Abstract
Rates of delay discounting (impulsive choice) have been shown to vary among individuals, particularly people who abuse drugs relative to those who do not, but factors that may contribute to these differences have not been identified. To explore a role for possible genetic and neurochemical determinants, Lewis (n=8) and Fischer 344 (n=8) rats were allowed to choose between one food pellet delivered immediately and three food pellets delivered after increasing delays. The delays to the large reinforcer (0, 10, 20, 40, 60 s) were increased across five blocks of trials in daily experimental sessions. For both groups of rats, choice for the larger reinforcer decreased as the delay to presentation increased. However, the Lewis rats were more likely to choose the smaller, immediate reinforcer earlier in the session, i.e., at shorter large-reinforcer delays, than the Fisher 344 rats. This difference in choice was statistically significant. Repeated administration of 3.0 mg/kg, i.p. clomipramine (mean of last five sessions) did not significantly alter choice, relative to baseline, for either strain. The present findings suggest that differences in delay discounting/impulsive choice may involve genetic, e.g., neurochemical, differences.
Keywords: Choice, Clomipramine, Delay discounting, Fischer 344, Impulsivity, Lewis, Rat, Self-control
1. Introduction
Behavior labeled “impulsive” has been correlated with many disorders, e.g., drug abuse, gambling, attention-deficit-hyperactivity disorder (ADHD), aggression, suicide (cf. American Psychiatric Association Diagnostic and Statistical Manual-IV-TR, 2000). There are many operational definitions for impulsivity and many facets to such behaviors (see Evenden, 1999a). One definition that has received much attention states that impulsive behavior is observed when a smaller, more immediate reinforcer is chosen over a larger, delayed reinforcer (e.g., Ainlsie, 1974; Logue, 1988; Rachlin and Green, 1972). This definition has led to the development of a quantitative model referred to as delay discounting (Mazur, 1987) that suggests that the value of a reinforcer is discounted or devalued as a result of its delay to presentation. This model has been supported by the findings from numerous experiments (e.g., Green et al., 1994; Madden et al., 1997; Mazur, 1987, 1998; Myerson and Green, 1995; Petry and Casarella, 1999).
Recent studies have shown that the degree to which delayed reinforcers are discounted or devalued is increased in individuals with a history of abuse of drugs. Petry and Casarella (1999) showed that substance abusers discounted delayed outcomes at greater rates than non-abusers. Similar findings have been reported with abusers of nicotine (Bickel et al., 1999), opioids (Madden et al., 1997), and alcohol (Petry, 2001; Vuchinich and Simpson, 1998). Despite the fact that a correlation between drug abuse (and other behaviors often characterized as impulsive) and delay discounting has been demonstrated, it is not clear whether increased delay discounting underlies drug abuse and other disorders or if long-term exposure to drugs or other variables underlie increased delay discounting. A third option is that some other factor(s) underlie increased delay discounting or impulsivity and the behavioral disorder, e.g., drug abuse. Such factors might stem from earlier experiences or historical variables, i.e., learning and conditioning histories, and/or genetic or neurochemical factors.
Past studies have indicated roles for serotonin (5-HT) and dopamine (DA) in impulsive behaviors. Low levels of 5-HT have been associated with suicide (Asberg, 1998), violence/aggression (Cherek et al., 1997), and other behaviors that may be deemed impulsive (Crean et al., 2002; see Lesch and Merschdorf, 2000, for a review). Cherek and Lane (1999) reported that fenfluramine (a 5-HT releasing agent) decreased aggressive and impulsive responses in adult male humans with conduct disorders and criminal behavior. In addition to numerous clinical findings, 5-HT has been shown to be involved in impulsivity in preclinical research. Wogar et al. (1993) and Mobini et al. (2000) reported that, compared to intact (control) rats, rats with lesioned 5-HT pathways selected more immediate, smaller reinforcers over delayed, larger reinforcers in a choice paradigm. The authors suggested that a decrease in central 5-HT contributes to increased rates of delay discounting. However, there have been mixed or conflicting reports regarding levels of 5-HT and impulsive behavior (see Evenden, 1998; Evenden and Ryan, 1996, 1999). Deficits in DA systems have also been implicated in impulsive behavior, but there are some conflicting reports as well (cf. Cardinal et al., 2000; Logue et al., 1992; Wade et al., 2000). Certainly more research is needed to help elucidate the roles of 5-HT and DA in impulsive behavior.
