Abstract
Background
The clinical importance of the association of HIV infection and antiretroviral therapy (ART) with low bone mineral density (BMD) in premenopausal women is uncertain because BMD stabilizes on established ART and fracture data are limited.
Methods
We measured time to first new fracture at any site with median follow-up of 5.4 years in 2391 (1728 HIV-infected, 663 HIV-uninfected) participants in the Women’s Interagency HIV Study (WIHS). Self-report of fracture was recorded at semiannual visits. Proportional hazard models assessed predictors of incident fracture.
Results
At baseline, HIV-infected women were older (40 ± 9 vs. 36 ± 10 years, P <0.0001), more likely to report postmenopausal status and be hepatitis C virus-infected, and weighed less than HIV-uninfected women. Among HIV-infected women, mean CD4+ cell count was 482 cells/μl; 66% were taking ART. Unadjusted incidence of fracture did not differ between HIV-infected and uninfected women (1.8 vs. 1.4/100 person-years, respectively, P = 0.18). In multivariate models, white (vs. African-American) race, hepatitis C virus infection, and higher serum creatinine, but not HIV serostatus, were statistically significant predictors of incident fracture. Among HIV-infected women, older age, white race, current cigarette use, and history of AIDS-defining illness were associated with incidence of new fracture.
Conclusion
Among predominantly premenopausal women, there was little difference in fracture incidence rates by HIV status, rather traditional risk factors were important predictors. Further research is necessary to characterize fracture risk in HIV-infected women during and after the menopausal transition.
Keywords: fracture, fragility fracture, HIV-infected women, premenopausal
Introduction
Low bone mineral density (BMD) is a recognized metabolic complication of HIV infection and its treatment [1]. Recent studies suggest that antiretroviral therapy (ART) initiation is associated with significant short-term bone loss in the range of 2–6% over 1–2 years, irrespective of the type of ART [2–7]. In contrast, longitudinal studies show that BMD is either stable or increases slightly over 2 years of follow-up in younger men and women on established ART [8–10].
It remains uncertain whether lower BMD associated with HIV infection or short-term bone loss associated with ART initiation will translate to increased fractures in younger HIV-infected individuals. There are several case series of fragility fractures in HIV-infected patients on ART [11–13], but only a few controlled studies evaluating fracture prevalence [14,15] and incidence [10,16–19]. Utilizing a large clinical database from the Partners HealthCare System with 8525 HIV-infected and 2 208 792 HIV-uninfected patients, Triant et al. [15] found that prevalence of spine, hip, and wrist fracture as determined by ICD9 codes was higher in HIV-infected compared to HIV-uninfected men and women in all age groups; however, that study only controlled for age, sex, and race/ethnicity. Smaller studies with patient-level data have found that differences in fracture rates were attenuated after controlling for known risk factors for fracture [16,20].
We compared incidence rates of fracture and determinants of fracture among HIV-infected and uninfected women from the Women’s Interagency HIV Study (WIHS), a large multicenter cohort of HIV-infected and uninfected women in the United States.
Methods
Study population
The WIHS, a multicenter prospective study of the natural and treated history of HIV infection in women, enrolled 3766 women (2791 HIV-infected and 975 HIV-uninfected) in 1994–1995 (n =2623) and 2001–2002 (n =1143) from six sites (Bronx/Manhattan, New York; Brooklyn, New York; Chicago, Illinois; Los Angeles, California, San Francisco, California; and Washington, DC). WIHS methods and baseline cohort characteristics have been described previously [21,22]. HIV-uninfected women in the WIHS were recruited because they were at risk for HIV infection, and were comparable for a wide array of variables, including drug use, history of chronic illness, perceived health status, reproductive history, and income [21]. At semiannual visits, participants complete physical examinations and provide biological specimens; they also undergo extensive questionnaires assessing clinical and demographic information. Informed consent was obtained using procedures approved by committees on human research at each of the collaborating institutions.
From April to September 2003 (visit 18), all WIHS participants were asked about personal history of fracture of the hip, wrist, and spine, both ever and within the prior 6 months. In those with a fracture history, fracture type(s) was determined, that is, fragility (resulting from fall from standing height or less) and nonfragility. In all subsequent study visits, participants were asked whether they had fractures of the hip, wrist, spine, and/or other site since the last visit. After exclusion of those who seroconverted during follow-up (n =17), 2391 (1728 HIV-infected, 663 HIV-uninfected) participants with at least one additional core visit after the index visit (visit 17, October 2002–March 2003) were included in the analysis. Visit 17 was designated as the index visit as incident fractures were only recorded after visit 17. We used fragility fractures, nonfragility fractures, and all fractures (fragility and nonfragility combined) at any body site as study outcomes given recent data that high-trauma fractures are associated with lower BMD and are similar to fragility fractures in predicting future fractures [23]. Our analysis included biannual observations from October 2002 (visit 17) to September 2008 (visit 28).
