Abstract
Background
Control of viral replication through combination antiretroviral therapy (cART) improves patient health outcomes. Yet many HIV-infected patients have co-morbidities that pose social and clinical barriers to achieving viral suppression. Integration of subspecialty services into HIV primary care may overcome such barriers.
Objective
Evaluate effect of Integrated HIV Care on suppression of HIV replication.
Research Design
A retrospective cohort study of HIV patients from five Veterans Affairs healthcare facilities 2000–2006.
Subjects
Patients with >3 months of follow-up, sufficient baseline HIV severity, on cART.
Measures
We measured and ranked Integrated Care at the facilities. These rankings were applied to patient visits to form an index of Integrated HIV Care utilization. We evaluated effect of Integrated HIV Care utilization on likelihood of achieving viral suppression while on cART, controlling for demographic and clinical factors using survival analysis.
Results
The 1,018 HIV-infected patients eligible for analysis had substantial barriers to responding to cART: 93% had co-morbidities with mean 3.2 co-morbidities per patient (S.D.=2.0); 52% achieved viral suppression in median 231 days (S.D.=411.6). Patients visiting clinics which offered hepatitis, psychiatric, psychological and social services in addition to HIV primary care were 3.1 times more likely to achieve viral suppression than patients visiting clinics which offered only HIV primary care (Hazard ratio=3.1, p<.001).
Conclusions
Patients who visited Integrated HIV Care clinics were more likely to achieve viral suppression while on cART. Future research should investigate which elements of Integrated Care are most associated with viral control and what role provider experience plays in this association.
INTRODUCTION
The ability of combination antiretroviral therapy (cART) to suppress replication of the Human Immunodeficiency Virus (HIV) has provided enormous clinical and survival benefits to HIV-infected persons.1–4 Unfortunately, not all patients derive this benefit. Although the dosing frequency and side effects of cART have improved since the introduction of cART in the mid-1990s, adherence to treatment regimen remains a challenge for many HIV-infected patients and is a principal reason for failure to suppress viral replication, emergence of antiretroviral resistance, and disease progression.5–9
Poor medication adherence in HIV-infected patients is related to the complexity of the treatment regimen, medication side effects, patient beliefs in the effectiveness of treatment, and access to medical care.10,11 In addition, co-morbidities such as substance abuse disorders, mental health disorders, hepatitis C infection, diabetes, and heart disease, which are common in HIV-infected patients, complicate treatment plans and are associated with lower adherence to cART.12–18
The consequences of poor adherence to antiretroviral therapy have led to the development of multiple patient-centered interventions to modify patient behavior such as providing pagers to remind patient to take their medications or education regarding the importance of adherence and management of side effect profiles.19,20 While these interventions are important, relatively less attention has been paid to addressing co-morbidities in order to improve adherence and consequent viral suppression. The most common way that HIV clinics address co-morbidities is by integrating non-infectious disease providers such as psychiatrists and social workers into HIV primary care. Various degrees of such integration have been implemented in portions of the Department of Veterans Affairs (VA) healthcare system as well as other healthcare systems in the U.S.21–30 In their most developed form, these VA clinics provide on-site pharmacy services, mental health care, urgent care, substance abuse treatment, women’s healthcare and case management. 30 Formal analyses that evaluate the benefit of such programs are lacking, however.31 To better understand the relationship of Integrated HIV Care to patient health outcomes, we conducted a retrospective cohort study to evaluate the association of patient utilization of Integrated HIV Care with the likelihood of achieving viral suppression among HIV-infected patients receiving care at five VA healthcare facilities in Southern California and Nevada.
METHODS
Conceptual model
We defined Integrated HIV Care (IHC) to be a care model in which specialists from multiple disciplines collaborate within a geographically and temporally constrained clinic environment to provide HIV-infected patients with onsite primary care, HIV specialty services and other services such as treatment for hepatitis C, mental health, substance abuse, social services, etc. We adopted the Donabedian model32 to examine the impact of IHC on viral suppression among HIV-infected patients. In this conceptual model, IHC (i.e., structure) influences the way in which providers manage patients’ co-morbidities and encourage patients to adhere to medications (i.e., process), hence influencing viral suppression (i.e., outcomes). We hypothesized that IHC enables providers within multidisciplinary teams to manage co-morbidities more effectively and promote better adherence to antiretroviral medication regimens; as a result, patients with co-morbidities who utilized IHC more frequently would be more likely to achieve suppression of HIV replication.
Study design
We conducted a retrospective cohort study of HIV-infected patients receiving care from five Veterans Affairs healthcare facilities in the Western United States from October 2000 to April 2006. We interviewed the chiefs of the HIV clinics at these facilities to measure and rank levels of IHC at their clinics. These rankings were applied to patient visits obtained from electronic medical records to form an index of IHC utilization. Using survival analysis, we analyzed the effect of IHC utilization on the likelihood of achieving viral suppression while on cART, controlling for demographic and clinical factors.
Data sources
We obtained administrative and clinical data of inpatient and outpatient encounters from a VA regional electronic data warehouse. The data were longitudinal and comprehensive, including patient demographics, date and time of health service, type of provider, location of service, laboratory tests and results, pharmacy prescriptions and refills, purpose of visit or reason for admission (diagnostic codes), and service provided (surgical and procedure codes). In addition to using these electronic medical data, we interviewed the chiefs of the Infectious Disease clinics at the five VA facilities to obtain the descriptions of integrated services that comprised their HIV clinics.
