Abstract
The objective of this cross-sectional study was to estimate the prevalence of and risk factors for osteoporosis in HIV+ postmenopausal women. Bone mineral density (BMD) by dual energy X-ray absorptiometry (DXA) and biochemical indices of mineral metabolism were measured in 31 Hispanic and African American HIV+ postmenopausal women. BMD was compared with 186 historical controls, matched for age, ethnicity and postmenopausal status. Mean BMD was significantly lower at the lumbar spine and total hip in the HIV+ group, as compared with controls. Prevalence of osteoporosis was higher in the HIV+ group than controls at the lumbar spine (42% vs 23%, p=0.03) and total hip (10% vs 1%, p=0.003). Among HIV+ women, time since menopause and weight were significant predictors of BMD, while duration or class of antiretroviral therapy (ART), AIDS diagnosis, nadir CD4, steroid use, and vitamin D deficiency were not. Prevalence of osteoporosis is substantially higher in HIV+ Hispanic and African-American postmenopausal women than in controls. Established osteoporosis risk factors were more important in predicting BMD than factors associated with HIV infection and ART. Long-term management of the growing female HIV population should include the evaluation for and management of osteoporosis.
Keywords: Bone metabolism, HIV, Osteoporosis, Postmenopausal women
Introduction
Low bone mineral density (BMD) is a recently recognized metabolic complication associated with HIV infection. The etiology of HIV-associated bone demineralization is complicated and undoubtedly multifactorial. It may include:
T cell activation and expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1 (IL-1), which stimulate osteoclast differentiation and resorption within the bone microenvironment [1-6]
Secondary effects of chronic infection such as weight loss, hypogonadism, and alterations in vitamin D metabolism [7-9]
Prominence of established risk factors for bone demineralization among HIV-positive (HIV+) patients
The independent effects of certain antiretrovirals, most notably protease inhibitors [10-12]
The attributable risk of each factor is difficult to ascertain, given the heterogeneity of study samples, study methodologies and the complex pathogenesis of osteoporosis. Several cross-sectional studies have reported lower mean BMD in HIV-infected, antiretroviral therapy (ART)-experienced and ART-naïve men in comparison with age-matched HIV-negative controls [13-16]. Fewer studies have focused upon women. In studies of normal weight, ambulatory, HIV-infected women who were predominantly premenopausal, BMD was reduced in ART-naïve HIV-infected women [17] as well as in ART-experienced women [18], compared with age-matched HIV controls. However, the absolute difference in bone density was relatively modest, and prevalence of osteoporosis does not appear to be higher in ART-experienced premenopausal HIV+ women [18,19]. There are no published estimates for HIV-infected postmenopausal women. Because their older age and estrogen-deficient status may place them at higher risk for bone loss, we assessed risk factors for osteoporosis and measured BMD and biochemical indices of mineral metabolism in a group of HIV-infected postmenopausal women.
Materials and methods
Subjects
Recruitment of HIV+ women
There are approximately 150 HIV+ women over the age of 50 followed regularly at the Columbia University Medical Center (CUMC) Infectious Disease Clinic. Over 90% of these women are Hispanic or African American. All women with a history of amenorrhea greater than 1-year’s duration were screened. Potential participants were excluded if they had a history of disorders known to affect the skeleton (Paget’s disease, primary hyperparathyroidism, Cushing’s syndrome), multiple myeloma or other malignancies metastatic to the skeleton, renal insufficiency (serum creatinine >2.5 mg/dl), a previous diagnosis of osteoporosis or a history of treatment with bisphosphonates, selective estrogen receptor modulators (SERMS), or calcitonin. Women with a history of current or past hormone replacement therapy or hysterectomy without ovariectomy were not excluded. Of the 40 women who met the criteria, 78% (31/40) were defined as postmenopausal, because they met defined hormonal or age criteria (serum follicular stimulating hormone (FSH) > 30 mIU/ml or FSH > 20 mIU/ml with estradiol <30 pg/ml or age > 55).
Selection of postmenopausal controls
BMD and body mass index (BMI) of HIV+ postmenopausal women were compared with data from 186 historical controls matched for ethnicity (Hispanic and African American) and age in years (± 5 years). Controls were determined to be postmenopausal based upon the diagnosis given on the DXA requisition and had been examined within the last year on the same DXA machine used for examination of HIV+ subjects. Controls with more than one DXA scan were excluded before matching. Data from local historical controls were used for comparison instead of data from the National Health and Nutrition Examination Survey (NHANES), in order to have comparable data for the local Hispanic population. Hispanics living in northern Manhattan, the main catchment area for CUMC, are predominantly from the Dominican Republic and may not be comparable with the Mexican-American norms available from NHANES. All subjects gave written informed consent, and the study was approved by the Institutional Review Board of CUMC.