Demographics, medical history, HIV treatment history, and laboratory data were extracted from the WIHS database at index visit and subsequent visits. Presence of prognostic factors for fracture [24] at the index visit were quantified from the database: age, menopause (no menses for greater than 1 year), personal history of fracture, parental history of hip fracture, weight, race/ethnicity, current use of cigarettes, greater than two drinks/day of alcohol, glucocorticoid use (ever), and history of rheumatoid arthritis, or other cause of secondary osteoporosis. In addition, we obtained history of diabetes, hepatitis C RNA, estimated glomerular filtration rate using the Modification of Diet in Renal Disease (MDRD) calculation, reported current calcium use or multivitamin supplementation, and any use of injection drugs (IDU), opiates or cocaine, statins, hormonal contraception, or hormone replacement therapy (HRT). We also considered HIV-specific variables: current and nadir CD4+ lymphocyte counts, history of AIDS-defining illness (ADI), type of ART at index visit, and cumulative ART exposure by type of ART (total years of exposure to nucleoside (NRTI) and nonnucleoside (NNRTI) reverse transcriptase inhibitors, protease inhibitors, and tenofovir from initiation, whether before or after enrollment into WIHS, to either last visit or fracture event).
Statistical methods
Means, medians, standard deviations, interquartile ranges, and proportions were derived to summarize study variables, depending on whether they were continuous or categorical. The primary outcome was time to self-reported fracture event, defined as a fragility fracture at any body site or any fracture (fragility or nonfragility) at any site. Incidence rate was defined as the number of participants with a new fracture divided by the total time (in person-years) at risk for new fracture during the study period. Visit 17 was considered the start time; the end of follow-up was either the visit, at which the first fracture event was observed, or, if no fracture was observed, the last visit for each subject. Thirty-nine participants (22 HIV-infected, 17 HIV-uninfected) reported more than one fracture during the study period; only the first fracture was included in the analysis.
Proportional hazard models with time-dependent covariates were constructed to determine predictors of incidence of new fracture. All covariates were assessed at the index visit, except for cumulative ART exposure, which was calculated from time of ART initiation to time of fracture event or last visit. Bivariate analyses comparing each predictor to the outcome were considered for HIV-infected and uninfected participants together, and for HIV-infected participants separately. Bivariate and multivariate proportional hazard models were performed for HIV-infected only and HIV-infected plus uninfected participants together. All variables were entered into multivariate models and a backward selection strategy with a stay P value 0.1 or less was used to determine which variables remained in the final model. HIV status was the only variable forced into the model. A P value less than 0.05 was considered statistically significant. Proportional hazard curves were constructed without and with adjustment for covariates using the Kalbfleisch–Prentice approach to estimate baseline hazards and were fit twice with all variables except HIV set to their mean values and HIV set to negative and positive [25]. All analyses were performed using SAS software (Version 9.1.3; SAS Institute Inc., Cary, North Carolina, USA).
Results
Participant characteristics at index visit
Table 1 presents participant characteristics at the index visit. HIV-infected women were older, of lower weight, and more likely to have detectable hepatitis C virus (HCV) RNA, to report postmenopausal status, and to use HRT, calcium and vitamin D supplementation. HIV-infected women were also less likely to report moderate and heavy alcohol consumption or current smoking than uninfected women. Among the 1728 HIV-infected women at the index visit, 39% reported a prior ADI and 66% reported taking ART (30% used a protease inhibitor-based regimen, 30% a NNRTI-based regimen, and 6% a NRTI-only regimen). Mean duration of ART exposure at the index visit (including self-reported ART use prior to enrollment in WIHS) was 4.31 ± 3.4 years. Among the 34% not receiving ART at the index visit, half were ART-naive and the other half reported prior ART use. Thirty-nine percent of participants on NNRTI-based regimens and 52% of those on protease inhibitor-based regimens at the index visit remained on the same class of ART throughout the duration of study.
Table 1.