Inclusion criteria
An HIV-infected patient had to have a viral load ≥5,000 at study entry, at least two HIV clinic visits per year, and at least a three-month supply of cART during the observation period. Patients who met these criteria were included in the analysis regardless of whether they achieved viral suppression or not. cART was determined by synchronous receipt of two nucleoside/nucleotide analogues and at least one protease inhibitor, non-nucleoside reverse transcriptase inhibitor, or fusion inhibitor. Patients were identified as being HIV-infected if they had qualified under any of the following criteria: two or more outpatient visits with a recorded diagnosis of HIV infection, one or more inpatient stays with a diagnosis of HIV infection,33 two or more positive plasma HIV-1 RNA assays, or a positive HIV antibody test with a confirmatory Western Blot test. Diagnoses of HIV infection were defined by the ICD-9-CM codes listed in Appendix 1.
Statistical methods
To assess the effect of IHC utilization on time to viral suppression adjusted for patient demographic and clinical characteristics, we used survival analysis with the Cox regression method in which the hazard function was determined by the rates at which patients achieved viral suppression over time. The dependent variable was the time from cART initiation to the first achievement of viral suppression. Although the study span was 2000–2006, each patient had their own observation period starting from the entry date when they started cART after their viral load was first recorded as being ≥5,000 HIV-1 RNA copies/mL to the date when they achieved viral suppression below 400 copies/mL. Patients who had not achieved viral suppression by the end of the study period (i.e. loss to follow-up) were assigned a “right-censored” time counting from study entry to their last visit. The independent variables included patient age, race and ethnicity, marital status, co-payment for VA medical costs as a proxy for income, lack of housing, sexually transmitted diseases, co-morbidities, baseline HIV viral load and CD4+ cell count, treatment-naive versus treatment-experienced, access to cART medication as determined by pharmacy data, and utilization of IHC as determined by clinic visits. Baseline CD4+ cell counts and viral loads were based on the first lab tests taken upon patient entry into the study. All viral load values were log10-transformed. Access to cART medication was computed by refill frequency during the observation period.34 Appendix 1 provides details of the diagnostic codes and laboratory tests used to determine the co-morbidities. Patients were considered as treatment naïve if they had an HIV positive antibody test and a subsequent viral load ≥5000 at study entry, followed by the first cART prescription. Patients were considered as treatment-experienced if they had records of receipt of cART prior to study entry. For those patients whose HIV status was determined by ICD-9 codes or positive viral loads and who initiated cART after study entry but did not have record of positive HIV antibody tests, we could not determine whether they were treatment-naïve or treatment-experienced; therefore we assigned such patients an unknown status.
To measure patient utilization of IHC, we first ranked the comprehensive level of IHC of the HIV clinics. We classified these clinics into four levels of comprehensiveness based on the number of services and the amount of resources devoted to each service (Table 1). Level I clinics offered walk-in services provided by a mid-level provider only (i.e., a nurse practitioner or physician assistant). Level II clinics offered more comprehensive care than Level I with the presence of an HIV physician specialist and a dedicated pharmacist. In addition to all services available in Level II clinics, Level III clinics offered psychiatric and social services. Level IV clinics offered the most comprehensive care with the addition of a psychologist. Various study facilities offered different IHC clinics on different days of the week at different locations. For example, facility E offered Level IV clinic on Tuesday afternoons, Level III clinic on Monday and Wednesday mornings, Level II clinic on Friday mornings, and Level I (walk-in) clinic on the other times of the week. The distribution of IHC users across the five VA facilities is shown in Table 2.
TABLE 1.
Four Levels of Integrated HIV Care Clinics
| Components of Integrated HIV Care |
Services | Levels | |||
|---|---|---|---|---|---|
| IV | III | II | I | ||
| Nurse practicioner, physician assistant | To follow patients on HIV treatment. | x | x | x | x |
| Clinical coordinator | To link patients to community-based programs for food, dental care, home care, etc. | x | x | x | x |
| HIV physician specialist | To provide diagnosis, counseling and treatment for HIV infection | x | x | x | |
| Dedicated pharmacist | To help patients with cART refills, side effects, food restriction, etc. | x | x | x | |
| Social worker | To help patients with housing, transportation, disability benefits, etc. | x | x | ||
| Psychiatrist | To provide psychiatric diagnosis and treatment for substance abuse and mental illnesses. | x | x | ||
| Psychologist | To provide counseling for substance abuse and mental illnesses | x | |||
Remark: ‘x’ indicates that the specialists are available at the HIV clinics to offer on-site services to HIV-infected patients.
TABLE 2.
Distribution of Integrated HIV Care Users across the Five VA Facilities
| Facilities | Number of users (column percent) | |||||
|---|---|---|---|---|---|---|
| Level I | Level II | Level III | Level IV | Multi levels | Total | |
| A | 131 (45.6%) | 131 (12.9%) | ||||
| B | 143 (49.8%) | 143 (14.1%) | ||||
| C | 1 (3.3%) | 36 (69.2%) | 85 (16.9%) | 122 (12.0%) | ||
| D | 8 (26.7%) | 91 (62.8%) | 201 (39.9%) | 300 (29.5%) | ||
| E | 21 (70.0%) | 13 (4.5%) | 16 (30.8%) | 54 (37.2%) | 218 (43.3%) | 322 (31.6%) |
| Total | 30 (100%) | 287 (100%) | 52 (100%) | 145 (100%) | 504 (100%) | 1018 (100%) |
Remark: Facilities A and B had Level II clinics only. Facility C had Level I and Level III clinics with a total of 122 patients, 1 of whom visited Level I only, 36 patients visited Level III only while the other 85 patients visited both levels. Similarly, facility D had Level I and Level IV clinics while facility E had all four levels.
Since more than half of the patients visited multiple IHC clinics, we evaluated their utilization of IHC by the following index:
In this index, the total days of observation referred to the number of days between study entry and viral suppression or loss to follow-up. We quantified the four IHC levels as 1, 2, 3, and 4. The index gave each patient a score that weighed the frequency of HIV clinic visits by these IHC levels. Patients who visited clinics of higher IHC level were assigned higher scores. Thus a patient with one visit to a Level IV clinic every 90 days would receive a score of 4 as would a patient with two visits to a Level II clinic during the same period.
