Abstract
Quantitative real-time-PCR (qRT-PCR) boasts many advantages over microarrays. For instance, very low amounts of total RNA are required to yield highly accurate and reproducible detection of transcript levels. As a consequence, qRT-PCR has become a popular technique for assessing gene expression levels and is now the gold standard. In this chapter, qRT-PCR using two distinct chemistries, SYBR Green and TaqMan, are described. We compare ABC transporter levels in various drug-resistant cancer cell lines by employing each method. SYBR Green yields reproducible results; nevertheless, TaqMan chemistry is superior to SYBR Green, as it displays higher specificity and sensitivity. Gene expression analysis by qRT-PCR is a powerful technique and shows potential as a diagnostic tool for predicting drug response in cancer patients.
Keywords: Quantitative real-time PCR (qRT-PCR), SYBR green, TaqMan, ABC transporters, gene expression profiling
1. Introduction
Gene expression analysis has become a valuable scientific tool. Microarrays and now quantitative Real-time PCR (qRT-PCR) are commonly used to profile gene expression patterns in both cell lines and tumors (1, 2). qRT-PCR allows the user to monitor the reaction in real-time as opposed to PCR, which relies on the end-point analysis (3). With the development of more sensitive and selective methodologies, qRT-PCR requires very little input material and provides accurate and reproducible detection of gene levels (4).
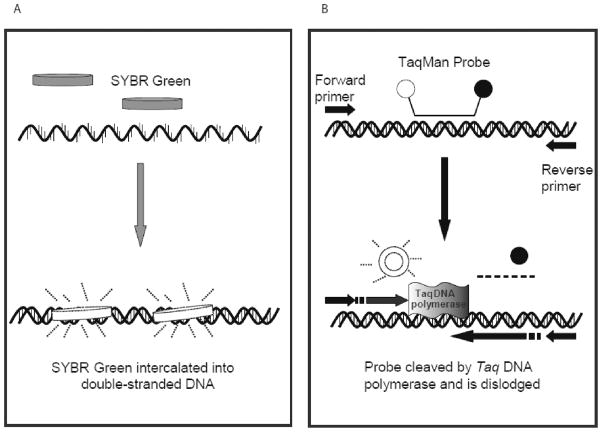
SYBR Green is an intercalating dye which binds to double-stranded DNA product during the PCR reaction. It binds to the minor groove of DNA (5) (Fig. 1A); however, it can also bind to primer dimers, causing false increases in fluorescence. This will lead to overestimation of the amount of actual product. In contrast, TaqMan chemistry has greater selectivity due to the use of both primers and a specific probe that has a reporter dye and quencher dye attached at opposite ends. The TaqMan probe hybridizes between the two PCR primers; no fluorescence is seen when the quencher dye is located near the reporter dye (6). When the Taq DNA polymerase cleaves the reporter dye from the probe with its 5′–3′ exonuclease activity, fluorescence intensity increases proportional to the amount of PCR product formed (Fig. 1B).
Fig. 1. Comparison of qRT-PCR chemistries.
A) Schematic showing SYBR Green qRT-PCR functions. SYBR Green will not bind to single stranded DNA, yet it binds to double-stranded DNA. In the bound form, it exhibits fluorescence, allowing for the PCR reaction to be monitored in real-time. B) Characterization of TaqMan qRT-PCR. The dual-labeled probe binds between the two primers, but the closeness of the quencher dye prevents the reporter dye from fluorescing. As the Taq polymerase cleaves the probe with its 5’ nuclease activity, the reporter dye is no longer blocked by the quencher dye. The fluorescence intensity is proportional to the amount of PCR products generated.