Demographic and clinical characteristics.
| Demographics | HIV-infected (N =1728) | HIV-uninfected (N =663) | P |
|---|---|---|---|
| Age | 40.4 ± 8.8 | 36.1 ± 9.9 | <0.0001 |
| Race | 0.30 | ||
| White | 230 (13.3%) | 71 (10.7%) | |
| Black | 972 (56.3%) | 387 (58.4%) | |
| Latina | 470 (27.2%) | 179 (27.0%) | |
| Other | 56 (3.2%) | 26 (3.9%) | |
| Weight (kg) (mean ± SD) | 74.5 ± 20.8 | 79.7 ± 22.9 | <0.0001 |
| BMI (kg/m2) (mean ± SD) | 28.5 ± 7.5 | 30.0 ± 8.2 | <0.0001 |
| Smoking at index visit | 783 (45.3%) | 337 (50.8%) | 0.017 |
| IDU use | 21 (3.2%) | 49 (2.8%) | 0.68 |
| Ever opiate use | 62 (3.6%) | 33 (5.0%) | 0.13 |
| Ever cocaine use | 50 (2.9%) | 27 (4.1%) | 0.15 |
| Alcohol use | <0.0001 | ||
| Abstainer | 918 (53.1%) | 265 (40.0%) | |
| Light (<3 drinks/week) | 618 (35.8%) | 259 (39.1%) | |
| Moderate (3–13 drinks/week) | 155 (9.0%) | 113 (17.0%) | |
| Heavy (≥ 14 drinks/week) | 37 (2.1%) | 26 (3.9%) | |
| Menstrual history | |||
| Self-reported menopause | 338 (19.6%) | 74 (11.2%) | <0.0001 |
| Hormone replacement therapy ever | 124 (7.2%) | 26 (3.9%) | 0.003 |
| Medical history | |||
| Previous fracture | 74 (4.5%) | 35 (5.6%) | 0.37 |
| Diabetes | 332 (19.2%) | 127 (19.2%) | 1.00 |
| HCV RNA positive | 438 (25.4%) | 96 (14.5%) | <0.0001 |
| Diastolic blood pressure (mmHg) | 75.6 ± 10.7 | 74.0 ± 10.5 | 0.006 |
| Serum creatinine (mg/dl) (mean ± SD) | 1.0 ± 1.2 | 1.2 ± 1.5 | 0.15 |
| Estimated GFR < 60 (MDRD) | 139 (8.5%) | 36 (5.9%) | 0.042 |
| Calcium supplementation | 95 (5.5%) | 21 (3.2%) | 0.019 |
| Vitamin D supplementation | 184 (41.9%) | 724 (27.8%) | <0.0001 |
| Statin use | 88 (5.1%) | 13 (2.0%) | 0.0004 |
| HIV | |||
| ADI ever | 671 (38.8%) | ||
| CD4+ cell count, at index visit (cells/μl) | 482 ± 320 | ||
| CD4+ cell count, nadir (cells/μl) | 233 ± 183 | ||
| ART at index visita | 1134 (65.6%) | ||
| Protease inhibitor-ART at index visit | 523 (30.4%) | ||
| NNRTI-ART at index visit | 510 (29.6%) |
ADI, AIDS-defining illness; ART, antiretroviral therapy; GFR, glomerular filtration rate; IDU, injection drugs; MDRD, Modification of Diet in Renal Disease; NNRTI, nonnucleoside reverse transcriptase inhibitor.
Including NRTI-only and combination ART.
Fractures
At the index visit, prevalence of self-reported history of fracture did not differ between HIV-infected and HIV-uninfected groups. Most fractures prior to the index visit occurred at the wrists in both groups. The median follow-up for the study was 5.4 (IQR 5.3, 5.5) years. During the study period, 217 new fractures occurred in 195 of 2391 participants (8.6% of HIV-infected and 7.1% of HIV-uninfected women; Table 2). In both groups, fractures were more common at sites other than the spine, hip, and wrist. Approximately one-third of the new fractures were classified as fragility fractures: 49/147 (33%) in HIV-infected and 18/47 (38%) in HIV-uninfected. Unadjusted incidence rates of fracture at any site were similar between HIV-infected and uninfected women (1.8 vs. 1.4/100 person-years, P = 0.13) as were rates of fragility fractures or rates of fracture limited to the hip, spine, or wrist (Table 2). Distribution of fractures at the spine, hip, and wrist did not differ by HIV serostatus.
Table 2.
Incidence rates of new fractures in HIV-infected and uninfected women.
| Fractures: number and site distribution | HIV-infected (N =1728)
|
HIV-uninfected (N =663)
|
P | ||
|---|---|---|---|---|---|
| Fractures N (%) | Fractures/100 person-year | Fractures N (%) | Fractures/100 person-years | ||
| Subjects with (any site): | |||||
| New fracture | 148 (8.6%) | 1.79 | 47 (7.1%) | 1.41 | 0.13 |
| New fragility fracture | 49 (2.8%) | 0.58 | 18 (2.7%) | 0.53 | 0.64 |
| Fracture sitesa | |||||
| Spine | 15 (9.4%) | 0.18 | 7 (12.3%) | 0.21 | 0.93 |
| Hip | 15 (9.4%) | 0.18 | 4 (7.0%) | 0.12 | 0.32 |
| Wrist | 25 (15.6%) | 0.29 | 11 (19.3%) | 0.32 | 0.94 |
| Other | 105 (65.6%) | 1.25 | 35 (61.4%) | 1.04 | 0.29 |
Includes only one fracture per person.