One method to further assess the impact of specific neurotransmitters on impulsive behavior is to compare strains of rats with neurochemical differences. Such an approach is the basis for the present study. Fischer 344 and Lewis rats were selected due to known neurochemical variations in the monoamine 5-HT and DA systems and because of reported behavioral differences with regard to drug self-administration (an animal model of drug abuse). Lewis rats have shown fewer 5-HT1A binding sites and lower levels of 5-HT in various brain regions (Selim and Bradberry, 1996; Burnet et al., 1996) than Fisher 344 rats. Lewis rats also have shown fewer DA transporters and reduced DA concentrations in specific brain regions (Flores et al., 1998; Lindley et al., 1999). In addition, Lewis rats show greater self-administration of cocaine, morphine, codeine, and ethanol than Fisher 344 rats (Li and Lumeng, 1984; Kosten et al., 1997; Martin et al., 1999; Suzuki et al., 1988a,b). Lewis rats also show increased conditioned place preference in response to cocaine (Kosten et al., 1994) and nicotine (Horan et al., 1997) than Fischer 344 rats. Thus, the genetic, neurochemical, and behavioral differences between these two strains suggest that they might be useful models for studying impulsive behavior.
The present paradigm, based on a procedure by Evenden and Ryan (1996), assessed choice between one food pellet delivered immediately (0-s delay) and three food pellets delivered after increasing delays to presentation. The Lewis and Fischer 344 rats were then repeatedly tested during daily administration of the highest dose of clomipramine that did not completely suppress responding. Clomipramine was selected as a first screen due to its established inhibition of 5-HT uptake. It was hypothesized that inhibition of 5-HT uptake, and hence increased extracellular concentrations of 5-HT, would decrease impulsive choices and that this effect would be the greater in the Lewis rats.
2. Method
2.1. Subjects
Eight experimentally naïve, male Lewis rats and eight experimentally naïve, male Fischer 344 rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing approximately 300 g served as subjects. Subjects were housed individually in stainless steel cages, with controlled environmental conditions (temperature, 24 °C; 12-h reverse light/dark cycle) and continuous access to water. In addition, subjects were fed approximately 15 g of food immediately following the experimental session and body weights were maintained or increased slightly throughout the duration of the experiment. Thus, the subjects were food-restricted for approximately 22 h preceding the experimental sessions and in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals. All procedures with animals were approved by the University of Mississippi Medical Center’s Institutional Animal Use and Care Committee.
2.2. Apparatus
Experimental sessions were conducted in eight identical rat operant chambers (Gerbrands, Arlington, MA). In each chamber, two response levers were mounted on one wall (12.5 cm apart, center to center, 10 cm above the floor) and a food receptacle was located between them. A minimal downward force of 0.30 N was required to activate each lever. Each chamber was illuminated at the onset of the session by a single 6-W light located on the wall opposite the two levers and by a white light located above each lever. Extraneous noise was diminished by enclosing each chamber in an insulated box and by operating a ventilation fan mounted on the outside of each box. Data collection and experimental events were controlled by a Macintosh computer with custom software and interfaces.