We conducted two survival analyses. In the first analysis, we included all study patients and evaluated the effect of IHC utilization index on time to viral suppression, adjusted for the demographic and clinical variables mentioned above. The second analysis was similar to the first one except that we included the subset of patients who visited only one IHC level and that we replaced the IHC utilization index by the categorical variable indicating the levels of the IHC clinics and the number of visits to the clinics per quarter. In both analyses, the effects were assessed by adjusted hazard ratios. Similar to the interpretation of risk ratios, hazard ratio values of less than 1 indicated negative association (i.e., less likely to achieve viral suppression) whereas a value greater than 1 indicated positive association (i.e., more likely to achieve viral suppression), and a value equal to 1 indicated no association (i.e., no change in likelihood of viral suppression). Since patients who received care at the same facilities might experience similar health outcomes, we adjusted the regression model for intra-facility clustering. Finally, we conducted a sensitivity analysis to examine how choices of baseline viral load threshold other than 5,000 counts would affect the study results. All statistical analyses were programmed in SAS35 and STATA36.
RESULTS
A total of 2,883 patients were identified as being HIV-infected. Of these, 1,018 (35.3%) patients met the inclusion criteria. Table 3 presents the baseline demographic and clinical characteristics of the study patients. Overall, the patients had substantial potential barriers to responding to antiretroviral therapy. Approximately 18% of the patients had a history of homelessness; 93% had one or more co-morbid conditions with a mean of 3.2 co-morbidities per patient (S.D.=2.0). The most prevalent co-morbidities were mental disorders (55.5%), prior or current hepatitis B infection (48.8%), hepatitis C infection (33.1%), and illicit drug or alcohol use (31.4%). Analysis of prior treatment indicated that 497 (48.8%) patients were treatment-experienced whereas 187 (18.4%) patients were treatment naïve. We could not determine whether the remaining 334 (32.8%) patients were treatment experienced or naïve because of insufficient information. Comparing users of the four IHC levels, we found that the four groups were different in their compositions of race/ethnicity, homelessness, baseline HIV viral load and CD4+ cell counts (all p-values<0.01). For example, users of Level III had the lowest baseline HIV viral loads while users of Level I were most likely to be homeless. Despite these differences, all four groups were similar in co-morbidity prevalence, access to cART, and frequency of visits to HIV clinics. These similarities indicate that the need for integrated HIV care was comparable among the four groups.
TABLE 3.
Baseline Demographic and Clinical Characteristics of Study Patients Stratified by Integrated HIV Care Levels
| Characteristics | Integrated HIV Care Users | Total n=1,018 |
||||
|---|---|---|---|---|---|---|
| Level I only n=30 |
Level II only n=287 |
Level III only n=52 |
Level IV only n=145 |
Multi- levels n=504 |
||
| Age (mean, s.d.) | 51.7 (9.5) | 50.9 (9.4) | 55.1 (8.8) | 49.4 (9.6) | 50.2 (9.2) | 50.6 (9.4) |
| Male (%) | 100.0 | 98.3 | 98.1 | 98.6 | 98.8 | 98.6 |
| Race/ethnicity (%) | ||||||
| ▪ Caucasian | 26.7 | 31.0 | 53.9 | 35.2 | 33.1 | 33.7 |
| ▪ African American | 43.3 | 19.5 | 15.4 | 28.3 | 29.4 | 26.1 |
| ▪ Hispanic | 3.3 | 6.3 | 0.0 | 4.8 | 6.6 | 5.8 |
| ▪ Asian, Native American | 13.3 | 3.5 | 3.9 | 4.8 | 5.4 | 4.9 |
| ▪ Missing | 13.3 | 39.7 | 26.9 | 26.9 | 25.6 | 29.5 |