Includes multiple fractures per person. There were a total of 160 fractures in 148 HIV-infected women and a total of 57 fractures in 47 HIV-uninfected women. (%) Percentage of total fractures occurring at body site.
Correlates of incident fracture
In bivariate analysis of all participants, traditional factors that were statistically associated with incidence of new fracture were older age, white race, self-report of menopause, history of fracture prior to index at any site, HCV infection, higher diastolic blood pressure at index, cigarette use at index, history of IDU or opiate use (Table 3). Use of HRT or oral contraceptives was associated with new fracture, but use of progestin-only formulations of hormonal contraception was not. HIV infection was not a significant predictor of incidence of new fracture [hazard ratio (HR) = 1.28; 95% confidence interval (CI): 0.93–1.78]. In multivariate analysis, only older age, white race, HCV infection, and higher serum creatinine were statistically significant predictors of incidence of new fracture.
Table 3.
Bivariate and multivariate models for incidence of new fractures in HIV-infected and uninfected women.
| Variables | Bivariate model HR (95% CI) | P | Multivariate modela HR (95% CI) | P |
|---|---|---|---|---|
| HIV status | 1.28 (0.93, 1.78) | 0.14 | 1.12 (0.78, 1.60) | 0.54 |
| Age (10 years) | 1.37 (1.18, 1.58) | <0.0001 | 1.21 (1.02, 1.43) | 0.031 |
| Race | ||||
| Black (Ref) | 1.0 | 1.0 | ||
| White | 1.50 (1.03, 2.17) | 0.033 | 1.60 (1.08, 2.39) | 0.020 |
| Hispanic | 0.70 (0.49, 1.01) | 0.057 | 0.80 (0.55, 1.18) | 0.26 |
| Other | 0.83 (0.37, 1.89) | 0.66 | 0.96 (0.39, 2.38) | 0.94 |
| BMI (kg/m2) | 1.00 (1.00, 1.01) | 0.66 | – | |
| Postmenopausal | 1.71 (1.23, 2.36) | 0.001 | – | |
| Fracture before index | 2.04 (1.24, 3.37) | 0.005 | 1.60 (0.94, 2.73) | 0.085 |
| HCV infection | 2.0 (1.49, 2.70) | <0.0001 | 1.56 (1.11, 2.20) | 0.011 |
| Serum creatinine (per mg/dl) | 1.09 (1.00, 1.19) | 0.063 | 1.11 (1.01, 1.21) | 0.031 |
| Diastolic blood pressure (per mmHg) | 1.01 (1.00, 1.03) | 0.038 | – | |
| Cigarette use (index) | 1.62 (1.22, 2.15) | <0.001 | – | |
| Alcohol use (index) | 1.09 (0.91, 1.30) | 0.34 | – | |
| Cocaine use (ever) | 1.78 (0.94, 3.36) | 0.076 | – | |
| Injected drugs (ever) | 2.21 (1.17, 4.18) | 0.014 | – | |
| Opiate use (ever) | 1.86 (1.04, 3.33) | 0.038 | – | |
| Calcium use (index) | 1.61 (0.95, 2.73) | 0.075 | – | |
| Vitamin D use (index) | 0.92 (0.68, 1.23) | 0.55 | – | |
| HRT (ever) | 1.67 (1.05, 2.66) | 0.029 | – | |
| Oral contraceptive use (ever) | 1.75 (1.12, 2.71) | 0.014 | – | |
| Statin Use (ever) | 1.43 (0.80, 2.56) | 0.231 | – | |
CI, confidence interval; HCV, hepatitis C virus; HR, hazard ratio; HRT, hormone replacement therapy.
All variables listed in table were considered in the multivariate model and eliminated if they did not meet backward selection criteria of P ≤ 0.1.
