Abstract
Exposure of rats to unpredictable, inescapable stress results in two distinct behaviors during subsequent escape testing. One behavior, suggestive of lack of stress resilience, is prolonged escape latency compared to non-stressed rats and is labeled learned helplessness (LH). The other behavior suggestive of stress resilience is normal escape latency and is labeled non-helpless (NH). This study examines the effects of unpredictable, inescapable tail-shock stress (TSS) on alpha2-adrenoceptor (α2-AR) and corticotropin-releasing factor 1 receptor (CRF1) regulation as well as protein levels of G protein-coupled receptor kinase 3 (GRK3), GRK2, tyrosine hydroxylase (TH) plus carbonylated protein levels in locus coeruleus (LC), amygdala (AMG), cortex (COR) and striatum (STR). In NH rats, α2-AR and CRF1 receptors were significantly down-regulated in LC after TSS. No changes in these receptor levels were observed in the LC of LH rats. GRK3, which phosphorylates receptors and thereby contributes to α2-AR and CRF1 receptor down-regulation, was reduced in the LC of LH but not NH rats. GRK2 levels were unchanged. In AMG, GRK3 but not GRK2 levels were reduced in LH but not NH rats, and receptor regulation was impaired in LH rats. In STR, no changes in GRK3 or GRK2 levels were observed. Finally, protein carbonylation, an index of oxidative stress, was increased in the LC and AMG of LH but not NH rats. We suggest that reduced stress resilience after TSS may be related to oxidative stress, depletion of GRK3 and impaired regulation of α2-AR and CRF1 receptor in LC.
Keywords: Learned Helplessness, GRK3, Alpha2A-AR, CRF1 receptor, Stress, Resilience
1. Introduction
It is well established that individuals are not equally resilient to stress and therefore some suffer greater neuropsychiatric pathophysiology than others[1–3]. Understanding the factors that influence stress resilience may help development of better pharmacotherapies to treat stress-related disorders. Moreover, recent evidence suggests that receptor phosphorylation by G protein-coupled receptor kinases (GRKs) may play a significant role in neuropsychiatric pathophysiology by modulating neurotransmitter signaling in the brain[4–9].
Strong evidence for the importance of stress in the development of affective disorders comes from animal models employed to test pharmacotherapies for these disorders. For example, application of stressors such as forced swim[10], tail shock[1], or tail suspension[10] in rodents all cause behavioral changes that are prevented or reversed by antidepressant drugs. Unpredictable, inescapable tail shock stress (TSS) prolongs escape latency when a rat or mouse is tested 24h later in a shuttle box, a condition called learned helplessness (LH)[1, 11]. However, only about 50% of animals subjected to TSS display LH. The remaining 50% exhibit normal escape latency, and are called non-helpless (NH). Therefore, TSS identifies stress resilient (NH) and stress resilience deficient (LH) groups within a population of stressed animals.
Research in our laboratory for several years has focused on regulation of cellular signaling in response to the stress hormones/neurotransmitters epinephrine (EPI) and corticotropin-releasing hormone by GRK3 [12–14]. GRK3 preferentially causes agonist-dependent phosphorylation of α2-AR and CRF1 receptors, thereby regulating the desensitization and down-regulation of these receptors (CRF1)[13, 15, 16]. Therefore, changes in GRK3 levels in the neurons have the potential to markedly alter signaling by these receptors. For example, a 2-fold increase in GRK3 levels renders the α2-AR 70-fold more sensitive to down-regulation whereas inhibition of GRK3 function can eliminate agonist-induced down-regulation[13]. Regulation of α2-AR and CRF1 receptors signaling by GRK3 is particularly relevant to stress resilience since each receptor is reported to modulate the response to stress.
Locus coeruleus (LC), a major source of noradrenergic inputs to higher brain centers, is activated during stress and is reported to be hyper-responsive in depression[17–19]. GRK3 is highly expressed in LC [20]. In LC, α2-ARs inhibit LC neurons firing while CRF1 receptors stimulate LC neuron firing[21, 22]. Therefore, it is conceivable that GRK3 regulates the balance between inhibitory and excitatory influences on LC. The present study was undertaken to test the hypothesis that stress resilient and stress resilience deficient animals could be identified based upon the effects of TSS on GRK3 and the regulation of α2-AR and CRF1 receptors in brain regions important in the stress response.
2. Materials and methods
2.1 Animals
Adult male Sprague–Dawley rats from Harlan Industries (Indianapolis, IN, USA) were acclimatized for 1–2 weeks before starting the behavioral testing procedures. Rats used were 250–350 g and had free access to food and water. Rats were housed in a humidity and temperature-controlled room (23°C) on a 12-h light/dark cycle (lights on at 7 A.M). All animal-related procedures were approved by the Creighton University Institutional Animal Care and Use Committee/University of Houston Institutional Animal Care and Use Committee and were performed in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Learned Helplessness Model
2.2.1 Inescapable shock paradigm
Rats were subjected to an inescapable shock session using a restrainer. The session consisted of 100 random un-signaled tail shocks from a constant-current (DC) shock generator. The duration of each shock was 5 s, assigned randomly at 25–110 s inter-trial periods (mean inter-trial period was 60s) for approximately 2 hr. The inescapable tail shock started at 1.2mA and was increased by 0.2mA/20 shocks to 2mA. Delivery of the shocks was controlled by software from Med Associates (SOF-735: MED-PC® IV software, Med Associates Inc. St. Albans, VT). After the shock session, rats were returned to their home cages. The rats were initially placed in 2 groups; a tested control (TC) group, which is restrained with electrodes attached to the tail but not shocked, and an inescapably stressed group (TSS). Twenty-four hours after restraint with or without TSS exposure, rats were divided in two sets, one set of rats tested for escape behavior using a shuttle-box test [23, 24], while the other set of rats were sacrificed before being tested for escape behavior.
2.2.2 Escape Behavior Testing
A shuttle box was used to create an escape task to assess responses of all rats. A door separated the two chambers of the box with a plastic divider enabling escape movement for rats to terminate delivery of shocks. Before the test, each rat was acclimated to the box for 2 min. The first 5 fixed ratio-1 (FR1) trials required a single crossing from one side of the box to the other to terminate shock. This was to assess normal motor functioning response to foot shock. After the final FR-1 trial, animals did not receive further shocks for 5 min. The subsequent 25 fixed ratio-2 (FR-2) trials required two crossings from one side of the shuttle box and back to terminate the foot shock. For both FR-1 and FR-2 trials, a foot shock of 0.56mA was randomly applied an inter-trial interval of 30–90 s using software from Med Associates. The time taken to perform the task required for terminating the shock during each trial (single, FR-1, or double crossing, FR-2) was the escape latency. If the rat did not terminate the shock within 30 s for any trial, shock was automatically terminated and an the escape latency of 30 s was recorded for that trial[24].