| Marital status (%) | ||||||
| ▪ Never married | 60.0 | 50.2 | 57.7 | 60.0 | 59.3 | 56.8 |
| ▪ Married | 6.7 | 7.7 | 7.7 | 5.5 | 5.8 | 6.4 |
| ▪ Widow, divorced, etc. | 33.3 | 42.2 | 34.6 | 34.5 | 34.9 | 36.8 |
| No co-payment for VA medical cost, proxy for low income (%) | 80.0 | 74.9 | 76.9 | 79.3 | 77.4 | 77.0 |
| Lack of housing (%) | 56.7 | 7.3 | 3.9 | 20.0 | 23.6 | 18.1 |
| Sexual transmitted diseases (%) | 20.0 | 11.2 | 23.1 | 16.6 | 19.4 | 16.9 |
| -Percentage of patients with any | 80.0 | 92.7 | 88.5 | 93.1 | 94.3 | 92.9 |
| co-morbidities | ||||||
| -Number of co-morbidities per patient (mean, s.d.) | 3.0 (2.1) | 3.0 (1.9) | 2.7 (1.8) | 3.0 (1.9) | 3.4 (2.0) | 3.2 (2.0) |
| Co-morbidities (%) | ||||||
| ▪ Mental disorders | 46.7 | 46.3 | 40.4 | 60.7 | 61.3 | 55.5 |
| ▪ HBV infection (prior/current) | 46.7 | 43.2 | 42.3 | 48.3 | 53.0 | 48.8 |
| ▪ HCV infection | 20.0 | 37.6 | 28.9 | 25.5 | 33.9 | 33.1 |
| ▪ Drug/alcohol use | 36.7 | 25.4 | 17.3 | 33.1 | 35.5 | 31.4 |
| ▪ Cancer | 10.0 | 14.6 | 15.4 | 14.5 | 17.9 | 16.1 |
| ▪ Heart disease | 20.0 | 14.3 | 21.2 | 15.9 | 12.5 | 14.2 |
| ▪ COPD | 16.7 | 13.9 | 17.3 | 13.1 | 9.1 | 11.7 |
| ▪ Tuberculosis | 13.3 | 3.8 | 0.0 | 15.2 | 16.5 | 11.8 |
| ▪ Diabetes | 10.0 | 10.1 | 19.2 | 13.1 | 10.5 | 11.2 |
| ▪ Cirrhosis | 3.3 | 23.7 | 1.9 | 2.8 | 3.8 | 9.1 |
| ▪ Stroke | 3.3 | 2.4 | 1.9 | 2.1 | 2.4 | 2.4 |
| Baseline HIV RNA (copies/mL) (%) | ||||||
| ▪ 5K–30K | 56.7 | 39.0 | 61.5 | 39.3 | 38.9 | 40.7 |
| ▪ 31K–60K | 16.7 | 17.1 | 17.3 | 16.6 | 17.9 | 17.4 |
| ▪ 61–90K | 10.0 | 9.1 | 19.2 | 15.2 | 23.2 | 17.5 |
| ▪ >90K | 16.7 | 34.8 | 1.9 | 29.0 | 20.0 | 24.6 |
| Median | 25.7K | 42.5K | 18.0K | 48.7K | 49.4K | 43.0K |
| Mean | 40.6K | 159.0K | 30.5K | 94.8K | 87.2K | 104.2K |
| S.D. | 38.5K | 335.5K | 27.0K | 13.7K | 14.3K | 214.3K |
| S.D. Baseline CD4+ cell count (%) | ||||||
| ▪ <50 | 10.0 | 15.7 | 1.9 | 6.9 | 12.1 | 11.8 |
| ▪ 50–200 | 10.0 | 25.8 | 23.1 | 31.7 | 23.6 | 25.0 |
| ▪ 201–350 | 23.3 | 25.4 | 26.9 | 27.6 | 30.4 | 28.2 |
| ▪ >350 | 56.7 | 33.1 | 48.1 | 33.8 | 33.9 | 35.1 |
| Median | 374.0 | 244.0 | 313.2 | 263.0 | 274.5 | 271.0 |
| Mean | 428.0 | 287.1 | 342.9 | 303.1 | 296.6 | 301.1 |
| S.D. | 298.5 | 231.0 | 202.0 | 222.7 | 212.2 | 222.5 |
| Treatment status (%) | ||||||
| ▪ Experienced | 40.0 | 46.7 | 76.9 | 44.1 | 49.0 | 48.8 |
| ▪ Naïve | 36.7 | 8.4 | 7.7 | 26.9 | 21.6 | 18.4 |
| ▪ Unknown | 23.3 | 45.0 | 15.4 | 29.0 | 29.4 | 32.8 |
| Frequency of cART refills (mean, s.d) | 77.8% (26.4) | 80.2% (26.9) | 82.9% (24.9) | 81.1% (28.5) | 74.1% (30.6) | 77.4% (29.1) |
| Number of visits to IHC clinics per quarter (mean, s.d) | 1.9 (2.1) | 2.6 (3.1) | 1.8 (2.0) | 3.1 (2.5) | 3.1 (3.6) | 2.8 (3.2) |
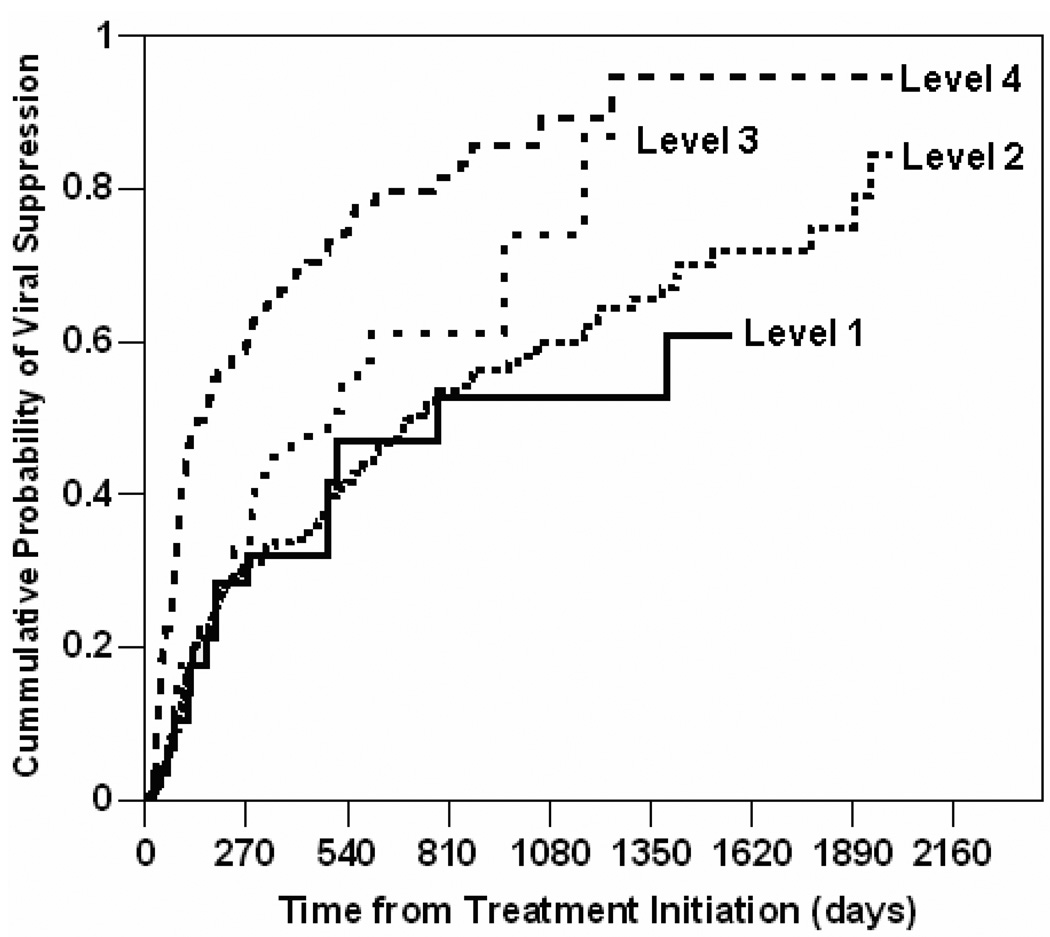
We used Kaplan-Meier method to estimate the cumulative probability of viral suppression among patients who accessed only one IHC level. Figure 1 shows the Kaplan-Meier curves depicting the cumulative probability of viral suppression stratified by IHC levels. The plot shows that by the 9th month (i.e. 270th day) of cART, Level IV users had 60% probability of viral suppression while Level I, II, and III users all had 32% probability of viral suppression. By the 27th month (i.e. 810th day) of cART, Level IV users had 80% probability of viral suppression; Level III users had 60% probability; Level I and II users both had 53% probability. By the end of the study, the probability of viral suppression was 95% for Level IV users, 87% for Level III users, 83% for Level II users, and 60% for Level I users. All comparative differences were statistically significant at p-values <0.01. In summary, the higher the IHC level, the higher the cumulative probability of viral suppression.