2.3. Procedure
After initial lever-press training, subjects were exposed to a discrete-trials choice paradigm in which subjects chose between one food pellet delivered immediately following a lever press and three food pellets delivered after a delay after pressing the opposite lever. This method was first described by Evenden and Ryan (1996) for use in studying impulsive choice in rats. Sessions consisted of five blocks of eight trials. Trials were two types, forced-choice and free-choice, and started every 100 s. This scheduling of the intertrial intervals (ITI) resulted in constant reinforcement frequency throughout the session. The first two trials in each block were forced-choice trials that resulted in the subject having exposure to both sets of contingencies before being allowed to choose between them. In the first forced-choice trial of each block, one of the two lever lights, randomly determined, was illuminated and the outcome associated with that lever was available for lever pressing under a fixed-ratio (FR) 1 schedule. For the second forced-choice trial in the block, the other outcome was available as a consequence of pressing the other lever (FR 1). For example, for one outcome, the lever press resulted in an immediate delivery of one food pellet. After the ITI had elapsed, lights were illuminated above the other lever and a lever press on that lever delivered three food pellets following a delay.
After exposure to both outcomes at the beginning of a block during forced-choice trials, the next six trials in each block were free-choice trials. During free-choice trials, lights were illuminated above both levers and the subject was allowed to choose one alternative (FR 1). The free-choice trial contingencies remained the same as programmed during the forced-choice trials in that block. After completion of the six free-choice trials within a block, the delay to the large reinforcer was increased and presented during the forced-choice trial in the following block. In the first block of trials, the delay to the large reinforcer was 0 s. This value was then increased in the following order: 0, 1, 2, 4, 6 s. This delay sequence was in effect until the rats were reliably finishing the sessions and the number of large reinforcer choices during the free choice trials in the equal-delay condition was 80% or greater for three consecutive sessions. Using that same protocol, the delays were then increased to 0, 2, 4, 8, 16 s followed by 0, 5, 10, 20, 40 s and ending with the terminal values of 0, 10, 20, 40, 60 s across the five blocks.
Sessions ended following 40 total (10 forced- and 30 free-choice) trials. If a subject failed to respond during the first 30 s of each trial, an omission was recorded and the ITI was started. Regardless of a left or right response or an omission, trials started every 100 s. Experimental sessions were conducted 5 days per week (Monday–Friday). Training conditions (each delay series) were in effect for at least five sessions and until responding was stable. Stable responding was defined as 80% or greater choice for the larger reinforcer under equal delay (0 s) conditions and less than 20% variation between the number of larger reinforcer choices in each block with no increasing or decreasing trends across the last three sessions of a condition. The terminal (baseline) condition (the 0-, 10-, 20-, 40-, 60-s delay series) was in effect for at least 10 sessions and until responding was stable. Counterbalancing of the lever associated with the larger reinforcer was established for half of the subjects of each strain. Mean percent choice for the larger reinforcer across the five blocks was the primary dependent variable and differences between the two rat strains were compared as described below.
2.4. Drug treatment
Prior to any drug exposure, saline was administered intraperiotoneally (i.p.) on two consecutive experimental sessions to ensure no behavioral disruption due to injection procedures. Then, clomipramine (30.0 mg/kg) was administered and the dose was decreased until the highest dose that did not completely suppress lever pressing was identified. This dose, 3.0 mg/kg, was given repeatedly for 11 days, during which time choice was assessed. Injections of drug were given every day of the week, unless otherwise stated, and experimental sessions were conducted 5 days a week (Monday–Friday). For all doses, administration of the drug (1.0 ml/kg, i.p.) occurred 15 min prior to the start of the experimental session and was delivered in a saline vehicle at a concentration of 1.0 mg/ml. Clomipramine was purchased from Sigma-Aldrich (St. Louis, MO).
2.5. Data analysis
Percent choice for the larger reinforcer as a function of delay to the large reinforcer was the primary dependent variable. Reporting data in this way is consistent with other published studies (cf. Evenden and Ryan, 1996, 1999; Cardinal et al., 2000, 2001) and thus, allows for comparisons among studies that have used the same or similar procedure. Statistical analysis of the data (repeated-measure ANOVA) were used to assess differences between the groups during baseline and drug treatment. For both comparisons, baseline and drug treatment, the means of last five sessions were used in the analyses. Indifference points (50% choice for each alternative) were interpolated for individual subjects and group means with 95% confidence intervals are reported. A t-test was conducted to determine a statistically significant difference in the mean indifference points between the strains.