2.2.3 Data Analysis for Learned Helplessness
Behavioral data were analyzed as described by Reed and coworkers[24]. The escape latency times in the FR-2 trials were averaged to determine the mean escape latency for each rat. Tested control rats (TC; not exposed to inescapable tail shock) were subjected to shuttle box test to determine mean escape latency times of non-stressed rats, i.e. normal behavior. For stressed rats, a mean escape latency time two standard deviations (SDs) greater than the mean escape latency time of TC rats in FR-2 trials, resulted in classification of a rat as learned helpless (LH). Rats with escape latencies within two SD of TC control rats are classified as non-helpless (NH). After assigning rats to the LH or NH group with these criteria, the escape latencies of these groups were significantly different, p<0.05, by the Fischer’s exact test [24, 25].
2.3 Brain Region Isolation and Preparation of Homogenates
2.3.1 Locus Coeruleus
After shuttle box testing, rats were returned to their home cages for 24 hr, and then euthanized under isoflurane anesthesia. The intact brains were removed, frozen on dry ice and stored at −80° C until assay. Isolated brains were equilibrated at −20° C in a cryostat (Leica CM 1850, Leica Microsystems Inc, Bannockburn, IL). The brain stem was removed by making a coronal cut at most 1.0 mm rostral to the cerebellum. The whole brain stem portion was embedded in optimal cutting temperature (OCT) compound (Tissue Tek, Sakura Finetek USA, Inc. Torrance, CA) at −20°C to form a uniform block of OCT/tissue that was adhered to a mounting disc. A series of 300μm sections from the tissue block were generated and sections containing the LC were identified using the floor of the fourth ventricle as a reference. 1 mm2 tissue plugs of LC were punched and transferred to Eppendorf tubes containing 100μl freshly prepared homogenization buffer (400 μL of 25 X complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) + 100 μL of 100 mM PMSF solution + volume up to 10 mL with Tris Buffer- pH 7.4 (10mM Tris Base +0.25M sucrose +1% SDS + 1mM EDTA). 1 mm2 plugs of cerebellum also were taken from the same slices. Samples were analyzed for presence of TH protein and 4-fold enrichment of TH over cerebellum samples from the same slides confirmed LC sample localization. A plastic Dounce homogenization pestle was used to homogenize the tissue manually and the homogenates were boiled at 100°C for 10 min. After cooling, the homogenates were centrifuged at 10,000 rpm/4°C for 10 min. Aliquots of the supernatant were taken to estimate protein content and the remaining sample was diluted with 4X sample buffer (50 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, and 0.1 mg/ml bromophenol blue) and stored at −80°C until analysis by Western Blot.
2.3.2 Cortex (COR), Amygdala (AMG), Striatum (STR)
The remainder of each frozen brain was put on a dry ice-cooled petri dish and a coronal cut at 0.75mm from the rostral end of the brain (ventral side down) was made to separate the COR. A sagital cut was made at 2.9 mm from midline followed by a second cut, an additional 1.5mm from midline. The two 1.4 mm slices from both hemispheres were separated. AMG and STR nuclei regions were isolated and removed from these slices. The isolated tissues were homogenized using freshly prepared homogenization buffer (300 μL for AMG/STR and 400μL for COR). Tissues were homogenized (PRO200 post-mounted laboratory homogenizer; MIDSCI, St. Louis, MO) using a 5 × 75 mm (DXL) flat style probe (2 × 10 sec, 12000 rpm). After this step, homogenates were processed as described previously for LC samples.
2.4 Protein Estimation
A Pierce protein detection kit (Pierce, Rockford, IL) containing BCA protein assay reagent A and reagent B was used to determine protein concentrations[26].
2.5 Western Blot Analysis
Homogenates diluted with 4x sample buffer were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and resolved proteins were electrophoretically transferred at 90V for 2.5 hrs (4°C) to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Biosciences, Piscataway, NJ). The PVDF membrane was then incubated for 1 h in 5% nonfat dry milk in TBS-T (20 mM Tris-HCl (pH 7.6), 137 mM NaCl and 0.1% Tween 20). Membranes were probed for GRK3, GRK2, TH and loading control (GAPDH) by simultaneous stripping (62.5 mM Tris-HCl, pH 6.8, 1% SDS, 100 mM 2-mercaptoethanol), blocking and reprobing the same membrane. The membranes were incubated with rabbit polyclonal anti- GRK3/GRK2 (1:500/1:1000) and antiTH/GAPDH (1:1000) for 1.5 hr. After washing the membranes were further incubated with an anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (GRK3/2; 1:500/1:1000) and anti-mouse HRP-linked secondary antibody (TH/GAPDH; 1:1000/1:1000) at room temperature for 1 hr. The images of immunoblots were captured by a Fluorchem imaging system (Alpha Innotech Corp., San Leandro, CA). Intensity of each immunoreactive band was determined using Alpha Ease FC 4.0 (Alpha Innotech Corp., San Leandro, CA) and were normalized to the GAPDH loading control.
Another set of the same tissue lysates samples were subjected to the same procedure for western blot and probed for α2A-AR. After stripping, the membranes were blocked and probed for CRF1 receptor protein and finally with GAPDH or β-actin for loading control. The membrane was incubated with anti-α2A-AR/CRF1 receptor (1:300/1:500) overnight at 4°C and anti- GAPDH or β-actin (1:1000/1:1000) for 1 hr. After washing the membrane was incubated with an anti-goat HRP-conjugated secondary antibody (α2A-AR/CRF1; 1:500/1:1000) and anti-mouse HRP-linked secondary antibody (GAPDH/β-actin; 1:1000) at room temperature for 1 hr. Images of blots were taken and processed as described above. Anti-GRK3/GRK2 (Rabbit Polyclonal), anti-α2A-AR/CRF1 receptor (goat polyclonal), anti-β-actin (mouse monoclonal), anti-rabbit, anti-mouse antibodies were from Santa Cruz Biotechnology Inc. (Santacruz, CA). Anti-TH/GAPDH (mouse monoclonal) antibodies were from Millipore (Billerica, MA).