Bone mineral density and Instant Vertebral Assessment
BMD of the lumbar spine (LS) (L1–4), proximal femur, non-dominant forearm and total body was measured by dual energy X-ray absorptiometry (DXA) on a QDR 4500 bone densitometer (Hologic, Waltham, MA, USA). At the metabolic bone diseases unit, reproducibility using a spine phantom is 0.51% (n= 178). Short-term in vivo reproducibility (coefficient of variation) is 0.68% for the spine, 1.36% for the proximal femur, and 0.70% for the radius. Osteoporosis and osteopenia were defined according to criteria developed by a World Health Organization (WHO) study group for postmenopausal Caucasian women: osteopenia (T -score <−1.0 and >−2.5) and osteoporosis (T -score < −2.5).
Assessment of vertebral fracture was performed utilizing morphometric DXA with Instant Vertebral Assessment (IVA). There is a strong overall agreement between visual evaluation of lateral DXA spine images and conventional lateral spine radiographs in postmenopausal women, although false-positive rates can be high [20,21]. All compression fractures diagnosed by IVA were confirmed with a lateral spine radiograph and interpreted by a skeletal radiologist.
Body composition measurement
Total and lean body mass were measured on a Delphi C QDR 4500 bone densitometer (Hologic, Waltham, MA, USA). The precision error is 3.0% for total fat body mass and 1.5% for total lean body mass [22].
Laboratory methods
Blood sampling was performed in the morning after a 12-hour overnight fast, aliquoted, frozen at −70° and analyzed in single assays in the core laboratory of the CUMC Irving Center for Clinical Research. Serum parathyroid hormone (PTH) was measured by a chemiluminescent method (Corning-Nichols Laboratory, San Juan Capistrano, CA, USA) that detects both a large, terminally truncated (7–84) fragment of PTH and the intact 1–84 molecule [23]. Intra-assay and inter-assay variabilities are 3.4% and 5.6%, respectively. Vitamin D metabolites were measured after extraction of serum. Serum 25-hydroxyvitamin D (25-OHD) was measured in a radio-binding assay (Corning-Nichols Laboratory). The intra-assay and inter-assay variabilities are 7.5% and 9.6%, respectively. Serum 1,25 dihydroxyvitamin D (1,25 (OH)2 D) was measured by a radioreceptor assay. The intra-assay and inter-assay variabilities are 7.6% and 9.8%, respectively. Bone alkaline phosphatase (BSAP) was measured by the Metra assay, which is an enzyme immunoassay (Quidel, San Diego, CA, USA). The intra-assay and inter-assay variabilities are 5% and 7.7%, respectively. Osteocalcin was measured by RIA (Corning-Nichols Laboratory) [24]. The intra-assay and inter-assay variabilities are 4.3% and 5.7%, respectively. Serum N-telopeptide (NTX) was measured using a competitive-inhibition, enzyme-linked immunosorbent assay (ELISA, Osteo-mark, Ostex, Seattle, WA, USA) [25]. The intra-assay and inter-assay variabilities are 4.6% and 6.9%, respectively.
CD4 counts were measured by flow cytometry (FACS Calibur, Becton Dickinson, San Jose, CA, USA). HIV-1 RNA was quantified by a polymerase chain reaction (PCR) assay utilizing the AMPLICOR HIV-1 MONITOR UltraSensitive Test (Version 1.5) with a linear range of 50–100,000 copies/ml (Roche Diagnostics, Indianapolis, IN, USA).
Statistical analysis
Comparisons between categorical groups were performed with use of chi-square and Fisher’s exact tests. Student’s t -test was used for continuous variables. All P values were two-tailed. Partial correlations testing was performed to estimate the strength of associations between various clinical and laboratory characteristics and BMD. Potential predictors of BMD were evaluated further by multiple regression analysis.