FIGURE 1.
Cumulative Probability of Viral Suppression Stratified by Integrated HIV Care Levels
In subsequent analyses, we adjusted for the demographic and clinical differences among the four groups when assessing the effect of IHC levels on time to viral suppression. Table 4 shows the adjusted hazard ratios obtained from two survival analyses, the first involving all study patients (n=1,018) and the second involving the subset of patients who accessed only one level of IHC clinics (n=514). From the first analysis, we found that once-married patients and those with high income were more likely to achieve viral suppression than those who were never married (Hazard ratio HR=1.34, CI=1.21–1.50) and had low income (HR=1.14, CI=1.01–1.30). Conversely, homeless patients were less likely to achieve viral suppression (HR=0.78, CI=0.67–0.90). Patients who had higher baseline CD4+ cell counts were more likely to achieve viral suppression (HR=1.06, CI=1.05–1.08). Most importantly, patients who had more access to cART were much more likely to achieve viral suppression (HR=3.13, CI=2.22–4.40). In analyses that controlled for these demographic and clinical factors, patients who had a higher index of IHC utilization were more likely to achieve viral suppression (HR=1.10, CI=1.09–1.11). From the second analysis, we found that there was no difference in viral suppression between patients who visited clinics of Level I and Level II. However, patients who visited clinics of Level III and Level IV were 1.7 times and 3.1 times more likely to achieve viral suppression than those who visited clinics of Level II (Level III: HR=1.74, CI=1.06–2.85. Level IV: HR=3.10, CI=1.76–5.47). Regardless of IHC levels, patients who visited clinics more frequently were more likely to achieve viral suppression (HR=1.42, CI=1.32–1.53).
TABLE 4.
Predictors of Time from Treatment Initiation to Viral Suppression
| Predictors | Adjusted Hazard ratios (95% CI) | |
|---|---|---|
| All study patients (n=1,018) |
Patients accessing only one IHC level (n=514) |
|
| Age (per one extra year) | 0.99 (0.98, 1.00) | 1.00 (0.99, 1.01) |
| Marital status (ref=Never married) | ||
| ▪ Married | 1.11 (0.90, 1.38) | 1.00 (0.60, 1.66) |
| ▪ Widow, divorced, separated, other | 1.34 (1.21, 1.50)* | 1.32 (1.17, 1.47)* |
| Co-payment for VA medical costs (yes vs. no) | 1.14 (1.01, 1.30)* | 1.02 (0.92, 1.13) |
| Lack of housing (yes vs. no) | 0.78 (0.67, 0.90)* | 0.96 (0.61, 1.50) |
| Sexual transmitted diseases (yes vs. no) | 1.20 (0.92, 1.55) | 1.52 (0.95, 2.42) |
| Number of co-morbidities (per one extra condition) | 1.01 (0.94, 1.09) | 0.97 (0.92, 1.02) |
| Baseline HIV viral load (per one Log10-unit increase) | 0.91 (0.72, 1.15) | 0.86 (0.76, 0.96)* |
| Baseline CD4+ cell count (per 100 count increase) | 1.06 (1.05, 1.08)* | 1.10 (1.05, 1.16)* |
| Treatment status (ref=naïve) | ||
| ▪ Experienced | 0.91 (0.77, 1.08) | 0.95 (0.70, 1.30) |
| ▪ Unknown | 1.04 (0.91, 1.19) | 1.08 (0.77, 1.52) |
| Frequency of cART refills (per one extra day of supply) £ | 3.13 (2.22, 4.40)* | 3.97 (2.44, 6.46)* |
| IHC utilization index (per one unit increase) | 1.10 (1.09, 1.11)* | N/A |
| IHC utilization (compared to Level II) | N/A | |
| ▪ Level I | 1.07 (0.90, 1.26) | |
| ▪ Level III | 1.74 (1.06, 2.85)* | |
| ▪ Level IV | 3.10 (1.76, 5.47)* | |
| Number of visits to IHC clinics per quarter (per one extra visit per quarter) | N/A | 1.42 (1.32, 1.53)* |
Remarks: Stars * indicate that the hazard ratios were significant at p-value <0.05. Symbol
refers to frequency of cART refills as a measure for the percentage of time in which patients had access to cART.