2.6 Protein Carbonylation-Marker of Oxidative Stress
Sample containing 20μg of protein was reacted with 2, 4 DNPH reagent. Carbonylated protein reacts to form a hydrazone; the reaction mixture was subjected to SDS-PAGE and resolved carbonylated proteins were detected by immunoblotting as per the Oxyblot (Millipore Billerica, MA) kit protocol. β-actin was detected in membranes after stripping for loading control.
2.7 Data analysis for Western Blots
Normalized intensities of immunoreactive bands were compared between groups by one-way ANOVA followed by Tukey’s post hoc test or Student’s t-test whichever is appropriate (Graph Pad Software, San Diego, CA), and groups were considered significantly different if p ≤ 0.05. Data points were excluded when Grub’s test indicated a statistical outlier at p ≤ 0.05.
2.8 Cluster Analysis
The biochemical (levels of proteins-GRK3, α2A-AR or CRF1) and behavioral (escape latencies) variables were used to independently identify two clusters using K-Means cluster analysis. ANOVA was performed on the TC group and the two identified clusters and the clusters were considered significantly different if p ≤ 0.05. The clusters identified on the basis of individual biochemical parameters alone were then compared with clusters identified by the behavioral parameter to evaluate the accuracy of the biochemical estimations (GRK3, α2A-AR or CRF1) in predicting the behavior of stressed animals. This whole procedure was done for each biochemical parameter in LC, AMG and COR. Cluster analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC). In the experiment wherein rats were sacrificed 24h post-TSS without testing for escape behavior, cluster analysis was used to identify the existence of two groups among the TSS rats. The levels of GRK3, α2A-AR, and CRF1 in each rat LC were used collectively as variables to identify clusters from the group of TSS rats.
3. Results
3.1 Behavioral Segregation of Rats Subjected to TSS
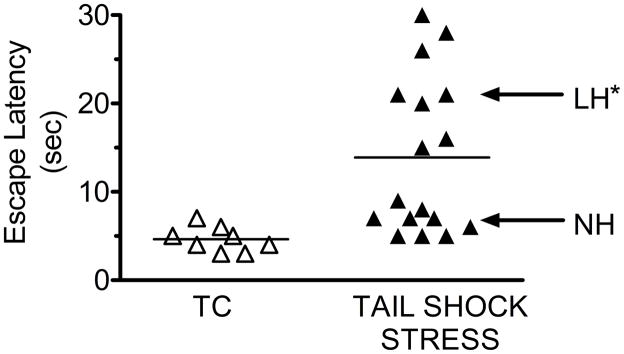
Rats used in this study were randomly separated into two groups. One group, the tested controls (TC), was restrained but did not receive TSS. The remaining group received TSS. Twenty-four hours after TSS or after restraint for TC, mean escape latency was determined by shuttle box testing. Based upon mean escape latencies for all rats subjected to TSS, 2 groups of escape latencies were identified in the TSS rats (Fig. 1). One TSS group had a mean escape latency not significantly different from TC, and was designated the NH group (Fig. 1). The other TSS group had a mean escape latency significantly larger than the TC or NH group, and was designated the LH group.
Figure 1.
Escape latency data for individual rats in the tested control (TC, n=8), and unpredictable inescapable tail shock stress (TSS) groups. Each point represents the FR-2 mean escape latency time (sec) for a single rat in the shuttle box test. Escape latency>13 sec defines a rat as learned helpless (LH, n=8). An escape latency <10 sec defines a rat as non-helpless (NH, n=9). The TSS paradigm resulted in approximately 50% of the rats becoming LH as a result of the TSS. * - LH significantly different from NH, p<0.05.
3.2 GRK3, GRK2 and TH Levels in LC, AMG and COR and STR of LH, NH, and TC Rats
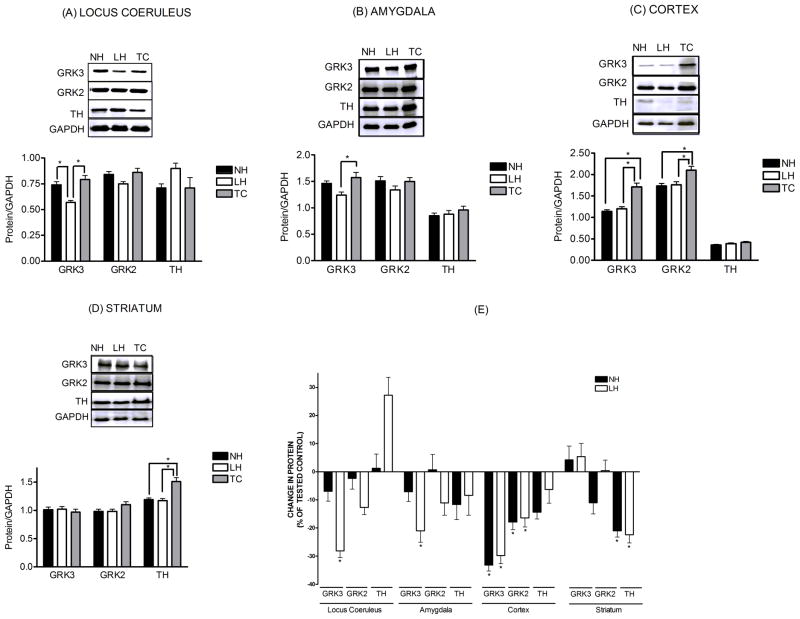
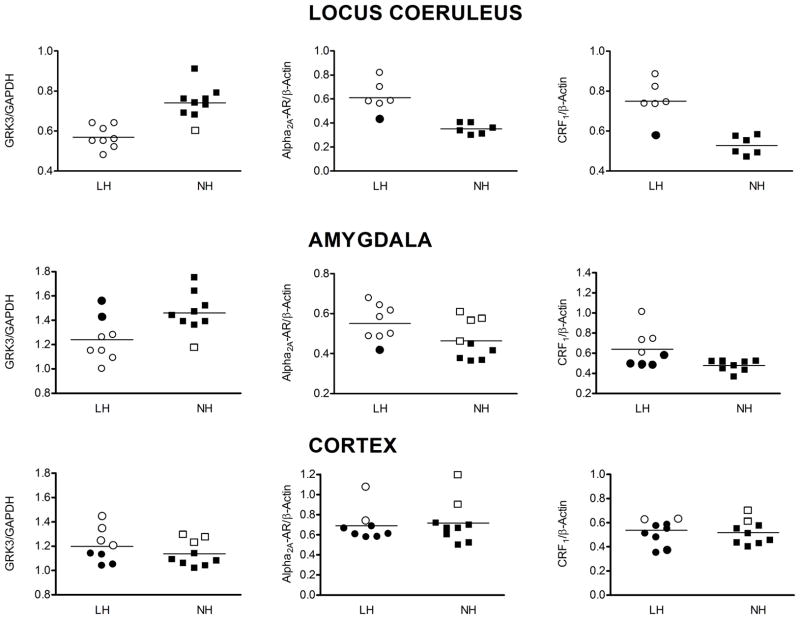
GRK3 levels were significantly reduced in the LC (−28.1%), AMG (−21.0%) and COR (−29.8%) of LH rats compared to TC (Fig. 2). In the LC and AMG these changes were related specifically to the LH group of TSS rats, because GRK3 was unchanged in the LC (−6.6%) and AMG (−7.1%) of the NH group of TSS rats. However, in COR, GRK3 was reduced in both LH and NH (−29.8% and −33.2%) groups compared to TC, suggesting a change that was TSS-induced and unrelated to the behavioral consequence of the TSS. In STR, no changes in GRK3 from TC were observed.