Results
Demographics and clinical characteristics
HIV+ and postmenopausal controls were well-matched with respect to age and ethnicity, but current weight and mean BMI were significantly higher in the control group (Table 1). Among the HIV+ women, 73% had at least one additional osteoporosis risk factor (alcohol consumption > one drink per day; family history of osteoporosis; current or past smoker) and 22% had more than two risk factors (Table 2). Most subjects (84%) were receiving ART at the time of the study, and 44% had undetectable viral loads. In addition to nucleosides, 34% had been exposed to protease inhibitors (PI) alone, 7% to non-nucleoside reverse transcriptase inhibitors (NNRTI) alone and 48% to both PIs and NNRTIs. Frequency of hepatitis C (HCV) co-infection was estimated by presence of HCV antibody by enzyme immunoassay (EIA), as determined by chart review. Of the 31 HIV+ postmenopausal subjects, 10% (3/29) had a positive HCV EIA, with two infections confirmed by reverse transcriptase (RT)-PCR. All presumably co-infected subjects had Child Class A disease, except for one who had Child Class B disease. No subject had a history of previous or current treatment for HCV.
Table 1.
Demographic and bone density variables for HIV+ and controls expressed as mean ± SEM or proportion (BMD bone mineral density, BMI body mass index)
| Variable | Postmenopausal HIV+ (N=31) | Postmenopausal control (N=186) |
|---|---|---|
| Age (years) | 56±1 | 57±1 |
| Ethnicity | ||
| Hispanic | 74% | 74% |
| African American | 26% | 26% |
| BMI (kg/m2) | 26±1* | 28±1 |
| Current weight (kg) | 64±3* | 73±1 |
| Lumbar spine BMD (g/cm2) | 0.85±0.03* | 0.94±0.01 |
| Lumbar spine T -score | −2.0±0.2* | −1.2±0.1 |
| Total hip BMD (g/cm2) | 0.83±0.03* | 0.91±0.01 |
| Total hip T -score | −1.0±0.2* | −0.5±0.1 |
| Distal radius BMD (g/cm2) | 0.65±0.02 | 0.68±0.01 |
| Distal radius T -score | −0.7±0.3 | −0.2±0.1 |
t -test p <0.05 between HIV+ and control postmenopausal groups
Table 2.
Summary of baseline data for HIV-infected postmenopausal women (N=31), according to presence or absence of lumbar spine osteoporosis. Data are reported as number of patients (percentage of group) or means ± SEM (ART antiretroviral therapy, HCV hepatitis C virus, IVA Instant Vertebral Assessment, NRTI nucleoside reverse transcriptase inhibitor, NNRTI non-nucleoside reverse transcriptase inhibitor, PI protease inhibitor)
| Characteristics | Lumbar spine osteoporosis
|
P | |
|---|---|---|---|
| No (N=18) | Yes (N=13) | ||
| Age | 54 ± 1 | 57±3 | 0.32 |
| Ethnicity | |||
| Hispanic | 13 (23) | 10 (77) | 0.77 |
| African American | 5 (28) | 3 (72) | - |
| HIV-associated variables | |||
| Months since HIV diagnosis | 100±10 | 87±11 | 0.4 |
| AIDS criteria | 10 (56) | 6 (46) | 0.61 |
| CD4+ T cell count | |||
| Nadir | 153±41 | 155±32 | 0.97 |
| Current | 458±72 | 501±88 | 0.71 |
| Current HIV viral load <50 copies/ml | 9 (50) | 5 (39) | 0.52 |
| Antiretroviral exposure | 16 (89) | 12 (92) | 0.75 |
| Months of ART by class | |||
| NRTI | 63±10 | 62±11 | 0.97 |
| NNRTI | 12±4 | 9±4 | 0.56 |
| PI | 35±6 | 32±8 | 0.75 |
| HCV co-infection | 3 (17) | 0 (0) | 0.27 |
| Bone metabolism-associated variables | |||
| Years since menopause | 9±2 | 11±2 | 0.42 |
| Prevalent vertebral compression fracture (IVA) | 1 (6) | 2 (15) | 0.56 |
| Osteoporosis risk factors | |||
| Family history of osteoporosis | 5 (28) | 0 (0) | 0.06 |
| Alcohol > 1 drink/day | 5 (28) | 4 (31) | 0.86 |
| Smoking, past or present | 5 (28) | 8 (62) | 0.06 |
| Steroid use | 3 (17) | 2 (15) | 0.92 |
| Hormone replacement therapy | |||
| Ever | 4 (22) | 2 (15) | 0.63 |
| Current | 1 (6) | 1 (8) | 0.77 |
| Multivitamin use | 10 (56) | 4 (31) | 0.17 |
| Calcium supplementation | 1 (6) | 2 (15) | 0.56 |
| Body mass index (kg/m2) | 27±1 | 24±1 | 0.2 |
| Current weight (kg) | 67±4 | 59±4 | 0.12 |
| Lowest historical weight (kg) | 67±4 | 57±3 | 0.07 |
| Total lean body mass (kg) | 41±4 | 36±2 | 0.06 |
| Total fat body mass (kg) | 22±2 | 19±3 | 0.46 |
| Percentage fat mass | 32±2 | 32±2 | 0.96 |
Bone mineral density
Mean BMD and T -scores were significantly lower in the HIV+ group at the lumbar spine (LS) and total hip (TH) (Table 1). Prevalence of LS osteoporosis was 42% in the HIV+ group, as compared with 23% in controls (p=0.03) (Fig. 1). Prevalence of TH osteoporosis was 10% in the HIV+ group, as compared with 1% in controls (p=0.003). Prevalence of osteopenia was similar among HIV+ and control groups at all measured sites: LS (36% vs 33%, p=0.77), TH (36% vs 29%, p=0.48), and distal radius (28% vs 16%, p=0.07). Among Hispanic HIV+ postmenopausal women, 44% had LS osteoporosis, and 4% had TH osteoporosis. Among African American HIV+ postmenopausal women, 38% had LS osteoporosis and 25% had TH osteoporosis.