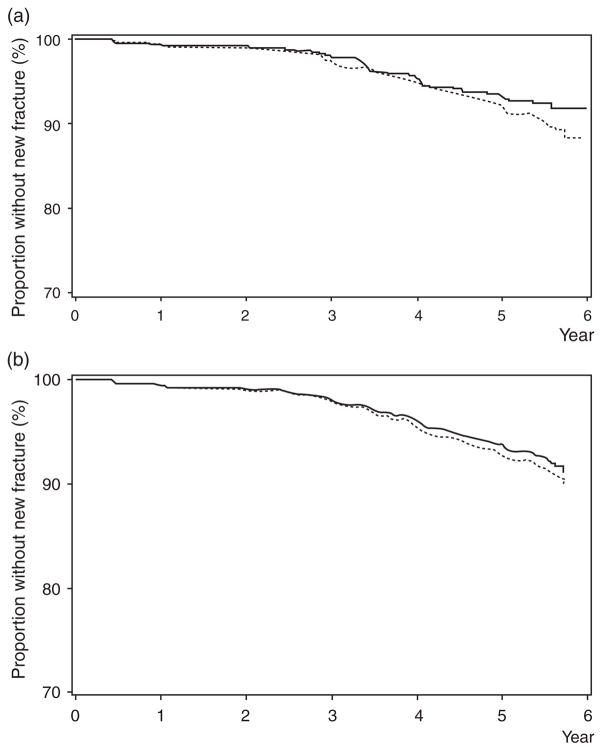
Proportional hazard curves depicting proportion without new fracture in HIV-infected and uninfected women are presented without adjustment (Fig. 1a). There is no statistically significant difference between groups (P = 0.13), although the curves appear to diverge with longer follow-up. After adjustment for significant covariates (age, white race, HCV infection, and serum creatinine) using the Kalbfleisch-Prentice approach [25] (Fig. 1b), the curves remain similar throughout the study period (P = 0.54)
Fig. 1.
(a) Incidence of new fracture (fragility or nonfragility) at any site, unadjusted in HIV-infected (dashed line) and uninfected (solid line) women (P = 0.13). (b). Incidence of new fracture (fragility or nonfragility) at any site adjusted for age, race, hepatitis C virus infection, and serum creatinine in HIV-infected (dashed line) and uninfected (solid line) women (P = 0.54).
In bivariate analyses restricted to HIV-infected participants older age, self-report of menopause, history of fracture prior to index at any site, HCV infection, cigarette use at index, history of IDU, and history of ADI were associated with incidence of new fracture (Table 4). In contrast, CD4+ cell count (at index visit or nadir), ART class treated as a categorical variable, cumulative protease inhibitor (HR = 1.03; 95% CI:, 0.98, 1.08, P = 0.29), NNRTI (HR = 0.95; 95% CI: 0.88, 1.02, P = 0.18), and tenofovir exposure (HR = 1.03, 95% CI: 0.98, 1.09, P = 0.26) were not associated with incidence of new fracture in bivariate analysis. In multivariate analysis, older age, white race, current smoking, and ADI were associated incidence of new fracture (Table 4). Cumulative NNRTI use was associated with a modest but statistically significant protective effect (HR = 0.92, 95% CI: 0.85, 0.99, P = 0.0327) (Table 4).
Table 4.
Bivariate and multivariate models for incidence of new fractures in HIV-infected women.
| Variables | Bivariate model HR (95% CI) | P | Multivariate modela HR (95% CI) | P |
|---|---|---|---|---|
| Age (10 years) | 1.36 (1.14, 1.62) | <0.001 | 1.24 (1.02, 1.51) | 0.033 |
| Race | ||||
| Black (Ref) | 1.0 | 1.0 | ||
| White | 1.49 (0.98, 2.27) | 0.06 | 1.56 (1.02, 2.39) | 0.041 |
| Hispanic | 0.74 (0.49, 1.11) | 0.15 | 0.84 (0.55, 1.27) | 0.40 |
| Other | 0.78 (0.29, 2.14) | 0.63 | 0.96 (0.35, 2.62) | 0.93 |
| BMI (per kg/m2) | 1.01 (0.99, 1.03) | 0.52 | 1.02 (1.00, 1.04) | 0.099 |
| Postmenopausal | 1.54 (1.06, 2.22) | 0.023 | – | |
| Fracture before index | 1.88 (1.02, 3.48) | 0.045 | – | |
| HCV coinfection | 1.86 (1.33, 2.61) | <0.001 | – | |
| Cigarette use (index) | 1.66 (1.20, 2.30) | 0.002 | 1.52 (1.09, 2.12) | 0.014 |
| IDU use (ever) | 2.17 (1.01, 4.62) | 0.046 | – | |
| CD4 cell count (per 100 cells/μl) | 0.97 (0.91, 1.03) | 0.29 | – | |
| Nadir CD4 (per 100 cells/μl) | 0.96 (0.87, 1.06) | 0.41 | – | |
| ADI | 2.10 (1.52, 2.91) | <0.0001 | 1.88 (1.34, 2.64) | <0.001 |
| NRTI use (index) | 0.76 (0.54, 1.07) | 0.12 | – | |
| TDF use (index) | 1.25 (0.90, 1.74) | 0.19 | – | |
| Protease inhibitor use (index) | 1.14 (0.82, 1.58) | 0.43 | – | |
| NNRTI use (index) | 0.79 (0.54, 1.16) | 0.23 | – | |
| Cumulative HAART (years) | 1.01 (0.97, 1.05) | 0.72 | – | |
| Cumulative NRTI (years) | 1.01 (0.97, 1.05) | 0.78 | – | |
| Cumulative protease inhibitor (years) | 1.03 (0.98, 1.08) | 0.29 | – | |
| Cumulative NNRTI (years) | 0.95 (0.88, 1.02) | 0.18 | 0.92 (0.85, 0.99) | 0.033 |
ADI, AIDS-defining illness; CI, confidence interval; HCV, hepatitis C virus; HR, hazard ratio; IDU, injection drugs; NNRTI, nonnucleoside reverse transcriptase inhibitor.