Figure 2.
Levels of GRK3, GRK2 and tyrosine hydroxylase (TH) expressed as a ratio relative to the loading control GAPDH. Shown above the quantified data are representative western blots of GRK3, GRK2 and TH for locus coeruleus, amygdala, cortex, and striatum. Also illustrated are the same data from each brain region, presented as changes in GRK3, GRK2 and TH levels of learned helpless (LH) and non-helpless (NH) rats expressed as a percent of the respective tested controls (TC). All data are presented as the mean± S.E.M., n=8–9. *-Significantly different from tested control (TC), p<0.05.
In contrast to GRK3, GRK2 levels in LC and AMG were unchanged compared to TC (Fig. 2). However, in COR GRK2 levels were reduced in both LH (−16.4%) and NH (−17.9%) groups compared to TC. These decreases were less than the changes in GRK3. This suggests that TSS does not change GRK2 in LC or AMG, and that TSS reduces GRK2 in COR regardless of the behavioral effect of the stress.
Tyrosine hydroxylase (TH) was used in these studies as a marker for catecholamine-containing neurons and particularly to indicate the concentrated presence of noradrenergic neurons in LC. In TC rats, TH levels in LC samples were 4-fold higher than in cerebellar samples punched from the same slice. TH levels were increased by TSS in LC (+27.2%) of LH rats, whereas they were unchanged in AMG (−8.4%) and COR (−6.3%). TH levels were not significantly altered in the LC, AMG and COR of NH rats. Levels of TH were significantly reduced by TSS in the STR (−22.4% and −21.0%), regardless of the behavioral consequence of the stress (Fig. 2).
3.3 Alpha2-AR and CRF1 Receptor Levels in LC, AMG and COR and STR of LH, NH, and TC Rats
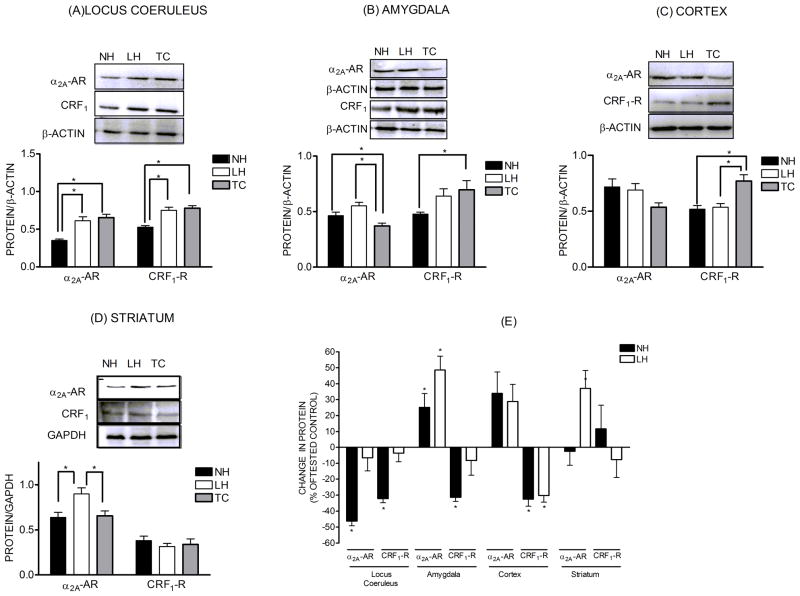
Alpha2-AR levels were significantly down-regulated in the LC of the NH rats (−46.3%) compared to TC (Fig. 3). However, α2-AR levels in the LC of LH rats were unchanged (−6.5%). This suggests that TSS results in an adaptive down-regulation of α2-AR in NH rats that is absent in LH rats. By contrast, TSS resulted in up-regulation of α2-AR in the AMG (+48.6%) and COR (+28.8%) of LH rats. In AMG, the up-regulation was greater in LH rats compared to NH rats (+25.2.0%). In COR, the increases in α2-AR levels were similar in the LH and NH groups (+28.8 vs. +34.0%). In STR, α2-AR levels were increased 37.1% in LH rats while no significant changes were observed in NH rats compared to TC. Therefore, in LC, AMG and STR, TSS produced behaviorally specific changes in α2-AR levels while the effects of TSS on α2-AR levels in COR were similar regardless of the behavioral effects of the stress.
Figure 3.
Levels of α2A-adrenoceptors (α2A-AR) and CRF1 receptors expressed as a ratio relative to the loading control GAPDH or β-actin. Shown above the quantified data are representative western blots of α2A-adrenoceptor and CRF1 receptors for locus coeruleus (n=5–6), amygdala (n=5–6), cortex (n=5–6), and striatum (n=8–9). Also illustrated are the same data from each brain region, presented as changes in α2A-AR and CRF1 receptors levels of learned helpless (LH) and non-helpless (NH) rats expressed as a percent of the respective tested controls (TC). All data are presented as the mean± S.E.M. *-Significant change in protein levels, p<0.05.
Similar to the α2-AR, TSS produced behaviorally specific changes in CRF1 levels in LC and AMG. In both regions, CRF1 receptors were down-regulated in the NH rats (−32.2% and −31.4%) but were unchanged in LH rats compared to TC. No changes in CRF1 receptors were observed the LC (−3.6%) or AMG (−8.2%) in NH rats. Also, TSS produced significant and comparable down-regulation of CRF1 receptors in COR compared to TC regardless of the behavioral effects of the stress (−32.6% and −30.2%). In STR, no changes in CRF1 receptors were observed after TSS.
