade IV | 10 | 10 | 71 |
Table 2.
Coagulation profiles of ALF patients undergoing ICP monitor placement. Coagulation abnormalities were significantly improved after treatment with blood product replacement.
| Median value on admission to PICU prior to correction (range) | Median value after correction prior to ICP monitor placement (range) | P value | |
|---|---|---|---|
| Partial thromboplastin time | 52 (30–160) | 38 (29–53) | P< 0.03 |
| Prothrombin time | 45(19–109) | 11.6(7–23) | P<0.04 |
| INR* | 3.2(1.5–4.6) | 0.8 (0.7–1.9) | P< 0.04 |
International normalized ratio
ICP Monitor Placement and ICP Management
ICP monitors were placed a median of 24 hours after admission to the PICU (range 10–32 hours). Median duration of ICP monitor use was 5.3 days (range 2.3–8.3). Median initial ICP immediately after placement was 14 mmHg (range 4–25). Median initial ICP of patients with an initial normal CT scan was 9 mmHg (range 6–17mm Hg), while median initial ICP in patients with cerebral edema by CT was 18 mmHg (range 8–47mmHg) (p<0.05). Of the 5 patients with cerebral edema, two had ICPs of 20 mmHg and two had ICPs of 18 mmHg. One patient with cerebral edema had a small subdural hematoma by CT following monitor placement; initial ICP was 47mm Hg. Initial ICP was not significantly correlated with initial serum sodium concentration. All 14 patients received some form of potentially neuroprotective osmotherapy for treatment of elevated ICP, decreased CPP, or abnormal sodium concentration (Table 3). Ten of 14 patients received at least one dose of 3% hypertonic saline (median dose 5 cc/kg) within the first 24 hours after ICP monitor placement for elevated ICP. Four patients received mannitol (dose 1 gm/kg). No patient received repeat mannitol dosing. Changes in physiologic variables in response to therapy are noted in Table 4. Median change in ICP following hypertonic saline was 7mm Hg (range 3–22 mmHg) and following mannitol was 5.5mm Hg (range 3–7mm Hg p = 0.436). Median change in CPP in response to hypertonic saline was 11 mm Hg (range 9–22 mm Hg) and to mannitol was 12.5mm Hg (range 8–16mm Hg p < 0.79). ICP was significantly decreased in association with increases in serum sodium concentration subsequent to hypertonic saline treatment (Figure; r = 0.934, p < .05). No patient had ICPs that were refractory to therapeutic interventions.
Table 3.
Neuroprotective therapy following ICP monitor placement in patients with ALF and severe hepatic encephalopathy
| # of patients (%) | |
|---|---|
| Mannitol | 4 (28.6) |
| 3% hypertonic saline | 10 (71.4) |
| Pentobarbital coma to treat ICP | 8(57.1) |
| Dopamine to maintain CPP* | 7(50) |
| Norepinephrine to maintain CPP* | 7(50) |
CPP* = cerebral perfusion pressure
Table 4.
Change in mean arterial pressure (MAP), intracranial pressure (ICP) and cerebral perfusion pressure (CPP) within 30 minutes following specific osmotherapy in children with ALF and ICP monitoring. All measurements in mm Hg.