Backward selection with stay criteria P ≤ 0.1.
Discussion
In our cohort of predominantly premenopausal women, we did not find a statistically significant increase in incident fractures in HIV-infected women, even though the HIV-infected women were on average 4 years older than the HIV-uninfected women. Our findings are consistent with a prior analysis in a subset of pre-menopausal WIHS women, which found little difference in rates of bone loss or self-reported incident fracture over 2 years [10]. Furthermore, rates of fracture observed at the hip and wrist in our HIV-infected women over a median of 5.4 years (0.2/100 and 0.3/100 person-years, respectively) are consistent with large epidemiological studies of healthy premenopausal women in the United Kingdom, where rates of hip and wrist fracture were 0.3/100 [26] and 0.1/100 person-years [27], respectively. Finally, we demonstrated that traditional risk factors including older age, white race, and renal insufficiency were statistically significant predictors of fracture, as was HCV infection. Among HIV-infected women, history of an ADI was also an independent predictor of fracture.
Our fracture incidence rates are somewhat higher than those reported in two other studies of HIV-infected patients of comparable age. A French cohort of 1281 ART-treated, mainly HIV-infected men with a median age of 36 years and follow-up of 7.1 years reported an incidence density rate of 0.33/100 person-years [17]. This study also found that the observed rates within their HIV-infected cohort were similar to that observed in the general population [17]. In the HIV Outpatient Study (HOPS), Dao et al. [19] reported a fracture incidence rate of approximately 0.8/100 person-years in HIV-infected patients (median age of 37, 81% men, 56% white), which was higher than rates from the National Hospital Discharge Survey. Estimates from our study (1.8/100 person-years for all fractures, 0.6/100 person-years for fragility fractures) are likely higher than previous studies because of more complete ascertainment resulting from use of a dedicated fracture questionnaire at semiannual visits, and possibly because all participants were women. Another critical difference between our study and the aforementioned studies is that our HIV-uninfected controls were prospectively enrolled and evaluated with the same questionnaire as our HIV-infected participants. The French study also defined fracture as having a fracture leading to severe or complete limitation of activity [17].
Two studies have also examined fracture rates in older HIV-infected men. In a study of 559 men 49 years of age or older, rates of self-reported incident fractures were similar for HIV-infected and uninfected groups (3.1/100 versus 2.6/100 person-years, P = 0.69) during a 2-year follow-up [16]. In contrast, the much larger Veterans Aging Cohort Study observed that HIV-infected veterans have higher rates of fragility fracture at the spine, hip, and wrist than uninfected veterans after the age of 50 [18]. There are no published data on fracture risk in HIV-infected postmenopausal women.
We found that in addition to white race and renal insufficiency, HCV infection but not HIV infection was associated with fracture. Several studies in HIV-uninfected individuals have described the association between noncirrhotic HCV infection and low bone mass [28] or increased fracture [29,30]. Putative mechanisms are likely multifactorial and may be associated with increased alcohol use in patients infected with HCV, alterations in calciotropic and gonadotropic hormone levels, weight loss, and increased fall risk [28–30]. In the setting of HIV infection, Lo Re et al. [31] found that HIV/HCV-coinfected women had lower BMD than HIV-monoinfected women, but did not observe a similar difference in men. Collin et al. [17] found an association of HCV coinfection and higher alcohol consumption with increased fracture risk. In our cohort, HIV/HCV-coinfected and HIV monoinfected women were similar in age, weight, and alcohol consumption; we were unable to assess for group differences in calciotropic or gonadotropic hormones.