Multidrug resistance (MDR) is a major cause of chemotherapy failure. Although MDR can be attributed to various mechanisms, the predominant mechanism is the overexpression of ATP-binding cassette (ABC) transporters (7). Of the 48 ABC transporter genes in the human genome, there are at least 12 transporters that function as drug efflux pumps (8). Members of this superfamily can efflux an assortment of substrates, including ions, sugars, amino acids, lipids, toxins and anticancer drugs. The three dominant ABC transporters that contribute to MDR are ABCB1, ABCC1 and ABCG2 (9). Remarkably, these three transporters demonstrate great overlap in substrate specificity although their sequence homology varies. Microarray analysis for profiling ABC transporter genes has been utilized by several investigators (10-13). Microarrays, however, demonstrate poor probe specificity for genes in families possessing high homology such as ABC transporters (14), whereas qRT-PCR can more readily quantify and identify individual genes in such a homologous superfamily (2, 15). qRT-PCR has become known as the gold standard because of its sensitivity and reproducibility with very low amounts of sample; thus, it shows great utility in the clinic (16). Although both SYBR Green and TaqMan chemistries can quantitate ABC transporter expression, TaqMan provides greater sensitivity and selectivity. In the future, gene expression analysis using qRT-PCR of ABC transporters could aid in the diagnosis of MDR in clinical samples and help predict response to treatment.
We describe here the materials and methods that can be employed to analyze the gene expression of drug resistance-linked ABC transporters. To illustrate the various steps involved, we will refer to a sample unpublished study performed in our laboratory.
2. Materials
2.1. Cell Culture and RNA Isolation
In our sample study, the cervical epidermal carcinoma cell line KB-3-1 and its drug resistant sublines, KB-V1, KB-C1, and KB-8.5-11, were generous gifts of Michael M. Gottesman (Laboratory of Cell Biology, National Cancer Institute, NIH, Bethesda, MD) (17).
These cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% (v/v) fetal calf serum supplemented with 2 mM glutamine and 100 units of penicillin/ streptomycin/ml at 37°C in 5% CO2 humidified air.
Vinblastine and colchicine were dissolved in DMSO and added to the medium of the resistant sublines at the appropriate concentrations.
Phosphate buffered saline.
RNeasy isolation kit and the RNAse-free DNAse kit (Qiagen, Valencia, CA). A table-top microcentrifuge with a rotor radius of 7 cm was used in the RNA isolation step.
Beta-mercaptoethanol and ethanol.
2.2. RNA quantification and RNA integrity determination
The purified RNA was quantified using the Nanodrop ND-1000 Spectrophotometer (Wilmington, DE).
The integrity of the RNA was verified using the Agilent 2100 Bioanalyzer (Palo Alto, CA) with the Eukaryote Total RNA Nano assay, which contains all necessary reagents for the assay.
2.3. SYBR Green qRT-PCR
The LightCycler RNA Master SYBR Green Kit and LightCycler 480 instrument (Roche Biochemicals, Indianapolis, IN) were utilized in our sample study.
Primers (Table 1) were designed using the LightCycler Probe Design Software 2.0 (Roche Biochemicals). Plasma membrane calcium ATPase 4 (PMCA4) was used as the reference gene (18) (see Note 1).
Table 1.
List of Primers used for Sybr Green RT-PCR (18).