| Patient | Therapy with 3% Hypertonic Saline (HS) | ||||||
|---|---|---|---|---|---|---|---|
| MAP before HS | MAP after HS | ICP before HS | ICP after HS | CPP before HS | CPP after HS | Change in CPP with HS | |
| 1 | 67 | 80 | 17 | 8 | 50 | 72 | 22 |
| 2 | 76 | 77 | 20 | 12 | 56 | 65 | 9 |
| 3 | 70 | 70 | 20 | 10 | 50 | 60 | 10 |
| 4 | 68 | 70 | 18 | 10 | 50 | 60 | 10 |
| 5 | 77 | 85 | 47 | 25 | 30 | 60 | 30 |
| 6 | 64 | 70 | 8 | 2 | 56 | 68 | 12 |
| 7 | 69 | 74 | 8 | 4 | 61 | 70 | 9 |
| 8 | 54 | 57 | 9 | 5 | 45 | 52 | 17 |
| 9 | 58 | 64 | 8 | 4 | 50 | 60 | 10 |
| 10 | 61 | 63 | 6 | 3 | 55 | 60 | 5 |
| Therapy with mannitol | |||||||
|---|---|---|---|---|---|---|---|
| Patient | MAP before mannitol | MAP after mannitol | ICP before mannitol | ICP after mannitol | CPP before mannitol | CPP after mannitol | Change in CPP with mannitol |
| 11 | 70 | 72 | 12 | 6 | 58 | 66 | 8 |
| 12 | 62 | 71 | 10 | 5 | 52 | 66 | 14 |
| 13 | 58 | 62 | 11 | 4 | 47 | 58 | 11 |
| 14 | 57 | 70 | 9 | 6 | 48 | 64 | 16 |
Complications of ICP Monitor Placement
One of the 14 patients (7%) had a bleeding complication associated with ICP monitor placement. The patient was a 15-year-old adolescent with ALF and Grade 4 encephalopathy who was intubated and mechanically ventilated. Patient INR was 1.5. She received IV phytonadione, FPP and recombinant activated factor VIIa within 30 minutes prior to ICP monitor placement. Immediately following ICP monitor placement, she was noted to have pupillary asymmetry. Opening pressure of 47 mmHg was noted. Computerized tomography revealed a small subdural hematoma. She was treated aggressively with fresh frozen plasma and factor seven concentrate, head of bed elevation, low normal ventilation(PCO2 32–38mmHg), and hypertonic saline. A repeat CT scan 24 hours later showed no progression in the hemorrhage and no other intracranial pathologic process. She received a liver transplant 3 days later. ICPs remained stable except for a very brief spike of 45mm Hg postoperatively which responded to medical therapy. The ICP monitor was removed in 7 days after placement. The patient was extubated, had an otherwise uneventful postoperative course and recovered with normal neurological function at follow-up.
No intracranial infections occurred from placement of ICP monitors. All patients received prophylactic antibiotics while the ICP monitor was in place.
Liver transplant and outcomes
Twelve of 14(85.7%) patients survived. Two patients died due to progression of multiple organ dysfunction syndrome and were not considered for liver transplantation. The two deaths were determined to be unrelated to ICP monitor placement. Initial ICP in these 2 patients was 20 and 18 mmHg, and did not differ from ICPs in survivors. The decision not to offer transplant option in these 2 patients was not based on ICP measurements. Four patients demonstrated improvement in ALF and did not require transplant. Eight of 12 (66%) patients received orthotropic liver transplant; all survived to hospital discharge. The twelve survivors had magnetic resonance imaging (MRI) of brain after ICP monitor removal. In 10 of 12, MRI findings were normal. One patient who did not receive a transplant was noted to have diffuse cerebral hypoxic injury. One patient had evidence of posterior reversible encephalopathy syndrome (PRES); opening ICP in this patient had been 10mm Hg. The etiology of these morphologic changes was determined to be unrelated to ICP monitor placement.
Discussion
The experience reported from our center provides encouraging information for placement of ICP monitors in children with ALF and severe encephalopathy to guide assessment and management prior to liver transplantation. No randomized controlled trials of ICP monitoring in ALF have been performed, and no consensus exists on indications and its use from the PALFSG findings. However, an adult ALF study group has recently endorsed the use of ICP monitors in adult ALF patients listed for orthotopic liver transplant with stage III/IV encephalopathy.18 The severe coagulopathy of ALF makes invasive procedures, including placement of ICP monitors risky. Preparation of patients with blood products in that case series was not reported.12 We observed a relatively low incidence of associated bleeding, and no associated neurologic sequelae in children, although our series was relatively small. Only one patient in our series had a small SDH noted after placement. This SDH could represent an incidental radiologic finding as shown in a series by Vaquero et al.11
This success may have been related to aggressive correction of coagulopathy prior to monitor placement. All our patients received recombinant activated factor VII before placement. Use of this product has been shown to be useful for restoring hemostasis in patients with ALF undergoing invasive procedures.19,20 It is possible that our use of intravenous phytonadione, fresh frozen plasma and single low dose activated recombinant factor VII was beneficial in decreasing likelihood of bleeding. Studies have shown that frequent use of activated recombinant factor VII in high doses can result in life threatening arterial and venous thromboembolism.21, 22 The Pediatric AFLSG supported correction of INR in ALF only for significant bleeding, or prior to invasive procedures. In an adult study of ALF patients who underwent ICP monitor placement, the risk of bleeding was proportional to the depth of insertion of the device.12 All ICP monitors placed in our ALF patients were intraparenchymal in location. The ALFSG found insufficient data to recommend a standard location for the ICP monitor placement. ICP monitors placed in the epidural space may decrease bleeding incidence but are less accurate than monitors traversing the dura and also tend to overestimate ICP.23,24 Avoidance of intraventricular placement is generally recommended due to the risk of bleeding. The lack of associated intracranial infections in our series is encouraging, as prevention of infections in ALF is important especially given that sepsis with SIRS is a major risk factor for developing cerebral edema and intracranial hypertension.25
Utilization of ICP monitoring allowed assessment and maintenance of an age appropriate CPP in these ALF patients. Insufficient data exist to recommend strict ICP and CPP goals in children with hepatic encephalopathy. At our center, desired ICP values are between 20–25mm Hg and CPP between 50 to 80 mm Hg. Obtaining higher CPP (> 70) may necessitate use of vasopressors to increase mean arterial pressure. An elevated ICP greater than 40mm Hg and a prolonged CPP less than 50mmHg are associated with poor neurological outcome in ALF patients and are not considered to be candidates for liver transplantation.26 Use of an ICP monitor would be critical for making these determinations.