Sensitivity analysis
We examined the sensitivity of our results to the choice of baseline viral load. We repeated the survival analysis with baseline viral load values varying between 1,000 and 10,000 counts (instead of 5,000). Regardless of the viral load choices, the results were consistent.
DISCUSSION
We evaluated the virological response to combination antiretroviral therapy in 1,018 HIV-infected veterans who received care at five VA healthcare facilities. We found that patients who visited HIV clinics with more integrated specialty services were more likely to achieve viral suppression. In particular, patients visiting clinics which offered hepatitis, psychiatric, psychological and social services in addition to primary care and HIV specialty services were three times more likely to achieve viral suppression than patients visiting clinics which offered only primary care and HIV specialty services. This effect had been adjusted for patients’ access to antiretroviral medication and demographic and clinical factors.
We believe that our results are generalizable to care settings beyond the VA. VA HIV-infected patients are older and have more co-morbidities than generally reported.37–39 However, with the improved outcomes being seen with HIV treatment, the HIV-infected patient population is generally aging and becoming more susceptible to co-morbidities, and the VA population may represent the future of the U.S. HIV epidemic in that respect.40–42 Except for being overwhelmingly male, the income status and racial/ethnic distribution of HIV-infected veterans is similar to that of many other HIV-infected populations in the U.S. The median time to viral suppression of our study patients is longer than that reported in modern clinical trials of treatment-naïve patients, reflecting the fact that majority of our study patients experienced multiple co-morbid conditions and prior failures to respond to treatment.
The principal limitation of our study is that unmeasured differences between the patients seen at the differing clinics and unmeasured differences in the skills of the providers who staff those clinics, rather than the comprehensiveness of care, may have accounted for our findings.43,44 However, we note that our statistical models have corrected for patient demographic and clinical factors that are previously reported to modulate the effectiveness of antiretroviral therapy. In addition, provider surveys indicate that the distribution of physician experience and expertise in the management of HIV infection is similar among persons staffing the Level II, III, and IV clinics. Indeed, in many instances the same physicians provide services in more than one type of clinic. Another limitation is that we did not explicitly compare the potency of antiretroviral therapy (e.g., the use of ritonavir-boosted protease inhibitors, triple nucleoside therapy or hard-gel saquinavir) in the patient population2,6 or the complexity of antiretroviral therapy (i.e., doses per day, pills per day or dietary restrictions). However, the availability of laboratory and pharmacy records monitoring patient viral loads and access to antiretroviral medications is the same at all clinic levels; as is the policy to provide care as recommended by national guidelines.6,45 Although we accounted for treatment-experienced status in the analysis, we could not account for the unmeasured duration of cART prior to study entry, which might be associated with viral suppression. The limitations of our statistical analysis are that the quantification of the four IHC levels in the IHC utilization index was arbitrary and that the retrospective nature of this study limited our ability to consider whether temporal changes in clinic structure and operations might have impacted our results.
This study made use of the electronic medical records maintained by the Veterans Health Administration.46,47 Since HIV-infected veterans who are VA heath care users receive over 70% of their total care and obtain 98% of their prescriptions through the VA37, these records allowed us to access nearly complete records of patient demographics, receipt of antiretroviral prescriptions, clinic visits, relevant laboratory results, and medical co-morbidities such as hepatitis B and C, mental illnesses, drug or alcohol use, STDs, diabetes, heart disease, COPD, tuberculosis, cirrhosis, stroke, and cancer. However, the VA electronic database had several limitations such as high rates of missing race data and diagnostic coding errors, which could affect the quality of the present data analysis.48,49
Literature shows that co-morbid conditions such as alcohol abuse, substance use and mental health disorders, all of which impede patient adherence to cART,13–15,18 are highly prevalent in HIV-infected patients.41 The Veteran Affairs Healthcare system has implemented Integrated HIV care in which subspecialty programs are integrated into HIV clinics to address co-morbid conditions and medication adherence. However, our study is one of the few to evaluate the relationship between the level of services provided by an integrated HIV care clinic and patient health outcomes.31 We believe that IHC clinics at the VA facilities may have effectively addressed co-morbidities and encouraged medication adherence. Based on our finding that frequency of visits was a strong predictor for viral suppression, we suggest not only that resources should be allocated to integrate subspecialty services into HIV primary care clinics but also that providers should channel patients toward these clinics and retain them in care. Future studies should examine which specific elements of IHC are most associated with viral control and what role provider experience plays in this association. Finally, these results may be relevant not only to the care of HIV-infected patients but also to the provision of care of patients with other complex medical issues that require principal care in subspecialty clinics.
Acknowledgement
This work was supported by Quality Enhancement Research Initiative for HIV and Hepatitis, Department of Health Services and Research Development, Veterans Affairs. Dr. Korthuis’ time was funded through a grant from the National Institutes of Health, National Institute on Drug Abuse (K23DA019808).
APPENDIX 1
Diagnostic codes and laboratory tests
HIV infection
ICD-9 codes: 042., 042.1, 042.2, 042.9, 043.0, 043.1, 043.2, 043.3, 043.9, 044.9, 079.53, V08
Lab tests: Positive HIV antibody test, positive confirmatory Western Blot, viral load
Hepatitis B infection
ICD-9 codes: 070.20, 070.21, 070.22, 070.23, 070.3, 070.30, 070.31, 070.32, 070.33, 070.52.
Lab tests: positive HBV surface antigen.