3.4 Effect of TSS on Carbonylated Protein Levels in LC, AMG, COR and STR
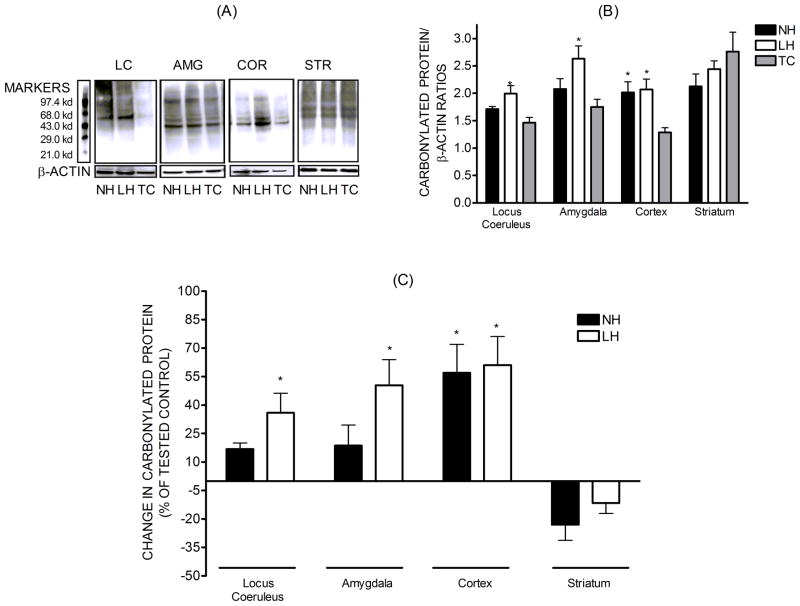
Protein carbonylation results from the oxidative reaction between proteins and reactive oxygen species, forming aldehyde or ketone groups, and is an index of oxidative stress[27]. The levels of carbonylated proteins were greater in the LC and AMG of LH rats compared to TC, while they were unchanged in NH rats. In COR, TSS increased the levels of carbonylated proteins to a similar degree in LH and NH rats (Fig. 4). In STR there were no differences in the levels of carbonylated proteins.
Figure 4.
Levels of carbonylated protein, a marker of oxidative stress, in tissue homogenates of locus coeruleus (n=3), amygdala (n=8–9), cortex (n=7–9), and striatum (n=8–9). The carbonylated protein levels were normalized against β-actin and presented as the mean± S.E.M. Also illustrated are the percent changes in carbonylated protein levels in each brain region relative to the respective TC. * - Significant change in protein levels, p<0.05.
3.5 Segregation of TSS Rats by GRK3, α2-AR, or CRF1 Receptor Levels in LC, AMG and COR After Shuttlebox Testing
K-means cluster analysis was used to define two groups for each of the biochemical markers in LC, AMG and COR of TSS rats. Then, these groups were compared by ANOVA to each other and to the TC. The overlaps of these groups with the stress resilient (NH) and stress resilience deficient (LH) populations identified by escape testing also were considered.
Analysis of GRK3 levels suggested two populations in LC and AMG of TSS rats, one similar to TC and one with reduced GRK3 compared to TC. There was 94% and 82% overlap, respectively, of these groups with the LH and NH groups (Fig. 5A). In LC, all of the samples with reduced GRK3 levels were in the LH group. In AMG 6 out of 8 samples in the group with reduced GRK3 levels compare to TC were in the LH group. In COR, the GRK3 groups identified were not significantly different from each other, although the TSS GRK3 values are different from TC. Collectively these results suggest that GRK3 levels in LC and AMG, and escape testing, will identify two populations of rats with greater than 80% overlap. In each case LH behavior was associated with reduced GRK3 levels.
Figure 5.
K-means cluster analysis for identification of two clusters based on GRK3, α2A-AR, or CRF1 receptor level in locus coeruleus, amygdala or cortex of TSS rats. In each panel, circles represent data points from LH rats while squares represent data points from NH rats as determined by behavioral testing. Open and filled symbols represent the two clusters identified on the basis of the biochemical parameter represented in each panel.
Analysis of α2-AR levels in LC, AMG and COR revealed that in AMG and COR two statistically different groups based upon α2-AR levels could not be identified. In LC, one group of α2-AR levels was similar to TC and the other was significantly lower than TC. Moreover, there was 92% overlap between these groups in LC and the LH and NH groups identified by escape testing. The NH group overlapped with the group with lower α2-AR levels while the LH group overlapped with the α2-AR group not different from TC. In both AMG and COR there were not two groups of α2-AR levels in TSS rats but the α2-AR levels in TSS rats were significantly higher than in TC.
Analysis of CRF1 receptor levels in LC and AMG identified two significantly different groups within the TSS rats. In both areas, one group had CRF1 receptor levels similar to TC while the other group had levels significantly lower than TC. The reduced CRF1 receptor level groups in LC and AMG overlapped 92% and 74%, respectively, with the NH group.