The specific mechanism responsible for cerebral edema in ALF is not clearly understood but is believed to be multifactorial in etiology. Two commonly accepted theories are the glutamine hypothesis and the cerebral vasodilatation hypothesis.27,28 As liver function deteriorates, cerebral hyperemia develops, leading to increased cerebral blood volume and intracranial hypertension. This could lead to subsequent development of cytotoxic edema, falling cerebral blood flow and increased ICP. Edema and increased ICP could be worsened by infection, hypoxemia, hemodynamic instability, metabolic disturbances and seizures.29 The cerebral edema and accompanying ICH, if untreated, may result in uncal herniation, which is uniformly fatal. While the impact of treatment on ICP in this setting is uncertain, it is reasonable to surmise that earlier recognition of increased ICP would seem valuable to provide aggressive treatment to prevent progression. Clinical signs and computed tomography are insensitive to diagnose significant ICP in such patients necessitating the need for invasive ICP monitoring.30,31 While ICP monitoring could be most important for the peri-operative transplant period, presence of monitoring could be of particular importance in the operating room during transplantation, where the risk of developing worsening cerebral edema is highest during the anhepatic portion of the procedure.32,33 This may be compounded by hemodynamic instability in a critically ill ALF patient.34
Use of hypertonic saline has shown benefit in treatment of pediatric patients with elevated ICP, and its use offers a theoretical advantage over mannitol, which can cause volume depletion due to its diuretic effect. 35 Hypertonic saline may decrease ICP by its osmotic effect on cerebral blood flow restoration of resting membrane potential and stabilization of cerebral endothelial cells thereby helping inhibit the occurrence of vasogenic cerebral edema.,36,37,38,39 The role of hypertonic saline in the management of cerebral edema in hepatic encephalopathy has not been studied in children. Hyponatremia is also common in ALF, especially in patients with liver failure secondary to acetaminophen toxicity and is reported to be a predictor of poor outcome in such patients.40,41,42 Use of hypertonic saline in the treatment of increased ICP could also have a salutatory effect on associated hyponatremia. However, the rate of correction of hyponatremia should be inversely proportional to the duration of hyponatremia to minimize risk of osmotic demyelination.43, A variety of interventions were used in these patients to manage ICP. The PILOT score44 has been developed to quantify the amount of interventions as an indicator of intensity in patients with traumatic brain injury. Given the lack of experience for this score’s use in nontraumatic brain injury, we chose not to perform this score.
A previous report noted an inverse correlation between opening ICP and admission serum sodium in patients with ALF.45 In our study we did not find this association. However, the improvement in median ICP with increases in sodium concentration from hypertonic saline use in our series suggests the benefit of monitoring to guide therapy. The ability to monitor ICP would allow for identifying and treating more subtle changes in ICP and acting pre-emptively compared to relying only on gross evidence of worsening edema such as acute pupillary dilation.
Conclusions
Invasive ICP monitoring can be performed in children with ALF with low complication, and its presence allows guidance of therapy for increased ICP during assessment and management of children awaiting liver transplantation. Correction of coagulopathy prior to placement could limit bleeding complications. Given the limitations of retrospective analysis of a small patient series from a single institution, further study is warranted to determine the optimal use of ICP monitoring in these patients.
Figure 1.