Hepatitis C infection
ICD-9 codes: 070.41, 070.44, 070.51, 070.54, 070.6, 070.70, 070.71, 070.9, 571.40, 571.41, 571.49, 571.5, 571.8, 571.9, 573.3, 573.8, V02.62
Lab tests: positive HCV antibody test or HCV viral load test
Depression
ICD-9 codes: 295, 297, 298, 311, 296.2, 296.3, 296.9
PTSD
ICD-9 codes: 309.81, 296.1, 296.4, 296.5, 296.6, 296.7, 296.80, 296.81, 296.89, 296.11, 296.41, 296.51, 296.45, 296.65, 296.46, 296.56, 296.66
Schizophrenia
ICD-9 codes: 295
Drug/alcohol use:
ICD-9 codes: 304.00, 304.01, 304.02, 304.03, 304.20, 304.21, 304.22, 304.23, 304.40, 304.41, 304.42, 304.43, 304.60, 304.61, 304.62, 304.63, 304.70, 304.71, 304.72, 304.73, 304.90, 304.91, 304.92, 304.93, 305.50, 305.51, 305.52, 305.53, 305.60, 305.61, 305.62, 305.63, 305.70, 305.71, 305.72, 305.73, 305.90, 305.91, 305.92, 305.93, 291.0, 291.1, 291.2, 291.3, 291.4, 291.5, 291.81, 291.89, 291.9, 571.2, 292.0, 292.11, 292.12, 292.2, 292.81, 292.82, 292.83, 292.84, 292.89, 292.9
Sexually Transmitted disease includes gonorrhea, chlamydia, syphilis, herpes ICD-9 codes: 054.10, 054.11, 054.12, 054.13, 054.19, 098.xx, 099.40, 099.41, 099.50, 099.51, 099.52, 099.53, 099.54, 099.55, 099.56, 099.59, 099.8, 099.9, 090.0, 090.1, 090.2, 090.3, 090.40, 090.41, 090.42, 090.49, 099.56, 090.5, 090.6, 090.7, 090.9, 091.0, 091.1, 091.2, 091.3, 091.4, 091.50, 091.51, 091.52, 091.61, 091.61, 091.69, 091.7, 091.81, 091.82, 091.84, 091.89, 091.9, 092.0, 092.9, 093.0, 093.1, 093.20, 093.21, 093.22, 093.23, 093.24, 093.81, 093.82, 093.89, 093.9, 094.0, 094.1, 094.2, 094.3, 094.51, 094.52, 094.7, 094.81, 094.82, 094.83, 094.84, 094.85, 094.86, 094.87, 094.89, 094.9, 095.0, 095.1, 095.2, 095.3, 095.4, 095.5, 095.6, 095.7, 095.8, 095.9, 096.0, 097.0, 097.1, 097.9
Diabetes
ICD-9 codes: 362.01, 362.02, 250, 250.94, 250.95, 250.96, 250.97, 250.98, 250.99
Heart disease
ICD-9 codes: 410, 413, 414.8, 414.9, 415.0, 416, 422, 421, 423, 425, 428, 429
Chronic obstructive pulmonary disease
ICD-9 codes: 491, 492, 493, 494
Tuberculosis
ICD-9 codes: 010, 011, 012, 013, 014, 015, 016, 017, 018
Stroke
ICD-9 codes: 430, 431, 432, 432.0, 432.1, 432.9, 434, 434.0, 434.00, 434.01, 434.1, 434.10, 434.11, 434.9, 434.90, 434.91, 435, 435.0, 435.1, 435.2, 435.3, 435.8, 435.9, 436, 997.02, 438, 438, 438.0, 438.11, 438.12, 438.20, 438.21, 438.22, 438.30, 438.31, 438.32, 438.40, 438.41, 438.42, 438.50, 438.51, 438.52, 438.53, 438.81, 438.82, 438.84, 437, 437.0, 437.1, 437.3, 437.4, 437.5, 437.6, 437.7, 437.8, 437.80, 437.81, 437.89, 437.9, 438.10
Cirrhosis
ICD-9 codes: 456, 572.2, 070.20, 070.21, 070.22, 070.41, 070.42, 070.44, 070.49, 070.6, 070.71, 070.0, 348.30, 348.31, 348.39, 572.3, 572.4, 572.8
Cancer
ICD-9 codes: 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160 161, 162, 163 164, 165, 166, 167, 168, 169, 170, 171, 172, 174, 175, 176, 177, 178, 179, 180, 181, 182 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208
Lack of housing
ICD-9 codes: V60.0
REFERENCES
- 1.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Barlett JA, Fath MJ, DeMasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naïve HIV-infected adults. AIDS. 2006;20:2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 3.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 4.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 5.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 6.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-infected adults and adolescents. [Accessed October 11, 2006]; Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 7.Garcia de OP, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 8.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 9.Piacenti FJ. An update and review of antiretroviral therapy. Pharmacotherapy. 2006 Aug;26(8):1111–1133. doi: 10.1592/phco.26.8.1111. [DOI] [PubMed] [Google Scholar]
- 10.Gordillo V, Del amo J, Soriano V, et al. Sociodemographic and psychological variables influencing adherence to anti-retroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 11.Waldrop-Valverde D, Valverde E. Homelessness and psychological distress as contributors to antiretroviral nonadherence in HIV-positive injecting drug users. AIDS Patient Care STDS. 2005 May;19(5):326–334. doi: 10.1089/apc.2005.19.326. [DOI] [PubMed] [Google Scholar]
- 12.Mugavero M, Ostermann J, Whetten K, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006 Jun;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 13.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook RL, Serika SM, Hunt SC, et al. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker JS, Burnam MA, Sherbourne CD, et al. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003 May;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 16.Perronne C. Antiviral hepatitis and antiretroviral drug interactions. J Hepatol. 2006;44(1) Suppl:S119–S125. doi: 10.1016/j.jhep.2005.11.025. Epub 2005 Dec 1. [DOI] [PubMed] [Google Scholar]
- 17.McCance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: the importance of drug interactions between opioids and antiretroviral agents. Clin Infect Dis. 2005 Jul 1;41 Suppl 1:S89–S95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- 18.Wagner GJ, Kanouse DE, Koegel P, et al. Adherence to HIV antiretrovirals among persons with serious mental illness. AIDS Patient Care STDS. 2003 Apr;17(4):179–186. doi: 10.1089/108729103321619782. [DOI] [PubMed] [Google Scholar]
- 19.Mannheimer SB, Morse E, Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention. Results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43 Suppl 1:S41–S47. doi: 10.1097/01.qai.0000245887.58886.ac. [DOI] [PubMed] [Google Scholar]