3. Results
All eight Fischer 344 rats and seven of the eight Lewis rats completed the study. (One of the Lewis rats died before the study was completed.) Prior to drug administration, statistically significant differences were observed between the two strains with regard to large reinforcer choices. The main effect of strain was significant, F(1,13)=13.3, p<0.01, and the main effect of delay was was significant, F(4,52)=70.4, p<0.001. For both groups, percent choice for the large reinforcer decreased as the delay to presentation increased (see Fig. 1). This rate of decline was greater for the Lewis rats when compared to the Fischer 344 rats and there was a significant strain × delay interaction, F(4,52)=6.2, p<0.001.
Fig. 1.
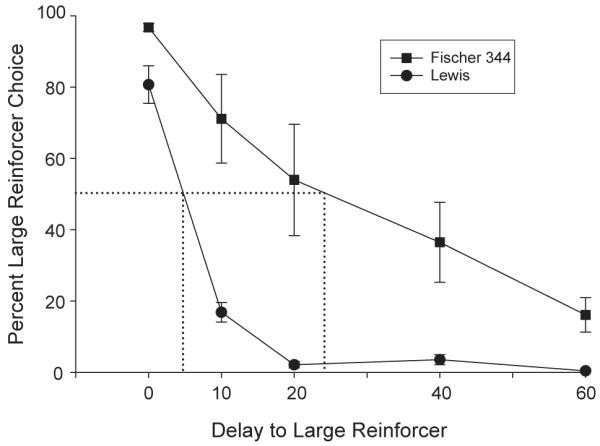
Percent choice for the larger food reinforcer as the delay to its presentation was increased across the session. Mean choices for the Lewis rats (n=7) are identified by filled circles. Mean choices for the Fischer 344 rats (n=8) are identified by filled squares. Vertical bars represent the standard errors of the means. Dotted drop lines indicate the delays to the large reinforcer when choice was indifferent, i.e., 50% choice for either alternative.
Interpolated indifference points (where the two outcomes are considered equal in value, i.e., percent large reinforcer choice is estimated to be 50%) were 28.0 s (95% CI: 11.2–44.9 s) for the Fischer 344 rats and 4.76 s (95% CI: 3.6–5.8 s) for the Lewis rats. These differences were statistically significance (t(13)=−3.038, p=0.01). The dashed drop lines in Fig. 1 represent group averages. Greater variability between subjects, as indicated by the SEM, was seen in the Fischer 344 rats (7.12) compared to the Lewis rats (0.44).
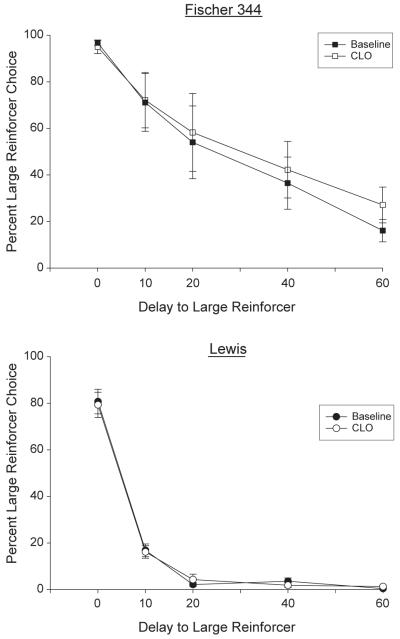
Following six consecutive days of exposure to 30.0 mg/kg clomipramine, lever pressing (and general activity in the home cage) was completely suppressed in both strains. Reducing the dose to 15.0 mg/kg for 3 days did not restore lever pressing. However, for most rats in both strains, responding returned to or near baseline levels following five days with no drug exposure (a “washout period”). Following repeated exposure (11 days) to 3.0 mg/kg clomipramine, choice for the large reinforcer was ultimately unchanged from baseline in both groups (see Fig. 2). The mean choice during last five sessions of 3.0 mg/kg clomipramine exposure did not differ statistically from mean baseline choice for either the Fischer 344 rats ( F(1,7)<1) or the Lewis rats (F(1,6)<1). For some rats, responding was disrupted or suppressed during the initial sessions, but it is unclear if this was a result of the 3.0 mg/kg dose or effects of earlier suppression by the larger doses. In any case, the effect was attenuated by the end of the condition. Throughout the experiment, omitted trials occurred infrequently and unsystematically, with the exception of when the largest doses of clomipramine were administered, in the early part of the drug “wash out” phase, and the early sessions of 3.0 mg/kg administration. There were no systematic differences in omissions observed between the two strains.