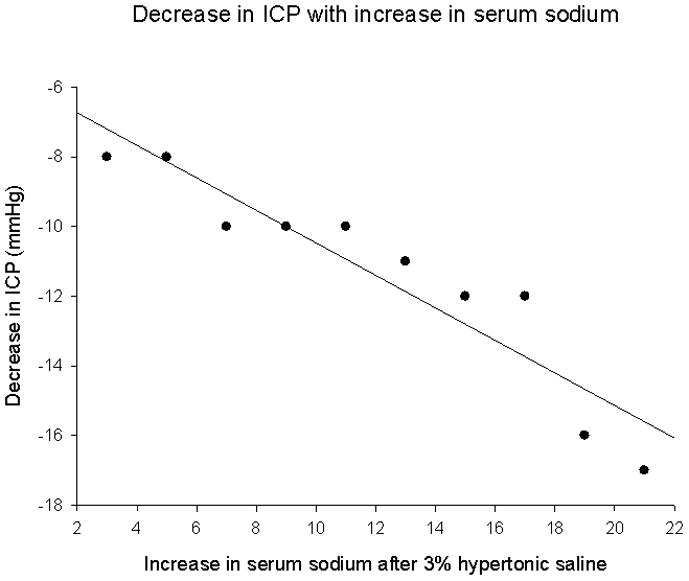
Change in intracranial pressure (mm Hg) in acute liver failure patients receiving intracranial pressure monitoring. Intracranial pressure decrease was associated with increase in serum sodium concentration (mmol/L) from baseline prior to monitor placement (r = −0.932, p<0.05)
Fig 2.
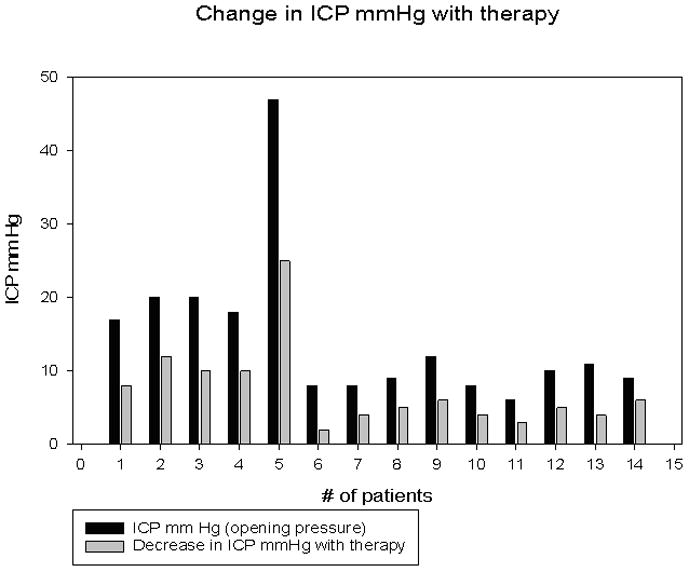
Change in intracranial pressure (mm Hg) with therapy. Patients 11, 12, 13 & 14 were treated with one dose of IV mannitol (1gm/kg). All other patients received 3% hypertonic saline (5cc/Kg).
Acknowledgments
Funding:
Dr. Vos is funded by NIH- K23DK080953
Dr Romero is funded by NIH 5 U01 DK084585-02
References
- 1.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, Dhawan A, Rosenthal P, Rodriguez-Baez N, Murray KF, Horslen S, Martin MG, Lopez MJ, Soriano H, McGuire BM, Jonas MM, Yazigi N, Shepherd RW, Schwarz K, Lobritto S, Thomas DW, Lavine JE, Karpen S, Ng V, Kelly D, Simonds N, Hynan LS. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006 May;148(5):652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera-Penera T, Moreno J, Skaff C, et al. Delayed encephalopathy in fulminant hepatic failure in the pediatric population and the role of liver transplantation. J Pediatr Gastroenterol Nutr. 1997;24(2):128–134. doi: 10.1097/00005176-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bucuvalas J, Yazigi N, Squires RH., Jr Acute liver failure in children. Clin Liver Dis. 2006 Feb;10(1):149–68. vii. doi: 10.1016/j.cld.2005.10.006. Review. [DOI] [PubMed] [Google Scholar]
- 4.Durand P, Debray D, Madeal R, et al. Acute liver failure in infancy: a 14-year old experience of a pediatric liver transplantation center. J Pediatr. 2001;139(6):871–6. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan A. Etiology and prognosis of acute liver failure in children. Pediatr Transplant. 2008;12:167–173. [Google Scholar]
- 6.Squires RH., Jr Acute liver failure in children. Semin Liver disease. 2008 May;28(2):153–66. doi: 10.1055/s-2008-1073115. [DOI] [PubMed] [Google Scholar]
- 7.Ware AJ, D’Agostino AN, Combes B. Cerebral edema: a major complication of massive hepatic necrosis. Gastroenterology. 1971 Dec;61(6):877–84. [PubMed] [Google Scholar]
- 8.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002 Dec 17;137(12):947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Downard C, Hulka F, Mullins RJ, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654–58. doi: 10.1097/00005373-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Barlow KM, Minns RA. The relation between intracranial pressure and outcome in non-accidental head injury. Dev Med Child Neurology. 1999;41:220–5. doi: 10.1017/s0012162299000481. [DOI] [PubMed] [Google Scholar]
- 11.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, Han S, Harrison ME, Stravitz TR, Muñoz S, Brown R, Lee WM, Blei AT. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005 Dec;11(12):1581–9. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 12.Blei AT, Olafsson S, Webster Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993;341:157–158. doi: 10.1016/0140-6736(93)90016-a. [DOI] [PubMed] [Google Scholar]