- 20.Tuldra A, Fumaz CR, Ferrer MJ, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:221–228. doi: 10.1097/00126334-200011010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bennett CL, Curtis JR, Achenbach C, et al. U.S. hospital care for HIV-infected persons and the role of public, private, and Veterans Administration hospitals. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Dec 15;13(5):416–421. doi: 10.1097/00042560-199612150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Meredith KL, Larson TA, Soons KR, et al. Building comprehensive HIV/AIDS care services. AIDS Patient care and STDs. 1998;12(5) doi: 10.1089/apc.1998.12.379. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi JS, Standridge WL. An integrated program for comprehensive HIV care. New Dir Ment Health Serv. 2000 Fall;87:111–118. doi: 10.1002/yd.23320008715. [DOI] [PubMed] [Google Scholar]
- 24.Malone SD, Osborne JJ. Improving treatment adherence in drug abusers who are HIV-positive. Lippincotts Case Manag. 2000 Nov–Dec;5(6):236–245. doi: 10.1097/00129234-200011000-00006. quiz 245-7. [DOI] [PubMed] [Google Scholar]
- 25.Stringari-Murray S, Clayton A, Chang J. A model for integrating hepatitis C services into an HIV/AIDS program. J Assoc Nurses AIDS Care. 2003 Sep–Oct;14(5) Suppl:S95–S107. doi: 10.1177/1055329003255581. [DOI] [PubMed] [Google Scholar]
- 26.Andrews D. Management of HIV/AIDS on the Mid North Coast: a collaborative model of care involving general practitioners and the public health system. Aust J Rural Health. 2002 Oct;10(5):244–248. doi: 10.1046/j.1440-1584.2002.00448.x. [DOI] [PubMed] [Google Scholar]
- 27.Le CT, Winter TD, Boyd KJ, et al. Experience with a managed care approach to HIV infection: effectiveness of an interdisciplinary team. Am J Manag Care. 1998 May;4(5):647–657. [PubMed] [Google Scholar]
- 28.Laraque F, Greene A, Triano-Davis JW, et al. Effect of comprehensive intervention program on survival of patients with human immunodeficiency virus infection. Arch Intern Med. 1996 Jan 22;156(2):169–176. [PubMed] [Google Scholar]
- 29.Samet JH, Friedmann P, Saitz R. Benefits of Linking Primary Medical Care and Substance Abuse Services Patient, Provider, and Societal Perspectives. Arch Intern Med. 2001;161:85–91. doi: 10.1001/archinte.161.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Yano EM, Asch SM, Phillips B, et al. Organization and management of care for military veterans with Human Immunodeficiency virus/acquired immunodeficiency syndrome in department of veterans affairs medical centers. Military medicine. 2005;170(11):952. doi: 10.7205/milmed.170.11.952. [DOI] [PubMed] [Google Scholar]
- 31.Handford CD, Tynan AM, Rackal JM, et al. Setting and organization of care for persons living with HIV/AIDS. Cochrane Database of Systematic Reviews. 2006;(Issue 3) doi: 10.1002/14651858.CD004348.pub2. Art. No.: CD004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donabedian A. The definition of quality and approaches to its assessment. Vol 1. Ann Arbor, MI: Health Administration Press; 1980. [Google Scholar]
- 33.Fultz SL, Skanderson M, Mole L, et al. Development and verification of a "virtual" cohort using the national VA health information system. Medical Care. 2006 Aug;44(8) suppl 2:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 34.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 35.SAS version 9.1. Cary, NC, USA: SAS Institute; [Google Scholar]
- 36.Stata/SE 9.2 for Windows. College Station, TX: StataCorp LP.; [Google Scholar]
- 37.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gange SJ, Kitahata MM, Saag MS, et al. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007 doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goulet JL, Fultz SL, McGinnis KA, et al. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19 Suppl 3:S99–S105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 40.CDC. HIV/AIDS Surveillance Report. 2005;Vol. 17 Rev ed. 2007. [Google Scholar]
- 41.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 43.Kitahata MM, Koepsell TD, Deyo RA, et al. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients. N Engl J Med. 1996;1996:701–706. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- 44.Kitahata MM, Van Rompaey SE, Dillingham PW, et al. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med. 2003;18:95–103. doi: 10.1046/j.1525-1497.2003.11049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 46.Boyko EJ, Koepsell TD, Gaziano JM, et al. US Department of Veterans Affairs medical care system as a resource to epidemiologists. Am J Epidemiol. 2000;151:307–314. doi: 10.1093/oxfordjournals.aje.a010207. [DOI] [PubMed] [Google Scholar]
- 47.Brown SH, Lincoln MJ, Groen PJ, et al. VistA--U.S. Department of Veterans Affairs national-scale HIS. Int J Med Inform. 2003;69:135–156. doi: 10.1016/s1386-5056(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher RD, Dayhoff RE, Wu CM, et al. Computerized medical records in the Department of Veterans Affairs. Cancer. 2001;91:1603–1606. doi: 10.1002/1097-0142(20010415)91:8+<1603::aid-cncr1173>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 49.Murphy PA, Cowper DC, Seppla G, et al. Veterans Health Administration inpatient and outpatient care data: an overview. Eff Clin Pract [serial online] 2002;5 Available at http://www.acponline.org/journals/ecp/mayjun02/murphy.htm. [PubMed] [Google Scholar]