Among HIV-infected women in our cohort, history of ADI was associated with incidence of new fracture. This may reflect more advanced HIV disease stage, or the impact of hormonal, nutritional or neurological alterations associated with AIDS, opportunistic infections or their treatment on fracture risk. AIDS wasting syndrome has also been associated with lower BMD [32] and loss of muscle mass and functional status [33] that may lead to increased falls. Interestingly, we found an association of cumulative exposure to NNRTIs with decreased fracture risk in multivariate analyses. It is unclear if these results represent a direct effect of NNRTI on fracture risk. First, there was only a weak, nonsignificant association in the unadjusted models; therefore, this may be a spurious finding. Second, women on NNRTI-based regimens may be less sick than those on protease inhibitor-based regimens; protease inhibitors may more likely be used in those with advanced disease. Studies comparing bone loss with initiation of protease inhibitor versus NNRTI-based regimens have yielded inconclusive results [34–36]. Moreover, a prior analysis in a subset of WIHS women suggested that BMD may be slightly higher in those on an NNRTI-based regimen [14], but no statistically significant difference in change in BMD occurred over 2 years between ART groups [10]. We found no association between tenofovir use at index and new fractures.
There are several strengths to our study. WIHS is one of the only cohorts that has collected detailed data semiannually regarding fractures in HIV-infected women and a well matched comparison group of HIV-uninfected women. Traditional risk factors for fracture have also been collected at regular intervals, and participant retention is excellent. Limitations include the lack of characterization of fracture sites other than spine, hip, and wrist and of radiographic confirmation of fractures. We also did not assess muscle strength or fall risk, which are other potential determinants of fracture. As the majority of women in WIHS are African-American and overweight, our results may not be generalizable to all HIV-infected women (although our enrolled cohort is representative of the HIV epidemic among US women). Finally, our sample size may be insufficient for comparisons of fracture rates by ART exposure.
In conclusion, after 5.4 years of median follow-up in predominantly premenopausal women of color, fracture rates were not significantly increased in HIV-infected as compared to uninfected women. Traditional risk factors were important predictors of fracture, whereas HIV status was not. Among HIV-infected women, history of ADI was more predictive of fracture than ART exposure. Our data provide some reassurance that fracture risk is modest in predominantly premenopausal HIV-infected women. However, further research is necessary to assess fracture risk as these women transition through menopause and to clarify whether fracture risk differs among antiretroviral regimens.
Acknowledgments
All authors played a role in editing the article and approved the text as submitted to AIDS. M.T.Y. designed the study and wrote the manuscript. Q.S. and D.R.H. performed the data analysis and assisted in the interpretation of statistical data. K.A., A.S., M.Y., A.L., M.H.C., E.S., E.T.G., and P.C.T. reviewed and edited the manuscript.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (K.A.); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Chicago Consortium (M.H.C.); Data Coordinating Center (Stephen Gange).
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). Drs. Yin and Tien are supported by the National Institute of Allergy and Infectious Diseases through K23 AI 059884 and K23 AI 066943, respectively.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 2.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 3.Cassetti I, Madruga JV, Suleiman JM, Etzel A, Zhong L, Cheng AK, Enejosa J. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials. 2007;8:164–172. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 4.Duviver C, Kolta S, Assoumou L, Gohosn J, Rozenberg S, Murphy R, et al. First-line PI-containing regimens enhance decreased bone mineral density greater than NNRTI-containing regimen in HIV-1 infected patients: A substudy of the HIPPOCAMPE-ANRS 121 Trial. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 5.Grund B, Peng G, Gibert CL, Hoy JF, Isaksson RL, Shlay JC, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23:1519–1529. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivas P, Gorgolas M, Garcia-Delgado R, Diaz-Curiel M, Goyenechea A, Fernandez-Guerrero ML. Evolution of bone mineral density in AIDS patients on treatment with zidovudine/lamivudine plus abacavir or lopinavir/ritonavir. HIV Med. 2008;9:89–95. doi: 10.1111/j.1468-1293.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 7.Tebas P, Umbleja T, dube MP, Parker RA, Mulligan K, Roubenoff R, et al. Initiation of ART is associated with bone loss independent of the three specific ART regimens: Results of ACTG A5005s. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, US. 2007. [Google Scholar]