| Gene | Position of primer | Forward oligo sequence | Reverse oligo sequence |
|---|---|---|---|
| ABCA2 | 238-684 | CATCCCCCTGGTGCTGTTCTT | GCTTGGGCCGTGCTATTGG |
| ABCA3 | 437-939 | GCCCTCTTTACACTCAGTTTTCA | GACGAGCAGTTGTCGTACCTAAT |
| ABCA4 | 3380-3555 | TCTGGGATCTGCTCCTGAAGTATCG | GGTTAAGTACAAGCCTGTGCCAAAG |
| ABCB1 | 834-1086 | GCCTGGCAGCTGGAAGACAAATAC | ATGGCCAAAATCACAAGGGTTAGC |
| ABCB11 | 2204-2371 | CTTCCATCCGGCAACGCT | CACTGAATTTCAGAATCCTCCTAACTGGG |
| ABCC1 | 1119-1670 | AGTGGAACCCCTCTCTGTTTAAG | CCTGATACGTCTTGGTCTTCATC |
| ABCC2 | 872-1027 | AATCAGAGTCAAAGCCAAGATGCC | TAGCTTCAGTAGGAATGATTTCAGGAGCAC |
| ABCC3 | 2572-2725 | TCCTTTGCCAACTTTCTCTGCAACTAT | CTGGATCATTGTCTGTCAGATCCGT |
| ABCC4 | 3880-4124 | TGATGAGCCGTATGTTTTGC | CTTCGGAACGGACTTGACAT |
| ABCC5 | 3692-3864 | AGAGGTGACCTTTGAGAACGCA | CTCCAGATAACTCCACCAGACGG |
| ABCC11 | 3025-3560 | CCACGGCCCTGCACAACAAG | GGAATTGCCAAAAGCCACGAACA |
| ABCG2 | 266-646 | CCGCGACAGTTTCCAATGACCT | GCCGAAGAGCTGCTGAGAACTGTA |
| PMCA4 | 2309-2465 | ATCTGCATAGCTTACCGGGACT | TGCCAGCTTGTTTGCATTTGGCAATA |
2.4 TaqMan qRT-PCR
A High-Capacity cDNA Reverse Transcription Kit with RNAse Inhibitor, TaqMan Universal PCR Master Mix, optical adhesive covers, ABI 7900HT and TaqMan Gene Expression Assays (Table 2).
Table 2.
TaqMan Assays used for qRT-PCR
| Gene symbol | Assay ID | Gene name |
|---|---|---|
| ABCB1 | Hs00184491_m1 | ATP-binding cassette, sub-family B (MDR/TAP), member 1 |
| ATP2B4 | Hs00608066_m1 | ATPase, Ca++ transporting, plasma membrane 4 |
The TaqMan assays were obtained from Applied Biosystems (Foster City, CA). For each reaction, 40 ng cDNA for each cell line was used (See Figure 3).
3. Methods
3.1. RNA Isolation
RNA is isolated from the starting concentration of 200,000 cells/well grown in 6-well plates for 72 hours to characterize the gene expression of select ABC transporters in parental KB-3-1 cells and three multi-step selected KB sublines. RNA samples should be isolated in at least duplicate.
The medium and any detached cells are first removed from the wells, and the cell monolayer is washed with PBS.
RNA isolation is performed on the cells that remain attached using the Qiagen Rneasy kit, as follows: Add 600 μl of Buffer RLT with beta-mercaptoethanol to each well. This buffer is prepared by adding 10 μl of beta-mercaptoethanol for each milliliter of Buffer RLT used. Use a pipette tip to first scrape the cells from the wells and then to transfer to an RNAse-DNAse free, sterile microcentrifuge tube.
Add 600 μl of 70% ethanol to this lysate and pipette up and down several times to ensure complete mixing. Add 700 μl of this mixture to the mini-column and centrifuge 15 seconds at ≥7000 × g using a microcentrifuge. Pull the mini-column from the collection tube and discard the flow-through. Return the mini-column to the collection tube. Add the remaining lysate (500 μl) to the mini-column and spin 15 seconds at ≥7000 × g and discard flow-through.
Gently add 350 μl of RW1 buffer to the filter of the mini-column and centrifuge 15 seconds at ≥7000 × g, and discard flow-through. Add 80 μl of the DNAse/RDD mixture onto the column and incubate for 15min at room temp. The DNAse mixture is prepared by adding 10 μl of DNASe stock to 70 μl Buffer RDD per tube of RNA. Mix by gently inverting (see Note 2). Add 350 μl of RW1 buffer to the filter of the mini-column at the completion of the DNAse step. Centrifuge 15 seconds at ≥7000 ×g, then discard flow-through as before. Transfer the mini-column into a new collection tube and pipet 500 μl of RPE buffer. Centrifuge 15 seconds at ≥7000 ×g, then discard flow-through.