3.6 Segregation of LC data into Two Groups by cluster analysis 24h Post TSS Without Shuttlebox Testing
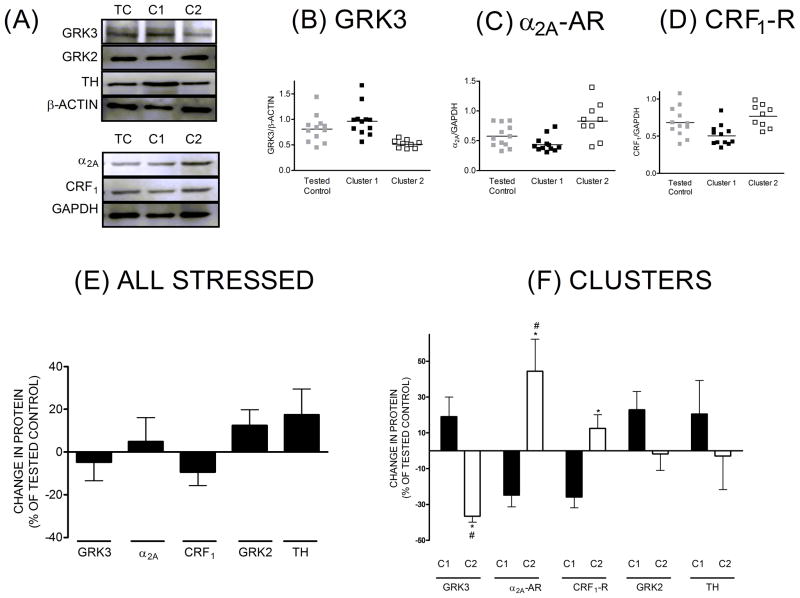
LH rats, as a result of prolonged escape latency relative to NH rats after TSS, receive approximately 4 times as many shocks as NH rats during escape testing in the shuttlebox. Therefore, it is possible that differences in brain biochemistry after TSS and escape testing are an artifact of shuttle box testing, and not a true consequence of TSS leading to differences in escape latency. To address this possibility, rats were subjected to TSS and 24h later, at the time escape testing had been performed in previous groups, brains were harvested and protein levels in the LC were measured (Figure 6). When all stressed rats were compared to TC, there were no significant differences in GRK3, α2A-AR and CRF1-R, GRK2 and TH levels in LC (panel E). However, when the data for GRK3, α2A-AR, CRF1-R for each rats were collectively subjected to K-means cluster analysis, two clusters were identified, cluster C1 having n=12 (57%) and cluster C2 having n=9(43%) (Figure 6, panels B, C, D and F). The total number of stressed animals was 21. The percentage of rats in each identified cluster was similar to the groups identified after behavioral analysis into LH and NH in the previous experiment. After cluster analysis, the data for each variable, GRK3, α2A-AR or CRF1-R, were grouped according to the identified clusters and these clustered data were compared to the TC group for each variable. GRK3 levels in cluster C1 were slightly higher that TC while GRK3 levels in cluster C2 were significantly decreased (−37%) relative to TC (panels B and F). Cluster C1, associated with a slight increase in GRK3, was characterized by non-significant decreases in α2A-AR (−25%) and CRF1-R (−26%) levels when compared to TC. Cluster C2, associated with a significant decrease in GRK3, was characterized by a significant increase in α2A-AR levels (+44%) and a smaller but significant increase in CRF1-R levels (+12%) when compared to TC. In contrast to GRK3, α2A-A or CRF1-R, neither GRK2 nor TH were significantly different from TC, whether grouped as all stressed rats or segregated into the identified clusters at 24h post-stress. Taken together, these data support our hypothesis that TSS identifies two groups of rats, one with significantly reduced levels of GRK3 in LC and the other with unchanged levels of GRK3 in LC, and these groups are present prior to escape behavior testing in the shuttle box.
Figure 6.
Locus Coeruleus Post stress-24h. Representative western blots for GRK3, GRK2, TH, α2A-AR, CRF1-R, and loading controls (A), immunoreactive band intensity for GRK3 (B), α2A-AR (C), CRF1-R (D) proteins normalized to loading controls after cluster identification by cluster analysis in comparison to tested controls, change in proteins for all stressed animals together expressed as % of tested control (E), and change in protein for clusters identified from stressed animals, expressed as % of tested control (F). *-significantly different from tested control (p≤0.05), # - Clusters (C1-cluster 1, C2-Cluster 2) significantly different from each other (p≤0.05).
4. Discussion
The present study was undertaken to test the hypothesis that changes in GRK3 levels in the LC, and perhaps other stress-responsive brain regions, accompany behavioral changes observed after inescapable unpredictable tail shock stress (TSS). Our results indicate that reduced levels of GRK3 in locus coeruleus (LC) and amygdala (AMG) are associated with the development of learned helplessness (LH) in a sub-population of rats subjected to TSS, whereas non helpless (NH) rats exhibit normal GRK3 levels in these brain areas. Moreover, these reduced levels of GRK3 are associated with differences in the regulation of α2-AR and CRF1 receptors in LC and AMG of LH and NH rats. Finally, these changes in GRK3 in LC can be used to independently identify stress resilient NH and non-resilient LH groups within a population of rats following TSS.
Cluster analysis of the levels of GRK3, α2-AR and CRF1 receptors suggests that, of the regions studied, namely LC, AMG, STR and COR, changes in the LC are most predictive of the behavioral effect of inescapable unpredictable TSS. In COR we observed 59, 53, and 53% agreement between the groups identified by cluster analysis for GRK3, α2-AR and CRF1 receptors, respectively, and the groups behaviorally identified as LH and NH. Given that 50% overlap would occur randomly, changes in COR appear not related to LH vs. NH behavior. Rather, the effects in COR appear generally related only to TSS exposure. In AMG, we observed 82, 71 and 77% agreement between the groups identified by cluster analysis for GRK3, α2-AR and CRF1 receptors, respectively, and the LH and NH groups. This overlap is considerably lower than the 94, 92, and 92% agreement for GRK3, α2-AR and CRF1 receptors, respectively, that we observed in LC. We therefore conclude that, amongst the brain regions we studied, the changes in GRK3, α2-AR and CRF1 receptors in LC are most closely associated with the development of LH or NH behavior after TSS and therefore the remainder of the discussion will focus on LC.
The suggestion that LC plays a critical role in LH and stress resilience is compatible with a significant body of literature. First, LH is the result of the application of inescapable unpredictable stress and inescapable stress results in marked activation of the LC [28]. Second, administration of α2-AR agonists into the LC can reverse the behavioral effects of exposure to inescapable stress[29]. Third, intra-LC administration of α2-AR antagonists produces behavioral effects that mimic those of inescapable stress[30]. Finally, chronic treatment with many currently employed antidepressants agents reduces the activity of LC neurons[17, 30]. A role of the LC in determining stress resilience also has been suggested by work related to gender and stress. For example, Valentino and co-workers recently reported that sex differences in the regulation of CRF1 receptor signaling in LC may play a role in female vulnerability to stress-related psychopathology [31]. This group also has suggested that CRF in LC may contribute to the role of stress in vulnerability to opiate abuse[32]. Therefore, the results of the present study add to the evidence supporting a role for the LC in the development of psychopathology as a result of stress.