- 8.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91:2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 10.Yin MT, Lu D, Cremers S, Tien PC, Cohen MH, Shi Q, et al. Short-term bone loss in HIV-infected premenopausal women. J Acquir Immune Defic Syndr. 2010;53:202–208. doi: 10.1097/QAI.0b013e3181bf6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guaraldi G, Ventura P, Albuzza M, Orlando G, Bedini A, Amorico G, Esposito R. Pathological fractures in AIDS patients with osteopenia and osteoporosis induced by antiretroviral therapy. AIDS. 2001;15:137–138. doi: 10.1097/00002030-200101050-00025. [DOI] [PubMed] [Google Scholar]
- 12.Stephens EA, Das R, Madge S, Barter J, Johnson MA. Symptomatic osteoporosis in two young HIV-positive African women. AIDS. 1999;13:2605–2606. doi: 10.1097/00002030-199912240-00022. [DOI] [PubMed] [Google Scholar]
- 13.McComsey GA, Huang JS, Woolley IJ, Young B, Sax PE, Gerber M, et al. Fragility fractures in HIV-infected patients: need for better understanding of diagnosis and management. J Int Assoc Physicians AIDS Care (Chic Ill) 2004;3:86–91. doi: 10.1177/154510970400300303. [DOI] [PubMed] [Google Scholar]
- 14.Anastos K, Lu D, Shi O, Mulligan K, Tien PC, Freeman R, et al. The association of bone mineral density with HIV infection and antiretroviral treatment in women. Antivir Ther. 2007;12:1049–1058. doi: 10.1177/135965350701200701. [DOI] [PubMed] [Google Scholar]
- 15.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21:617–623. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin F, Duval X, Le Moing V, Piroth L, Al Kaied F, Massip P, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23:1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Womack J, Goulet J, Gilbert C, Brandt C, Mattocks K, Rimland D, et al. HIV-infection and fragility fracture among male veterans. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 16–19 February 2010. [Google Scholar]
- 19.Dao C, Young B, Buchacz K, Baker R, Brooks J. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study compared to the general US population, 1994 to 2008. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 16–19 February 2010. [Google Scholar]
- 20.Prior J, Burdge D, Maan E, Milner R, Hankins C, Klein M, Walmsley S. Fragility fractures and bone mineral density in HIV positive women: a case-control population-based study. Osteoporos Int. 2007;18:1345–1353. doi: 10.1007/s00198-007-0428-7. [DOI] [PubMed] [Google Scholar]
- 21.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 22.Hessol NA, Schneider M, Greenblatt RM, Bacon M, Barranday Y, Holman S, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol. 2001;154:563–573. doi: 10.1093/aje/154.6.563. [DOI] [PubMed] [Google Scholar]
- 23.Mackey DC, Lui LY, Cawthon PM, Bauer DC, Nevitt MC, Cauley JA, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 24.Kanis JA, McCloskey EV, Johansson H, Oden A, Strom O, Borgstrom F. Development and use of FRAX in osteoporosis. Osteoporosis Int. 2010;21 (Suppl 2):S407–S413. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- 25.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. Hoboken: Wiley; 2002. [Google Scholar]
- 26.Banks E, Reeves GK, Beral V, Balkwill A, Liu B, Roddam A. Hip fracture incidence in relation to age, menopausal status, and age at menopause: prospective analysis. PLoS Med. 2009;6:e1000181. doi: 10.1371/journal.pmed.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson PW, Taylor J, Dawson A. The annual incidence and seasonal variation of fractures of the distal radius in men and women over 25 years in Dorset, UK. Injury. 2004;35:462–466. doi: 10.1016/S0020-1383(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 28.Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, et al. Reduced bone mineral density and altered bone turnover markers in patients with noncirrhotic chronic hepatitis B or C infection. World J Gastroenterol. 2005;11:1843–1847. doi: 10.3748/wjg.v11.i12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46:1271–1278. doi: 10.1002/hep.21852. [DOI] [PubMed] [Google Scholar]
- 30.Nanda KS, Ryan EJ, Murray BF, Brady JJ, McKenna MJ, Nolan N, et al. Effect of chronic hepatitis C virus infection on bone disease in postmenopausal women. Clin Gastroenterol Hepatol. 2009;7:894–899. doi: 10.1016/j.cgh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Lo Re V, 3rd, Guaraldi G, Leonard MB, Localio AR, Lin J, Orlando G, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 2009;23:2191–2198. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang JS, Wilkie SJ, Sullivan MP, Grinspoon S. Reduced bone density in androgen-deficient women with acquired immune deficiency syndrome wasting. J Clin Endocrinol Metab. 2001;86:3533–3539. doi: 10.1210/jcem.86.8.7728. [DOI] [PubMed] [Google Scholar]
- 33.Grinspoon S, Corcoran C, Rosenthal D, Stanley T, Parlman K, Costello M, et al. Quantitative assessment of cross-sectional muscle area, functional status, and muscle strength in men with the acquired immunodeficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 1999;84:201–206. doi: 10.1210/jcem.84.1.5375. [DOI] [PubMed] [Google Scholar]
- 34.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25 hydroxyvitamin D. Antiviral Ther. 2010;15:425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 35.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;27:817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 36.McComsey G, Kitch D, Daar E, Tierney C, Jahed N, Tebas P, et al. Bone and limb fat outcomes of ACTG A5224 s, a substudy of A5202: A prospective, randomized, partially blinded phase III trial of ABC/3TC or TDF/FTC with EFV or ATV/r for initial treatment of HIV-1 infection. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]