fig. 1.
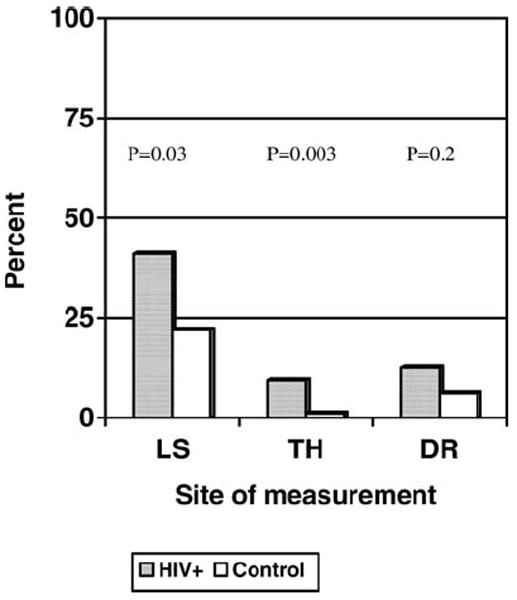
Prevalence of osteoporosis at specific sites in the HIV+ group as compared with controls (LS lumbar spine, TH total hip, DR distal radius)
Vertebral compression fractures
Three HIV+ women (9.7%) had evidence of prevalent vertebral compression fractures as determined by morphometric DXA with IVA. Only one woman had evidence of a vertebral compression fracture on a lateral spine radiograph.
Biochemical indices of mineral metabolism and bone turnover
Calciotropic and gonadotropic hormone levels and bone turnover markers for the HIV+ postmenopausal women are presented in Table 3. Vitamin D insufficiency (25-OHD between 10–20 ng/ml) and frank deficiency (25-OHDr=−0.4, p=0.03), suggesting that the majority of subjects had secondary hyperparathyroidism associated with vitamin D deficiency. Serum BSAP, osteocalcin, and NTX levels were elevated in 7/31 (22%), 6/31(19%), and 5/31(16%), respectively. Serum osteocalcin and N-telopeptide (NTX) levels were highly correlated (r=0.9, p<0.001), which suggests that bone formation and bone resorption were highly coupled in this sample.
Table 3.