Depletion of GRK3 and the accompanying changes in α2-AR and CRF1 receptor regulation in the LC in LH rats support a role of GRK3 as a regulator of stress resilience in LC. GRK3 modulates receptor signaling by catalyzing the agonist-induced phosphorylation of receptors, facilitating the recruitment of arrestin to the receptor. This process initiates G protein-mediated signaling desensitization[33, 34]. CRF1 receptors activate LC neurons, increasing neuronal firing, whereas α2-AR inhibits the firing of LC neurons[35, 36]. Therefore, depletion of GRK3 in LC would reduce the capacity of GRK3 to desensitize the signaling of both an excitatory and an inhibitory input to the LC. Because hyper-responsiveness of the LC neurons is associated with the behavioral consequences of inescapable stress, we hypothesize that rats resistant to LH would be able to make adaptive changes to limit LC hyper-responsiveness. This could be accomplished by either reducing the response to stimulatory inputs by CRF1 or by enhancing the response to inhibitory inputs via the α2-AR. We observed down-regulation of both the α2-AR and CRF1 receptors only in the stress resilient NH rats that do not develop LH. In rats that developed LH, GRK3 levels were reduced and there were no α2-AR or CRF1 receptor changes compared to TC rats. It is tempting to speculate that the changes in α2-AR and CRF1 receptors in NH rats contribute to a net decrease in LC responsiveness, preventing the hyper-responsiveness of the LC that is characteristic of LH animals. The fact that inhibitory α2-AR are down-regulated in the LC of resilient NH rats appears inconsistent with this suggestion. However, α2-AR in LC are present prejunctionally on recurrent collateral neurons and inhibit the release of NE onto LC neurons, and postjunctionally on LC neurons where they inhibit neuronal firing. Reduced prejunctional inhibition would increase NE release and enhance NE inhibition of the LC, while reduced postjunctional inhibition would reduce NE inhibition of LC. Direct assessment of neuronal responses to α2-AR and CRF1 receptors alone and in combination is required to determine the precise consequences of the changes in receptors to the firing of LC neurons.
The suggestion that reduced levels of GRK3 after TSS should be associated with the negative consequences of stress is not universally supported by the literature. For example, compared to wild type mice[37], GRK3 knockout mice exhibit reduced immobility, or reduced depression-like behavior after forced swim. This effect of GRK3 was localized to the nucleus accumbens, where GRK3 phosphorylation of the kappa opioid receptor was required for activation of p38 MAP kinase by the kappa-opioid receptor. Kappa opioid receptor activation also was required for increased immobility in response to forced swim stress[38]. Therefore, in nucleus accumbens activation of p38 MAP kinase was eliminated by GRK3 knock out. Because activation of kappa opioid receptors in nucleus accumbens causes aversive behavior that could contribute to the increased immobility after forced swim, we hypothesize that this explains the results in GRK3 knockout mice. Moreover, CRF injection into LC enhances swimming. This response to CRF in LC of GRK3 knock out mice could be enhanced and could antagonize the effect of forced swim on immobility[39,45]. The suggestion of an opposite effect of GRK3 depletion on receptor response, depending on brain region, is supported by previous studies. For example, GRK3 depletion by knock out enhances kappa opioid response in other regions of the central nervous system. Spinal neuropathic pain, which requires kappa opioid receptor desensitization, is not observed in GRK3 knock out mice[39]. Therefore, GRK3 may differentially effect GPCR signaling depending on the regional localization. We suggest that in LC GRK3 participates in the termination of G protein mediated signaling by the α2-AR and CRF1 receptors.
An important question is why were levels of GRK3 reduced in LC of LH rats after TSS. Previous reports indicate that inescapable unpredictable TSS causes pronounced activation of the LC[19]. Our observation that an index of oxidative stress, protein carbonylation, was increased in the LC only in LH rats may help explain this difference. We and others have investigated the regulation of cellular GRK3 levels[14, 40, 41]. Activation of CRF1 receptors leads to increased expression of GRK3 in cultured CATH.a neuronal cells, a mouse LC tumor cell line[14]. In addition, increased intracellular calcium, as might be expected during excessive activation of neurons and oxidative stress, is associated with increased degradation of GRK3 in neurons[42]. Supporting a role for oxidative stress in the development of LH, voluntary wheel running for 6 weeks prior to TSS prevents LH after TSS[43]. In addition, oxidant treatment causes anxiety-associated behaviors and oxidative stress in LC and involuntary moderate treadmill exercise eliminated both the anxiety-associated behavior and the oxidative stress in LC[44]. Therefore, we hypothesize that increased susceptibility to oxidative stress in LC after TSS leads to depletion of GRK3, dysregulation of α2-AR and CRF1 receptor signaling, and development of LH.
A final outcome of the present study is the suggestion that reduced levels of GRK3 in LC after TSS can be used as a marker to identify a stress resilience deficient group within a population of rats independent of any behavioral testing. Unfortunately, since we measured protein levels in LC and other brain regions after escape testing, where LH rats received many more shocks than NH rats, the changes in GRK3 and other proteins in the LH group could be a consequence of the escape testing and not the TSS alone. However, this does not appear to be the case as we demonstrated that GRK3 levels in LC at 24h after TSS, the time escape testing was performed in other rats, can be segregated into two groups based upon cluster analysis of collective GRK3, α2-AR and CRF1 receptor data in LC. Furthermore, the data in these clusters appear very similar to the data in the LH and NH groups identified by escape testing. Therefore future studies can focus on the time course for the development and disappearance of the behavioral changes after TSS relative to biochemical changes in the brain. For example, if oxidative stress in the LC and depletion of GRK3 are observed quickly after TSS, followed by changes in receptor regulation and LH behavior, this would suggest a contribution of these changes in signaling regulation to the stress-induced changes in behavior which are observed as LH. Moreover, could maintenance of GRK3 levels in LC increase stress resilience or could reduction of GRK3 levels in LC reduce stress resilience?
5. Conclusion
In summary, the present study suggests that reduced levels of GRK3 in LC after TSS lead to deficits in stress resilience and contribute to the development of LH behavior. Reduced stress resilience also is associated with lack of alterations of α2-AR and CRF1 receptor regulation in the LC after TSS, and increased oxidative stress within the LC. Future studies will be directed towards determining if changes in GRK3 levels in LC are indeed a cause for development of learned helplessness and whether interventions that enable GRK3 levels in LC to be maintained may reduce or prevent the development of LH.
Acknowledgments
The work was supported by AHA TX 0555032Y and NARSAD grant awarded to Douglas C Eikenburg, MH077224 grant awarded to David B Bylund and VA Merit Award granted to Fredrick Petty and H Kevin Happe.