Add another 500 μl of RPE buffer and centrifuge 2 min at 13,000 ×g. Discard both the flow-through and the collection tube. Put the column in a 1.5 ml tube w/ lid and add 50 μl of Rnase-free water and centrifuge 1 minute at 7000 ×g to elute the RNA (see Note 3).
Store RNA samples at −80 C until ready to use.
3.2. RNA quantification and RNA integrity determination
After isolating the RNA, it is then quantified using the NanoDrop 1000. The NanoDrop is turned on and the Nucleic Acid tab is selected. To initialize the instrument, place 2 μl of distilled water on the lower pedestal and bring down the top lever before clicking to initialize. The setting is changed to quantitate RNA. Clean the pedestal and place 2 μl of Rnase-free water on the pedestal using a Kimwipe. Click on blank. The pedestal is cleaned following each new sample. The sample is measured by placing 2 μl on the pedestal, closing the lever and clicking on measure.
The integrity of the RNA is verified using the Agilent 2100 Bioanalyzer with the Eukaryote Total RNA assay. Allow the reagents to come to room temperature for 30 minutes prior to beginning. The RNA 6000 ladder remains at −20°C. The gel (550 μl) is filtered for 10 minutes using the microcentrifuge at ~1500 × g.. Aliquot 65 μl of filtered gel into microcentrifuge tubes. Store additional aliquots for later use at 4°C (see Note 4). Add 1 μl of dye to 65 μl of filtered gel and vortex for 10 seconds. Centrifuge the gel-dye mixture for 10 minutes at 13,000 ×g.
While the gel-dye mixture is spinning, prepare the RNA samples for testing as follows: Place 2 μl of a sample into an individual small PCR tube and heat at 70°C for 2 minutes (see Note 5). Another small PCR tube should contain 2 μl of RNA 6000 ladder and should be heated with the other RNA samples.
Clean the electrodes by placing 350 μl of RNAse Zap in the Electrode Cleaner Chip 1 and insert into the Bioanalyzer for 1 minute. Remove this chip. Load the 2nd Electrode Cleaner Chip with 350 μl of RNAse-free water and insert into the Bioanalyzer for 10 seconds. Remove this chip and keep the instrument open for 10 seconds to allow the electrodes to dry. Close the instrument until the filled RNA Nano Chip is ready to insert.
Assemble the priming station by locking the syringe into the top of the priming station. Place the RNA Nano Chip into the priming station set on the “C” setting. Add 9 μl of gel-dye mixture to the well marked with “G” and a dark circle around it. Close the priming station for 30 seconds. Slowly release the pressure and open the priming station. Place 9 μl of gel-dye mixture into the 2 remaining wells marked with “G”. Place 5 μl of marker into each of the 12 wells and the ladder well. Place 1 μl of RNA 6000 ladder into the well marked as “ladder”. Add 1 μl of the first sample to a well marked by the number 1. Add 1 μl of the next sample to the well marked 2. Continue until all wells are filled. 12 samples can be analyzed per run. If fewer than 12 samples are analyzed, add an additional 1 μl of marker to the wells which do not contain sample.
Vortex the filled Lab-Chip for 1 minute at 2400 rpm in the special IKA vortexer provided by Agilent. Place the Lab-Chip in the BioAnalyzer and run the instrument. Total RNA with a RNA integrity (RIN) number above 7 can be utilized in qRT-PCR. The Agilent software assigns a RIN number, which presents a standardized RNA integrity evaluation, to each sample run on the BioAnalyzer (19).
3.3. SYBR Green qRT-PCR using the One-step Method
Following quantitation and analysis of the total RNA, the RNA is diluted to the desired concentration. For SYBR Green qRT-PCR, an optimal range is 75-100 ng/μl. Each reaction use 2 μl of total RNA. The Roche RNA Master SYBR Green kit and total RNA are used for the one step method.
-
The total volume for each reaction is 20 μl.