Serum indices of gonadal hormones, mineral metabolism and bone turnover reported as mean ± SD (BSE bone collagen equivalent, FSH follicle stimulating hormone, PTH parathyroid hormone, 25-OHD 25-hydroxyvitamin D, 1,25 (OH)2D 1,25 dihydroxyvitamin D, BSAP bone alkaline phosphatase)
| Indices | Lumbar spine osteoporosis
|
P | |
|---|---|---|---|
| No (N=18) | Yes (N=13) | ||
| FSH (mIU/ml) | 58±24 | 63±25 | 0.54 |
| Estradiol (pg/ml) | 18±11 | 18±16 | 0.54 |
| Corrected calcium (mg/dl) | 9.1±0.4 | 9.1±0.3 | 0.83 |
| Intact PTH (pg/ml) | 39±15 | 47±18 | 0.18 |
| 25 OHD (ng/ml) | 37±37 | 16±8 | 0.06 |
| 1,25 (OH)2 D (pg/ml) | 56±21 | 46±21 | 0.23 |
| BSAP (units/l) | 33±17 | 36±17 | 0.73 |
| Osteocalcin (ng/ml) | 6±4 | 8±5 | 0.19 |
| N-telopeptide (nmol/BCE) | 14±4 | 16±6 | 0.16 |
| Total hip osteoporosis | |||
| - | No (N=28) | Yes (N=3) | - |
| FSH (mIU/ml) | 60±24 | 54±34 | 0.72 |
| Estradiol (pg/ml) | 15±9 | 38 ±27 | 0.02 |
| Corrected calcium (mg/dl) | 9±0.4 | 9±0.4 | 0.33 |
| Intact PTH (pg/ml) | 40±3 | 63 ±4 | 0.01 |
| 25 OHD (ng/ml) | 29±31 | 17±4 | 0.49 |
| 1,25 (OH)2 D (pg/ml) | 52±21 | 54±21 | 0.82 |
| BSAP (units/l) | 34±16 | 41±27 | 0.49 |
| Osteocalcin (ng/ml) | 7±3 | 11±8 | 0.06 |
| N-telopeptide (nmol/BCE) | 14±4 | 19±9 | 0.12 |
Correlates of BMD in HIV-infected subjects
On univariate analysis, there were no significant associations between LS osteoporosis and duration of HIV diagnosis, AIDS criteria, CD4 levels (nadir or current), and duration or class of ART (Table 2). LS BMD was positively correlated with current weight (r=0.42, p=0.02), lowest historical weight (r=0.46, p=0.01), and total lean body mass (r=0.52, p=0.003), but not with BMI or total fat body mass. LS BMD was negatively correlated with years since menopause (−0.39, p=0.03), serum osteocalcin (r=−0.47, p=0.01) and NTX levels (r=−0.33, p=0.07), but not with 25-OHD or PTH levels. There were no significant correlations between LS BMD and any HIV or ART-associated variables. In a multivariate regression analysis, a significant association for LS BMD remained with years since menopause and lowest historical weight.
On univariate analysis, TH osteoporosis was associated with higher serum levels of PTH (p=0.01). TH BMD was positively correlated with all measurements of weight: current weight (r=0.57, p=0.001), BMI (r=0.49, p=0.004), lowest historical weight (r=0.48, p=0.01), total lean body mass (r=0.62, p < 0.001), and total fat body mass (r=0.41, p=0.02). TH BMD was negatively correlated with years since menopause (−0.44, p=0.03) and serum osteocalcin (r=−0.42, p=0.02), but not with NTX, BSAP, 25-OHD, or PTH levels. There were no significant associations between TH BMD and CD4 level (nadir or current), time since HIV diagnosis, and duration or class of ART. In multivariate regression analysis, a significant negative association for TH BMD remained with years since menopause and a positive association remained with lowest historical weight (Table 4). Addition of baseline osteocalcin level did not improve the model.
Table 4.
Multivariate modeling with total-hip bone mineral density (N=31, whole model, P < 0.001, r2=0.37; adjusted r2=0.33) (CI confidence interval)
| Variable | Estimate | 95% CI | SE | P value |
|---|---|---|---|---|
| Years since menopause (log transformed) | −0.07 | −0.13, −0.01 | 0.03 | 0.02 |
| Lowest historical weight | 0.005 | 0.001, 0.008 | 0.002 | 0.001 |
Discussion
This study suggests that the prevalence of osteoporosis is substantially higher in HIV-infected postmenopausal women than in healthy women of similar age, ethnicity, and menopausal status. We found that established osteoporosis risk factors, such as years since menopause and weight, were more important predictors of LS BMD than were variables associated with HIV infection and treatment, in this small, highly treatment-experienced sample of HIV-infected women.
Control subjects were more likely than women in similar populations to have lower bone mass, since their physicians believed that their underlying risk factors warranted bone density testing. As a result, the prevalence of LS osteoporosis in the controls was much higher than estimates of 5–16% for Hispanics and African Americans quoted in population studies [26,27]. Despite the likelihood that selection bias in the controls would have tilted towards the null, prevalence of osteoporosis was still higher in HIV+ postmenopausal women than in controls.