Fig. 2.
Percent choice for the larger food reinforcer as the delay to its presentation was increased across the session during baseline (no-drug) conditions is identified by filled symbols for the Fischer 344 rats (n=8; upper panel) and the Lewis rats (n=7; lower panel). Means from the last five sessions of repeated 3.0 mg/kg, i.p. clomipramine are represented by open symbols. Vertical bars indicate the standard errors of the means.
4. Discussion
The major finding of the present study is that increasing the delay to the large reinforcer decreased its value and this outcome was different between the Lewis and the Fischer 344 rats. Specifically, the Lewis rats were more likely to switch from the larger reinforcer to the smaller one when delays to the larger reinforcer were relatively short. This finding has implications regarding the use of various rat strains for studying genetic and/or neurochemical underpinnings for delay discounting and behavior that is often labeled “impulsive.” For instance, identifying specific neurochemical differences between rat strains and how they impact behavior may yield information useful to the development of pharmacological interventions to treat/prevent impulse-control disorders. Genetic factors in mice and rats have already been implicated in the correlation between performance on impulsivity tasks and drug (alcohol) self-administration (Logue et al., 1998; Poulos et al., 1995), but certainly more research is warranted to increase understanding of such relations, including any differential neurochemical contributions and roles of potential pharmacotherapeutic agents.
Previous work involving drug or lesion studies suggests that lower levels of 5-HT and DA are related to increased impulsive choice or increased rates of delay discounting (Evenden, 1999b; Cardinal et al., 2000, 2001; Harrison et al., 1997; Mobini et al., 2000; Wogar et al., 1993). Such neurochemical effects may contribute to the present outcome as Lewis rats have been shown to have lower levels of 5-HT and DA in various brain regions relative to Fisher 344 rats (Selim and Bradberry, 1996; Lindley et al., 1999). However, many variations have been noted. For instance, Lewis rats have shown lower levels of 5-HT in the nucleus accumbens (Selim and Bradberry, 1996), but a 3–4 fold higher baseline level of extracellular 5-HT in the hippocampus (Pollier et al., 2000) and significantly fewer 5-HT1A binding sites in the hippocampus and frontal cortex (Burnet et al., 1996) than Fischer 344 rats. With regard to DA, Lewis rats have shown lower concentrations in the dorsal striatum (Lindley et al., 1999), but there are mixed findings in the nucleus accumbens. Sziraki et al. (2001) reported a 3-fold increase in extracellular DA in the shell of the nucleus accumbens for Fischer 344 rats relative to Lewis rats. However, Lindley et al. (1999) found no differences in DA in either the core or the shell of the nucleus accumbens. Relative to Fischer 344 rats, Lewis rats have shown lower DA transporter levels in the striatum, nucleus accumbens, and olfactory tubercle (Flores et al., 1998). It remains to be seen, however, whether the extent of any or all of these, or some other difference, may be involved in impulsive choice. Further investigations into specific neurochemical differences may suggest targets for pharmacological interventions designed to modify impulsive choice.