RNAse-free water 4.2 μl Mn Acetate 1.3 μl SYBR Green Master Mix 7.5 μl Forward primer 2 μM stock 2.5 μl Reverse primer 2 μM stock 2.5 μl Then add 2μl of the appropriate Total RNA at the desired concentration.
The RT-PCR reaction is performed on 300 ng total RNA with 250 nM specific primers under the following conditions on the LightCycler 480: reverse transcription (20 min at 61°C), one cycle of denaturation at 95°C for 30 seconds, and PCR reaction of 45 cycles with denaturation (15 seconds at 95°C), annealing (30 seconds at 58°C), and elongation (30 seconds at 72°C with a single fluorescence measurement). The PCR reaction was followed by a melting curve program (65–95°C with a heating rate of 0.1°C per second and a continuous fluorescence measurement) and then a cooling program at 40°C.
Negative controls consisting of no-template (water) reaction mixtures should be run with all reactions. PCR products can also be run on agarose gels to confirm the formation of a single product of the desired size.
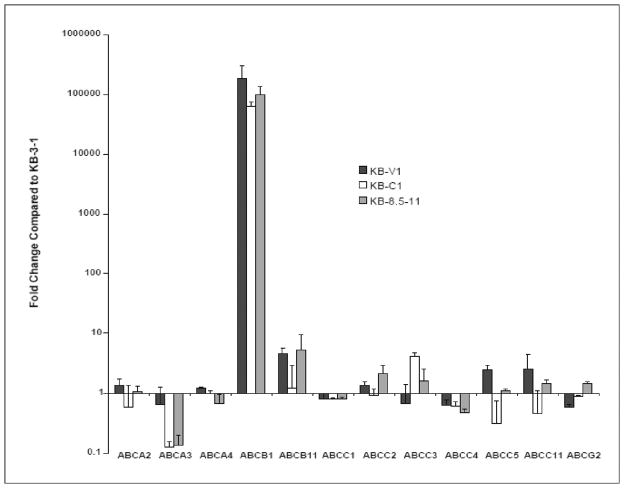
Crossing points for each transcript are determined using the 2nd derivative maximum analysis with an arithmetic baseline adjustment. Data are normalized to expression of only a single convenient reference gene. We used PMCA4. Data are presented as a comparison of gene expression for the resistant sublines relative to that for the parental cells using the delta delta Ct method. Fig. 2 displays the unpublished gene expression profile for each of the KB resistant sublines relative the parental KB-3-1 obtained in our sample study using SYBR Green q-RT-PCR.
Fig. 2. Analysis of ABC transporter expression in multi-step selected KB sublines using SYBR Green qRT-PCR.
The delta-delta CT method was used to determine the fold change of ABC transporter gene expression in the multi-step selected KB sublines (17), compared to their parental line, KB-3-1. 300 ng of Total RNA used per reaction. Data were normalized to expression of the reference gene, plasma membrane Ca2+ ATPase 4 (PMCA4) (18). The values represent the mean and standard deviation (n=2). The 12 ABC transporters known to transport drugs are indicated on the X-axis. (Calcagno AM and Ambudkar SV, unpublished data).
3.4. TaqMan qRT-PCR
-
The TaqMan assay requires a reverse transcription step to be performed using the High-Capacity cDNA Reverse Transcription Kit with RNAse Inhibitor. The quantitated RNA is utilized to generate cDNA. In a 20 μl reaction, up to 2 μg of RNA can be reverse transcribed. In our own study, 400 ng of RNA in a total volume of 10 μl was used in a PCR tube (dilute up to 10 μl with RNAse-free water), and mixed with a 10 μl 2X reverse transcription mix given below:
10X RT Buffer 2.0 μl 25X dNTP Mix (100mM) 0.8 μl 10X RT Random Primers 2.0 μl MultiScribe Reverse Transcriptase 1.0 μl Rnase Inhibitor 1.0 μl RNAse-free water 3.2 μl Total 10 μl Mix by pipetting and briefly centrifuge to ensure homogeneous mixing. The PCR tube with the 20 μl is placed in a thermal cycler for reverse transcription with the following temperatures and times: Step 1. 10 minutes at 25 C; Step 2. 120 minutes at 37 C; Step 3. 5 seconds at 85 C; Step 4. Hold at 4 C (see Note 6).