- 13.Heffron TG, Pillen T, Smallwood G, Rodriguez J, Sekar S, Henry S, Vos M, Casper K, Gupta NA, Fasola CG, Romero R. Pediatric liver transplantation for acute liver failure at a single center: A 10-yr experience. Pediatric Transplantation. 2010 Mar;14(2):p228–232. doi: 10.1111/j.1399-3046.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003 Jul 19;362(9379):192–7. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 15.Tissieres P, Devictir D. Fulminant hepatic failure and transplantation: Roger’s textbook of pediatric intensive care. 4. 2008. pp. 578–584. [Google Scholar]
- 16.Cochran JB, Losek JD. Acute liver failure in children: Pediatr Emerg Care. 2007 Feb;23(2):129–35. doi: 10.1097/PEC.0b013e3180308f4b. Review. [DOI] [PubMed] [Google Scholar]
- 17.Tasker RC. Head and spinal cord trauma: Roger’s textbook of pediatric intensive care. 4. 2008. pp. 888–911. [Google Scholar]
- 18.Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro L, Smith AD, Lee WM, Fontana RJ, McGuire BM Acute Liver Failure Study Group. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007 Nov;35(11):2498–508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 19.Porte R, Caldwell S. The role of recombinant factor VIIa in liver transplantation. Liver Transpl. 2005;11:872–874. doi: 10.1002/lt.20447. [DOI] [PubMed] [Google Scholar]
- 20.Quan D, Bass N, Hirose R, et al. The effect of recombinant factor VIIa and fresh frozen plasma on the INR in patients with acute and chronic liver failure. Hepatology. 2003;38(4):550A. [Google Scholar]
- 21.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T FAST Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008 May 15;358(20):2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 22.Alten JA, Benner K, Green K, Toole B, Tofil NM, Winkler MK. Pediatric off-label use of recombinant factor VIIa. Pediatrics. 2009 Mar;123(3):1066–72. doi: 10.1542/peds.2008-1685. [DOI] [PubMed] [Google Scholar]
- 23.Keays RT, Alexander GJ, Williams R. The safety and value of Extradural intracranial pressure monitors in fulminant hepatic failure. J Hepatol. 1993:205–209. doi: 10.1016/s0168-8278(05)80247-8. [DOI] [PubMed] [Google Scholar]
- 24.Poca MA, Sahuquillo J, Topczewski T, et al. is intracranial pressure monitoring in epidural space reliable? Fact and fiction. J Neurosurg. 2007;106:548–556. doi: 10.3171/jns.2007.106.4.548. [DOI] [PubMed] [Google Scholar]
- 25.Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 125:755–764. doi: 10.1016/s0016-5085(03)01051-5. [DOI] [PubMed] [Google Scholar]
- 26.McCashland TM, Shaw BW, Jr, Tape E. The American experience with liver transplantation for acute liver failure. Seminal Liver disease. 1996;16:427–433. doi: 10.1055/s-2007-1007255. [DOI] [PubMed] [Google Scholar]
- 27.Butterworth RF. Molecular neurobiology of acute liver failure. Semin Liver Dis. 2003 Aug;23(3):251–8. doi: 10.1055/s-2003-42643. [DOI] [PubMed] [Google Scholar]
- 28.Blei AT. The Pathophysiology of brain edema in acute liver failure. Neurochem Int. 2005;47:71–77. doi: 10.1016/j.neuint.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Steadman RH, Van Rensburg A, Kramer DJ. Transplantation for acute liver failure: perioperative management. Curr Opin Organ Transplant. 2010 Jun;15(3):368–73. doi: 10.1097/MOT.0b013e32833982dd. [DOI] [PubMed] [Google Scholar]
- 30.Ellis A, Wendon J. Circulatory, respiratory, cerebral, and renal derangements in acute liver failure: pathophysiology and management. Semin Liver Dis. 1996 Nov;16(4):379–88. doi: 10.1055/s-2007-1007251. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki M, Shaw B, Schafer D, et al. Advantages of intracranial pressure monitoring in patients with fulminant liver failure. Gastroenterology. 1992;102:A826. [Google Scholar]