Research focused on drug effects on behavior operationally defined as impulsive has yielded mixed results. Many drugs that have effects on 5-HT and DA systems have been evaluated, but generally it is their acute, rather than repeated, effects on behavior that have been the central focus (e.g., Evenden, 1998, 1999b; Evenden and Ryan, 1996, 1999; Poulos et al., 1996; Wade et al., 2000). Despite the mixed results, which could be due to various factors, e.g., operational definitions of impulsivity, route of administration, dose, species, it appears that drugs targeting 5-HT and DA systems can produce significant behavioral change, and thus, warrant further study.
In the present study, clomipramine’s effects were evaluated after repeated exposure to a single dose (3.0 mg/kg, i.p). No statistically significant effects in either strain following repeated administration were observed during the last 5 days of testing when compared to baseline data. It should be noted, however, that decreased large reinforcer choices may not have been as obvious in the Lewis rats given the already low baseline for large reinforcer choices, i.e., floor effects. It is possible that different effects would have been noted if another dose had been tested, if the testing phase had been lengthened, or if the delay values had been reduced. Clomipramine was chosen because of its ability to block reuptake of serotonin, but it also has an active metabolite that blocks uptake of norepinephrine, which may have affected the results. Perhaps, a drug with more selective effects on 5-HT and/or DA receptor subtypes would have produced a different outcome.
It should be noted that “impulsive” choice, as operationally defined in the present paper, involves two variables, reinforcement delay and reinforcement amount. Thus, any observed differences in choice between Lewis and Fischer 344 rats may be due differential sensitivities to reinforcer delay and/or magnitude. Future studies and quantitative analyses may elucidate some of the mechanisms that underlie choice deemed “impulsive” and allow for a teasing apart of factors that influence such choice, e.g., sensitivity to reinforcer magnitude and delay (see Ho et al., 1999 and Pitts and Febbo, 2004 for further discussions of quantitative analyses). Certainly, there are many operational definitions and paradigms to study impulsivity (cf. Evenden, 1999a; Monterosso and Ainslie, 1999) and more work is needed to elucidate the factors that may contribute to disorders characterized by “impulsive” behavior (e.g., drug abuse, gambling, ADHD).
In summary, Lewis rats emitted more responses resulting in delivery of an immediate, small food reinforcer (impulsive choice) than the Fischer 344 rats. Overall, repeated administration of 3.0 mg/kg, i.p. clomipramine did not affect the percentage of large, delayed reinforcer choices. Better characterization of neurochemical factors, the involvement of particular brain regions and specific receptor subtypes, and the impact of genetic contributions on choice deemed “impulsive” is needed and may have implications for the development of pharmacotherapies designed to address maladaptive behavior patterns associated with differential delay discounting, e.g., drug abuse. The correlation between differential drug self-administration (seen in other studies) and impulsive choice in Fischer 344 and Lewis rats (seen in the present study) may suggest future research endeavors aimed at identifying potentially common mechanisms and treatment protocols. This has relevance given the correlation between drug abuse and increased delay discounting in the human population.
Acknowledgements
The authors wish to acknowledge Christine Tiep for superb technical assistance, Kent Parker for assistance with the statistical analyses, Gregory A. Ordway and Ian Paul for their suggestions and comments during the course of the study. Funding for this project was provided to K.G.A. by the NIH Centers of Biomedical Research Excellence (COBRE), through the Center for Psychiatric Neuroscience, University of Mississippi Medical Center (P20-RR16438).