-
The step above will yield cDNA to be employed in the qRT-PCR.
For qRT-PCR using TaqMan assays on a 96-well plate, the following are mixed together.cDNA from Step 3.3.1 (40 ng cDNA) 2 μl TaqMan Universal PCR Master Mix 25 μl 20X TaqMan Gene Expression Assay Mix 2.5 μl RNAse-free water 20.5 μl TOTAL 50 μl The desired genes can be evaluated on a 96-well plate. After each reaction is loaded on a 96-well plate, the plate is sealed with an optical adhesive cover. The plate is centrifuged at ~500 × g for 1 minute in a centrifuge with adapters for a 96-well plate to ensure proper mixing of the reagents.
-
The plate is run on the ABI 7900HT with the following settings:
Step 1. 2 minutes at 50 C; Step 2. 10 minutes at 95 C and then 40 cycles of Step 3. 15 sec at 95 C and then 1 minute at 60 C.
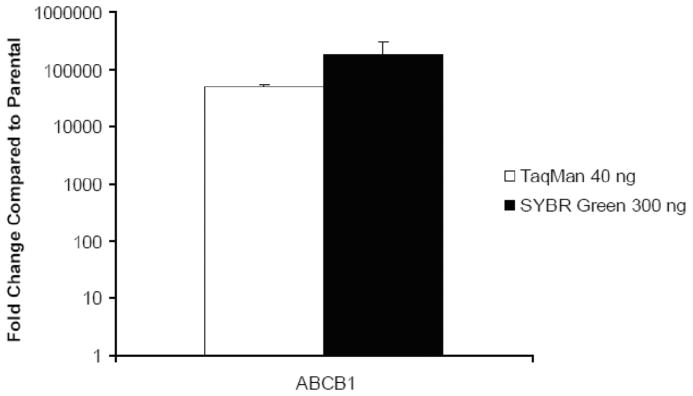
Crossing points for each transcript are determined using the SDS software. Data are normalized to the expression of a reference gene, PMCA4. In our sample study, data are presented as a comparison of ABCB1 gene expression for the resistant KB-V1 subline relative to that for the parental KB-3-1 cells using the delta delta Ct method (Fig. 3 and Table 3).
Fig. 3. Evaluation of ABCB1 gene expression in KB-V1 cells using SYBR Green and TaqMan qRT-PCR.
The delta-delta CT method was used to determine the fold change for ABCB1 gene expression in KB-V1 cells compared to the parental KB-3-1. The SYBR Green method utilized 300 ng of Total RNA whereas the TaqMan used 40 ng of cDNA. Both were normalized to the equivalent amounts of PMCA4 as the reference gene. The values represent the mean and standard deviation (n=2). (Calcagno AM and Ambudkar SV, unpublished data).
Table 3.
Comparison of CT values for ABCB1 and PMCA4 using SYBR Green and TaqMan for qRT-PCR
| ABCB1 CT value KB-3-1 | ABCB1 CT value KB-V1 | PMCA4 CT value KB-3-1 | PMCA4 CT value KB-V1 | |
|---|---|---|---|---|
| SYBR Green (300 ng) | 37.0 ± .49 | 19.8 ± .30 | 22.4 ± .64 | 22.4 ± .22 |
| TaqMan (40 ng) | 32.7 ± .05 | 16.2 ± .03 | 27.0 ± .05 | 26.1 ± .03 |
The values represent the mean CT value and the standard deviation (n=2).
Acknowledgments
We thank Mr. George Leiman for editorial assistance. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
All primer sets should be tested prior to use to ensure that only a single product of the correct size is amplified for all ABC transporter primer sets. Each product is run on a DNA gel to determine the product sizes.