- 32.Detry O, Arkadopoulos N, Ting P, Kahaku E, Margulies J, Arnaout W, Colquhoun SD, Rozga J, Demetriou AA. Intracranial pressure during liver transplantation for fulminant hepatic failure. Transplantation. 1999 Mar 15;67(5):767–70. doi: 10.1097/00007890-199903150-00024. [DOI] [PubMed] [Google Scholar]
- 33.Jalan R, Olde Damink SW, Deutz NE, Davies NA, Garden OJ, Madhavan KK, Hayes PC, Lee A. Moderate hypothermia prevents cerebral hyperemia and increase in intracranial pressure in patients undergoing liver transplantation for acute liver failure. Transplantation. 2003 Jun 27;75(12):2034–9. doi: 10.1097/01.TP.0000066240.42113.FF. [DOI] [PubMed] [Google Scholar]
- 34.Alper G, Jarjour IT, Reyes JD, Towbin RB, Hirsch WL, Bergman I. Outcome of children with cerebral edema caused by fulminant hepatic failure. Pediatr Neurol. 1998 Apr;18(4):299–304. doi: 10.1016/s0887-8994(97)00218-x. [DOI] [PubMed] [Google Scholar]
- 35.Khanna S, Davis D, Peterson B, Fisher B, Tung H, O’Quigley J, Deutsch R. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000 Apr;28(4):1144–51. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 36.Rizoli SB, Rotstein OD, Sibbald WJ. The immunological effects of hypertonic saline. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency medicine. Berlin: Springer; 2002. pp. 446–453. [Google Scholar]
- 37.Bhardwaj A, Ulatowski JA. Hypertonic saline solutions in brain injury. Curr Opin Crit Care. 2004 Apr;10(2):126–31. doi: 10.1097/00075198-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Vats A, Chambliss CR, Anand KJS, et al. Is hypertonic saline an effective alternative to mannitol in the treatment of elevated pressure in pediatric patients? Journal of Intensive Care Medicine. 1999;14:184–8. [Google Scholar]
- 39.Kamat P, Vats A, Gross M, Checchia PA. Use of hypertonic saline for the treatment of altered mental status associated with diabetic ketoacidosis. Pediatr Crit Care Med. 2003 Apr;4(2):239–42. doi: 10.1097/01.PCC.0000059340.19010.CE. [DOI] [PubMed] [Google Scholar]
- 40.Tandon BN, Joshi YK, Tandon M. Acute liver failure. Experience with 145 patients. J Clin Gastroenterol. 1986 Dec;8(6):664–8. doi: 10.1097/00004836-198612000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava KL, Mittal A, Kumar A, Gupta S, Natu SM, Kumar R, Govil YC. Predictors of outcome in fulminant hepatic failure in children. Indian J Gastroenterol. 1998 Apr;17(2):43–5. [PubMed] [Google Scholar]
- 42.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004 Feb;39(2):464–70. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 43.Sarnaik AP, Meert K, Hackbarth R, Fleischmann L. Management of hyponatremic seizures in children with hypertonic saline: a safe and effective strategy. Crit Care Med. 1991 Jun;19(6):758–62. doi: 10.1097/00003246-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Shore PM, Hand LL, Roy L, Trivedi P, Kochanek PM, Adelson PD. Reliability and validity of the Pediatric Intensity Level of Therapy (PILOT) scale: a measure of the use of intracranial pressure-directed therapies. Crit Care Med. 2006 Jul;34(7):1981–7. doi: 10.1097/01.CCM.0000220765.22184.ED. [DOI] [PubMed] [Google Scholar]
- 45.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004 Feb;39(2):464–70. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]