References
- Ainlsie GW. Impulse control in pigeons. J Exp Anal Behav. 1974;21:485–9. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders. 4th ed. Text Revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann New York Acad Sci. 1998;836:158–81. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Mefford IN, Smith CC, Gold PW, Sternberg EM. Hippocampal 5-HT1A receptor binding site densities, 5-HT1A receptor messenger riboneucleic acid abundance and serotonin levels parallel the activity of the hypothalamo-pituitary-adrenal axis in rats. Behav Brain Res. 1996;73:365–8. doi: 10.1016/0166-4328(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD. Effects of d,l-fenfluramine on aggressive and impulsive responding in adult males with a history of conduct disorder. Psychopharmacology. 1999;146:473–81. doi: 10.1007/pl00005493. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Moeller FG, Dougherty DM, Rhoades H. Studies of violent and nonviolent male parolees: II. Laboratory and psychometric measurements of impulsivity. Biol Psychiatry. 1997;41:523–9. doi: 10.1016/s0006-3223(96)00426-x. [DOI] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effects of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav Brain Res. 2002;136:349–57. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats: IV. The effects of selective serotonergic agents on a paced fixed consecutive number schedule. Psychopharmacology. 1998;140:319–30. doi: 10.1007/s002130050773. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999a;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats: VII. The effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology. 1999b;146:422–31. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: VI. The effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–21. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptor levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Green L, Fristoe N, Myerson J. Temporal discounting and preference reversals in choice between delayed outcomes. Psychon Bull Rev. 1994;1:383–9. doi: 10.3758/BF03213979. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–42. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Ho MY, Mobini S, Chiang TJ, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. 1999;146:362–72. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR. (−)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–4. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharm Exp Ther. 1994;269:137–44. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Haile CN, DeCaprio JL, Jatlow PI, Nester EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–29. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L. Alcohol preference and voluntary intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol, Clin Exp Res. 1984;8:485–6. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Bengoechea TG, Wong DL, Schatzberg AF. Strain differences in mesotelencephalic dopaminergic neuronal regulation between Fischer 344 and Lewis rats. Brain Res. 1999;832:152–8. doi: 10.1016/s0006-8993(99)01446-8. [DOI] [PubMed] [Google Scholar]
- Logue AW. Research on self-control: an integrating framework. Behav Brain Sci. 1988;11:665–709. [Google Scholar]
- Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S. Cocaine decreases self-control in rats: a preliminary report. Psychopharmacology. 1992;109:245–7. doi: 10.1007/BF02245509. [DOI] [PubMed] [Google Scholar]
- Logue SF, Swartz RJ, Wehner JM. Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol, Clin Exp Res. 1998;22:1912–20. [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharm. 1997;5:256–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–5. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The effect of delay and intervening events on reinforcement value. Quantitative Analyses of Behavior. vol. 5. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- Mazur JE. Choice with delayed and probabilistic reinforcers: effects of prereinforcer and postreinforcer stimulus. J Exp Anal Behav. 1998;70:253–65. doi: 10.1901/jeab.1998.70-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytrptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–7. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology. 1999;146:339–47. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: models of individual choice. J Exp Anal Behav. 1995;64:263–76. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug Alcohol Depend. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Febbo SM. Quantitative analyses of methamphetamine’s effects on self-control choices: implications for elucidating behavioral mechanisms of drug action. Behav Processes. 2004;66:213–33. doi: 10.1016/j.beproc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Pollier F, Sarre S, Aguerre S, Ebinger G, Mormede P, Michotte Y, et al. Serotonin reuptake inhibition by citalopram in rat strains differing for their emotionality. Neuropsychopharmacology. 2000;22:64–76. doi: 10.1016/S0893-133X(99)00092-5. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–4. [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le AD. Dexfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption. Behav Pharmacol. 1996;7:395–9. doi: 10.1097/00008877-199608000-00011. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice, and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–64. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988a;245:164–70. [PubMed] [Google Scholar]
- Suzuki T, Otani K, Koike Y, Misawa M. Genetic differences in preferences for morphine and codeine in Lewis and Fischer 344 inbred rat strains. Jap J Pharmacol. 1988b;47:425–31. doi: 10.1254/jjp.47.425. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, et al. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;26:609–17. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;150:90–101. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–43. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]