The RNase-free DNase Set was from Qiagen Catalog number 79254, and contains DNAse and Buffer RDD. This is an optional step; however, it seems to produce the best quality RNA.
The kit calls for two elution spins; however, one elution with a larger volume such as 50 μl at 7000 × g for 1 minute yields good results.
These aliquots expire 30 days following the filtering step. Store at 4°C. Usually 7 additional aliquots are made with one filtering step. Once the dye is added to the 65 μl tube of filtered gel, this is can be used for only 24 hours. This mixture must be centrifuged prior to using in the RNA Nano Chip.
Only 1 μl sample will be loaded on the RNA Nano Chip; however, 2 μl are placed in the tube for heat denaturing to ensure that enough remains for loading.
For this step, 100% conversion of RNA to cDNA is assumed. 400 ng cDNA would be present in the final volume of 20 μl; therefore, each μl would contain 20 ng cDNA.
References
- 1.Staunton JE, Slonim DK, Coller HA, et al. Chemosensitivity prediction by transcriptional profiling. Proc Natl Acad Sci U S A. 2001;98:10787–10792. doi: 10.1073/pnas.191368598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szakacs G, Annereau J, Lababidi S, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–137. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 4.Gyorffy B, Surowiak P, Kiesslich O, et al. Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. Int J Cancer. 2006;118:1699–1712. doi: 10.1002/ijc.21570. [DOI] [PubMed] [Google Scholar]
- 5.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22:130–131. 134–138. doi: 10.2144/97221bi01. [DOI] [PubMed] [Google Scholar]
- 6.Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Gottesman M, Fojo T, Bates S. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman M, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 9.Calcagno AM, Kim IW, Wu CP, Shukla S, Ambudkar SV. ABC drug transporters as molecular targets for the prevention of multidrug resistance and drug-drug interactions. Curr Drug Deliv. 2007;4:324–333. doi: 10.2174/156720107782151241. [DOI] [PubMed] [Google Scholar]
- 10.Annereau JP, Szakacs G, Tucker CJ, et al. Analysis of ATP-binding cassette transporter expression in drug-selected cell lines by a microarray dedicated to multidrug resistance. Mol Pharmacol. 2004;66:1397–1405. doi: 10.1124/mol.104.005009. [DOI] [PubMed] [Google Scholar]
- 11.Gillet JP, Efferth T, Steinbach D, et al. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res. 2004;64:8987–8993. doi: 10.1158/0008-5472.CAN-04-1978. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Anderle P, Bussey KJ, et al. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64:4294–4301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Peng H, Zhang JT. Expression profiling of ABC transporters in a drug-resistant breast cancer cell line using AmpArray. Mol Pharmacol. 2005;68:430–438. doi: 10.1124/mol.105.011015. [DOI] [PubMed] [Google Scholar]
- 14.Lee JK, Bussey KJ, Gwadry FG, et al. Comparing cDNA and oligonucleotide array data: concordance of gene expression across platforms for the NCI-60 cancer cells. Genome Biol. 2003;4:R82. doi: 10.1186/gb-2003-4-12-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmann T, Mauerer R, Zahn A, et al. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clinical chemistry. 2003;49:230–238. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- 16.Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci (Lond) 2005;109:365–379. doi: 10.1042/CS20050086. [DOI] [PubMed] [Google Scholar]
- 17.Shen D, Cardarelli C, Hwang J, et al. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986;261:7762–7770. [PubMed] [Google Scholar]
- 18.Calcagno AM, Chewning KJ, Wu CP, Ambudkar SV. Plasma membrane calcium ATPase (PMCA4): a housekeeper for RT-PCR relative quantification of polytopic membrane proteins. BMC Mol Biol. 2006;7:29. doi: 10.1186/1471-2199-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder A, Mueller O, Stocker S, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Molecular Biology. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]