Abbreviations
- AR
Adrenoceptor
- CRF1
Corticotropin-releasing factor 1 receptor
- LH
Learned Helpless
- NH
Non-Helpless
- TC
Tested Control
- LC
Locus Coeruleus
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- TH
Tyrosine hydroxylase
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:435–46. [PubMed] [Google Scholar]
- 2.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MR, Bychkov E, Gurevich VV, Benovic JL, Gurevich EV. Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment. J Neurochem. 2008;104:1622–36. doi: 10.1111/j.1471-4159.2007.05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, et al. Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. Journal of Neuroscience. 2009;29:1855–9. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grange-Midroit M, Garcia-Sevilla JA, Ferrer-Alcon M, La Harpe R, Huguelet P, Guimon J. Regulation of GRK 2 and 6, beta-arrestin-2 and associated proteins in the prefrontal cortex of drug-free and antidepressant drug-treated subjects with major depression. Brain Res Mol Brain Res. 2003;111:31–41. doi: 10.1016/s0169-328x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- 8.Ertley RN, Bazinet RP, Lee HJ, Rapoport SI, Rao JS. Chronic treatment with mood stabilizers increases membrane GRK3 in rat frontal cortex. Biol Psychiatry. 2007;61:246–9. doi: 10.1016/j.biopsych.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Barrett TB, Emberton JE, Nievergelt CM, Liang SG, Hauger RL, Eskin E, et al. Further evidence for association of GRK3 to bipolar disorder suggests a second disease mutation. Psychiatr Genet. 2007;17:315–22. doi: 10.1097/YPG.0b013e3282efeeb4. [DOI] [PubMed] [Google Scholar]
- 10.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–8. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Bawa-Khalfe T, Altememi GF, Mandyam CD, Schwarz LA, Eikenburg DC, Standifer KM. The presence of beta2-adrenoceptors sensitizes alpha2A-adrenoceptors to desensitization after chronic epinephrine treatment. BMC Pharmacol. 2007;7:16. doi: 10.1186/1471-2210-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai AN, Standifer KM, Eikenburg DC. Cellular G protein-coupled receptor kinase levels regulate sensitivity of the {alpha}2b-adrenergic receptor to undergo agonist-induced down-regulation. Journal of Pharmacology and Experimental Therapeutics. 2005;312:767–73. doi: 10.1124/jpet.104.076042. [DOI] [PubMed] [Google Scholar]
- 14.Salim S, Hite B, Eikenburg DC. Activation of the CRF(1) receptor causes ERK1/2 mediated increase in GRK3 expression in CATH.a cells. FEBS Lett. 2007;581:3204–10. doi: 10.1016/j.febslet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Dautzenberg FM, Braun S, Hauger RL. GRK3 mediates desensitization of CRF1 receptors: a potential mechanism regulating stress adaptation. Am J Physiol Regul Integr Comp Physiol. 2001;280:R935–46. doi: 10.1152/ajpregu.2001.280.4.R935. [DOI] [PubMed] [Google Scholar]
- 16.Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochemical and Biophysical Research Communications. 2000;268:572–6. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- 17.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 18.Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, et al. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–86. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 19.Weiss JM, Simson PG. Depression in an animal model: focus on the locus ceruleus. Ciba Found Symp. 1986;123:191–215. doi: 10.1002/9780470513361.ch11. [DOI] [PubMed] [Google Scholar]
- 20.Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Hollt V. Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res. 2001;95:129–37. doi: 10.1016/s0006-8993(01)03046-3. [DOI] [PubMed] [Google Scholar]
- 21.Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. Journal of Pharmacology and Experimental Therapeutics. 1997;281:163–72. [PubMed] [Google Scholar]
- 22.Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience. 1999;93:1263–70. doi: 10.1016/s0306-4522(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 23.Petty F, Chae Y, Kramer G, Jordan S, Wilson L. Learned helplessness sensitizes hippocampal norepinephrine to mild restress. Biol Psychiatry. 1994;35:903–8. doi: 10.1016/0006-3223(94)91235-1. [DOI] [PubMed] [Google Scholar]
- 24.Reed AL, Anderson JC, Bylund DB, Petty F, El Refaey H, Happe HK. Treatment with escitalopram but not desipramine decreases escape latency times in a learned helplessness model using juvenile rats. Psychopharmacology (Berl) 2009;205:249–59. doi: 10.1007/s00213-009-1535-2. [DOI] [PubMed] [Google Scholar]
- 25.Petty F, Kramer G, Wilson L. Prevention of learned helplessness: in vivo correlation with cortical serotonin. Pharmacol Biochem Behav. 1992;43:361–7. doi: 10.1016/0091-3057(92)90163-a. [DOI] [PubMed] [Google Scholar]
- 26.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 27.Goto S, Nakamura A, Radak Z, Nakamoto H, Takahashi R, Yasuda K, et al. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech Ageing Dev. 1999;107:245–53. doi: 10.1016/s0047-6374(98)00133-x. [DOI] [PubMed] [Google Scholar]
- 28.Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, et al. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Simson PG, Weiss JM, Hoffman LJ, Ambrose MJ. Reversal of behavioral depression by infusion of an alpha-2 adrenergic agonist into the locus coeruleus. Neuropharmacology. 1986;25:385–9. doi: 10.1016/0028-3908(86)90232-7. [DOI] [PubMed] [Google Scholar]
- 30.Weiss JM, Boss-Williams KA, Moore JP, Demetrikopoulos MK, Ritchie JC, West CH. Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin. Neuropeptides. 2005;39:281–7. doi: 10.1016/j.npep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Research. 2010;1314:162–74. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premont RT. Once and future signaling: G protein-coupled receptor kinase control of neuronal sensitivity. Neuromolecular Med. 2005;7:129–47. doi: 10.1385/NMM:7:1-2:129. [DOI] [PubMed] [Google Scholar]
- 34.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochimica et Biophysica Acta. 2007;1768:913–22. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Research. 1983;270:363–7. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- 36.Mateo Y, Pineda J, Meana JJ. Somatodendritic alpha2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J Neurochem. 1998;71:790–8. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- 37.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. Journal of Neuroscience. 2007;27:11614–23. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, et al. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. Journal of Neuroscience. 2004;24:4576–84. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dautzenberg FM, Wille S, Braun S, Hauger RL. GRK3 regulation during CRF- and urocortin-induced CRF1 receptor desensitization. Biochemical and Biophysical Research Communications. 2002;298:303–8. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- 41.Thakker DR, Standifer KM. Induction of G protein-coupled receptor kinases 2 and 3 contributes to the cross-talk between mu and ORL1 receptors following prolonged agonist exposure. Neuropharmacology. 2002;43:979–90. doi: 10.1016/s0028-3908(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 42.Salim S, Eikenburg DC. Role of 90-kDa heat shock protein (Hsp 90) and protein degradation in regulating neuronal levels of G protein-coupled receptor kinase 3. Journal of Pharmacology and Experimental Therapeutics. 2007;320:1106–12. doi: 10.1124/jpet.106.114835. [DOI] [PubMed] [Google Scholar]
- 43.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. Journal of Neuroscience. 1990;10:176–83. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]