Vitamin D insufficiency and deficiency were common in this study population and appeared to be associated with secondary hyperparathyroidism; however, the prevalence of low vitamin D levels was not outside the range observed in urban, low-income, African Americans living in northern latitudes [28]. Bone turnover, as evidenced by serum osteocalcin, BSAP and NTX levels, was also increased in a substantial proportion of the study population. Moreover, serum osteocalcin and NTX levels were highly correlated in our subjects, in contrast to the observations of Aukrust in treatment-naive patients [6]. In our sample, the number of ART-naïve subjects was too few to determine whether the high correlation of osteocalcin and NTX is a result of ART, as observed by Aukrust et al. [6] or of estrogen deficiency, which is also associated with increases in both osteocalcin and NTX levels [29]. Serum osteocalcin levels also vary with ethnicity, anthropometric measures, season and geographic locale [30]. Data from the Study of Women’s Health Across the Nation (SWAN) reveal that unadjusted osteocalcin levels are 11% higher in Caucasians than in African Americans, and 21–24% higher in Caucasians than in Asians [30]. Unfortunately, no data exist for Hispanic women for comparison. As with the calciotropic hormone data, osteocalcin and NTX levels from appropriate controls are necessary to fully interpret these findings.
Comparisons of BMD results from this study with other studies of HIV-infected women are difficult, given differences in the populations studied, and given the age and weight of subjects, menopausal status, and ethnic distribution [18, 19,31]. While differences in BMD were detectable in cross-sectional studies comparing pre-menopausal HIV+ women on ART with HIV-negative women, they were not as pronounced as observed in these postmenopausal subjects. The pathogenesis of low bone density in HIV+ postmenopausal women is unclear. It could be the result of confounders such as differences in weight, ethnic distribution, hormonal or nutritional status. Alternatively, it may be the result of a pathophysiologic interaction between estrogen deficiency and elements of HIV infection and/or treatment.
There are several ways in which estrogen could attenuate the effects of HIV-associated bone loss. Estrogen down-regulates many of the proinflammatory cytokines (TNF-α, IL-1, Il-6) that increase bone resorption [2,32]. These proinflammatory cytokines have all been found to be elevated in HIV+ individuals [1,4] and may not be completely suppressed after ART [33,34]. Receptor activator of nuclear factor kappa-B (RANK), its ligand (RANKL) and its decoy receptor osteoprotegerin (OPG) are three recently identified members of the TNF ligand and receptor-signaling family that are the final effectors of bone resorption [35,36]. Recent studies have demonstrated that RANKL production in the unstimulated peripheral blood mononuclear cells of HIV+ patients is higher than in HIV controls, and that sera from HIV+ patients demonstrated increased osteoclastogenesis and bone resorption in established investigational models [37,38]. Furthermore, pharmacologic levels of certain PIs, such as ritonavir and saquinavir, appear to increase RANKL activity by abrogating physiologic, IFN-γ-linked blocks to the degradation of the RANKL-signaling adapter protein, TNF receptor-associated protein 6 (TRAF-6) [38]. Estrogen appears to down-regulate bone-marrow cell expression of RANKL [39], and up-regulate gene expression and protein synthesis of OPG [40]. In pre-menopausal subjects, estrogen could attenuate the effects of proinflammatory cytokines and RANKL production on osteoclastogenesis, mitigating the accelerated bone demineralization associated with HIV infection and treatment. Conversely, the decline in estradiol levels that accompanies menopause would be expected to exacerbate any cytokine-mediated increase in bone resorption.
The results of in vitro and cross-sectional clinical studies and their significance with regard to long-term bone health require substantiation by well-controlled, longitudinal studies. Despite numerous studies suggesting an association between PIs and bone demineralization, recent longitudinal studies of men suggest that certain PI-based antiretroviral regimens may be associated with increases in BMD during short-term follow-up [41,42]. As yet, there is insufficient evidence to conclude that low BMD translates to increased fracture rates in the HIV+ population. Results from a retrospective study of phase III PI trials suggest that fracture rates are not higher in HIV+ subjects on treatment, compared with expected rates from the general population [43]. However, the majority of subjects in the longitudinal study of BMD and the fracture study were young men at peak bone mass. It is not possible to generalize these findings to predict the impact of HIV-associated bone loss in older, postmenopausal women who have greater baseline risk for fracture.
Incidence of HIV infection is increasing fastest in women, and these women are living longer with efficacious ART. Longitudinal studies are necessary to determine whether the rate of bone loss or fracture is higher in HIV-infected women, especially those who are approaching or have completed the menopausal transition. Whether or not biochemical abnormalities such as vitamin D deficiency, secondary hyperparathyroidism and increased bone turnover are related to or independent of HIV and its therapy, these women still require diagnosis and correction if possible. Finally, our results clearly suggest that recommendations to evaluate postmenopausal women for osteoporosis should be extended to postmenopausal women with HIV/AIDS.
Acknowledgments
This work was supported by Grant RR-006645 from the National Institutes of Health and the Tisch HIV/AIDS fellowship. We would like to thank Dr. Scott Hammer, Dr. Mary Ann Chiasson, and Donald McMahon for their helpful comments
Contributor Information
Michael Yin, PH8-876, Division of Infectious Diseases, Department of Medicine, Columbia College of Physicians and Surgeons, 622 West 168th Street, New York, NY 10032, USA; PH8-876, 630 West 168th Street, New York, NY 10032, USA, mty4@columbia.edu, Tel.: +1-212-3057185, Fax: +1-212-3057290.
Jay Dobkin, PH8-876, Division of Infectious Diseases, Department of Medicine, Columbia College of Physicians and Surgeons, 622 West 168th Street, New York, NY 10032, USA.
Karen Brudney, PH8-876, Division of Infectious Diseases, Department of Medicine, Columbia College of Physicians and Surgeons, 622 West 168th Street, New York, NY 10032, USA.
Carolyn Becker, Division of Endocrinology, Department of Medicine, Columbia College of Physicians and Surgeons, New York, NY, USA.
Janis L. Zadel, PH8-876, Division of Infectious Diseases, Department of Medicine, Columbia College of Physicians and Surgeons, 622 West 168th Street, New York, NY 10032, USA
Monica Manandhar, Division of Infectious Diseases, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Vicki Addesso, Division of Endocrinology, Department of Medicine, Columbia College of Physicians and Surgeons, New York, NY, USA.
Elizabeth Shane, Division of Endocrinology, Department of Medicine, Columbia College of Physicians and Surgeons, New York, NY, USA.
References
- 1.Lahdevirta J, et al. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 2.Manolagas SC. Role of cytokines in bone resorption. Bone. 1995;17([Suppl 2]):63S–67S. doi: 10.1016/8756-3282(95)00180-l. [DOI] [PubMed] [Google Scholar]
- 3.Roodman GD. Role of cytokines in the regulation of bone resorption. Calcif Tissue Int. 1993;53([Suppl 1]):S94–98. doi: 10.1007/BF01673412. [DOI] [PubMed] [Google Scholar]
- 4.Breen EC, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144(2):480–484. [PubMed] [Google Scholar]
- 5.Aukrust P, et al. Serum levels of tumor necrosis factor-alpha (TNF alpha) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994;169(2):420–424. doi: 10.1093/infdis/169.2.420. Erratum: J Infect Dis 169(5):1186-1187. [DOI] [PubMed] [Google Scholar]
- 6.Aukrust P, et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999;84(1):145–50. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 7.Dobs AS, et al. Endocrine disorders in men infected with human immunodeficiency virus. Am J Med. 1988;84(3(Part 2)):611–616. doi: 10.1016/0002-9343(88)90144-1. [DOI] [PubMed] [Google Scholar]
- 8.Haug CJ, et al. Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab. 1998;83(11):3832–3838. doi: 10.1210/jcem.83.11.5270. [DOI] [PubMed] [Google Scholar]
- 9.Huang JS, et al. Reduced bone density in androgen-deficient women with acquired immune deficiency syndrome wasting. J Clin Endocrinol Metab. 2001;86(8):3533–3539. doi: 10.1210/jcem.86.8.7728. [DOI] [PubMed] [Google Scholar]
- 10.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem. 2002;277(22):19247–19250. doi: 10.1074/jbc.C200069200. [DOI] [PubMed] [Google Scholar]
- 11.Wang MWH, Teitelbaum SL, Tebas P, Powderly WG, Ross FP. Indinavir inhibits bone formation while ritonavir inhibits osteoclast differentiation and function. Eighth Conference on Retroviruses and Opportunistic Infections. 2001 Abstract 541. [Google Scholar]
- 12.Wang MWH, Wei S, Teitelbaum SL, Ross FP. HIV protease inhibitor indinavir impairs osteoblastic bone formation, in vivo and in vitro. ASBMR 24th Meeting. 2002 Abstract SA333. [Google Scholar]
- 13.Paton NI, et al. Bone mineral density in patients with human immunodeficiency virus infection. Calcif Tissue Int. 1997;61(1):30–32. doi: 10.1007/s002239900288. [DOI] [PubMed] [Google Scholar]
- 14.Tebas P, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14(4):F63–67. doi: 10.1097/00002030-200003100-00005. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knobel H, et al. Osteopenia in HIV-infected patients: Is it the disease or is it the treatment? AIDS. 2001;15(6):807–808. doi: 10.1097/00002030-200104130-00022. [DOI] [PubMed] [Google Scholar]
- 16.Lawal A, et al. Equivalent osteopenia in HIV-infected individuals studied before and during the era of highly active antiretroviral therapy. AIDS. 2001;15(2):278–280. doi: 10.1097/00002030-200101260-00022. [DOI] [PubMed] [Google Scholar]
- 17.Teichmann J, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect. 2003;46(4):221–227. doi: 10.1053/jinf.2002.1109. [DOI] [PubMed] [Google Scholar]
- 18.Dolan SE, Huang JE, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Fifth International Workshop on Adverse Drug Reactions and Lipodystrophy. Paris: 2003. Reduced bone density in HIV-infected women. Abstract 23. [DOI] [PubMed] [Google Scholar]
- 19.Arnsten JH, Freeman R, Santoro N, Schoenbaum EE. HIV infection and protease-inhibitor use are not associated with reduced bone mineral density in older HIV-infected women. Tenth Conference on Retroviruses and Opportunistic Infections. 2003 Abstract 103. [Google Scholar]
- 20.Rea JA, et al. Visual assessment of vertebral deformity by X-ray absorptiometry: a highly predictive method to exclude vertebral deformity. Osteoporos Int 2000. 2001;11(8):660–668. doi: 10.1007/s001980070063. Erratum: Osteoporos Int 12(4):336. [DOI] [PubMed] [Google Scholar]
- 21.Rea JA, et al. Morphometric X-ray absorptiometry and morphometric radiography of the spine: a comparison of prevalent vertebral deformity identification. J Bone Miner Res. 2000;15(3):564–574. doi: 10.1359/jbmr.2000.15.3.564. [DOI] [PubMed] [Google Scholar]
- 22.Mazess RB, et al. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 23.Gao P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16(4):605–614. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 24.Gundberg CM, et al. Determination of osteocalcin in human serum: results with two kits compared with those by a well-characterized assay. Clin Chem. 1985;31(10):1720–1723. [PubMed] [Google Scholar]
- 25.Clemens JD, et al. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997;43(11):2058–2063. [PubMed] [Google Scholar]
- 26.Murillo-Uribe A, D-HM, Aguirre E, et al. Osteoporosis en la mujer postmenopausica mexicana: Magnitud del problema. Estudio Multicentrico. Ginecol Obstet Mex. 1999;67:227–233. [PubMed] [Google Scholar]
- 27.Bauer RL, Deyo RA. Low risk of vertebral fracture in Mexican-American women. Arch Intern Med. 1987;147(8):1437–1439. [PubMed] [Google Scholar]
- 28.Harris SS, et al. Vitamin D insufficiency and hyperparathyroidism in a low-income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 29.Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int. 2000;11([Suppl 6]):S30–44. doi: 10.1007/s001980070004. [DOI] [PubMed] [Google Scholar]
- 30.Finkelstein JS, et al. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3051–3056. doi: 10.1210/jcem.87.7.8480. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson D, Knox T, Shevitz S, Gorbach S. Low bone mineral density in HIV-infected women. Tenth Conference on Retroviruses and Opportunistic Infections. 2003 Abstract 102. [Google Scholar]
- 32.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11(8):1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 33.Aukrust P, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: Persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179(1):74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 34.Ledru E, et al. Alteration of tumor necrosis factor-alpha T-cell homeostasis following potent antiretroviral therapy: contribution to the development of human immunodeficiency virus-associated lipodystrophy syndrome. Blood. 2000;95(10):3191–3198. Erratum: Blood 2000 96(5):1673. [PubMed] [Google Scholar]
- 35.Hofbauer LC, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15(1):2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 37.Fakruddin JM, Laurence J. HIV-1 modulates TRANCE expression in vitro and in vivo: potential mechanism for the osteopenia of HIV disease. Ninth Conference on Retroviruses and Opportunistic Infections. 2002 Abstract 714-T. [Google Scholar]
- 38.Fakruddin JM, Laurence J. HIV envelope gp 120-mediated regulation of osteoclastogenesis via RANKL secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278(48):48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 39.Eghbali-Fatourechi G, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111(8):1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofbauer LC, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140(9):4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 41.Nolan D, et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS. 2001;15(10):1275–1280. doi: 10.1097/00002030-200107060-00009. [DOI] [PubMed] [Google Scholar]
- 42.Mondy K, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 43.Struble KMJ, Schneider B, Soon G. Bone fracture rates in HIV+ patients receiving PI vs non-PI regimens. Forty-first Interscience Conference on Antimicrobial Agents and Chemotherapy. 2001 Abstract I-1329. [Google Scholar]


