Abstract
Sildenafil, a phosphodiesterase-5 inhibitor, and simvastatin, a cholesterol lowering drug, both have therapeutic effects on PAH; however, the combination of these drugs has not been tested in the treatment of PAH. The purpose of this study was to determine whether the combination of sildenafil and simvastatin is superior to each drug alone in the prevention of MCT-induced PAH. Phosphorylated Smad levels were decreased in lung tissue in MCT-injected rats, whereas ERK protein levels were increased. This indicates a possible role for an increase in mitogenic ERK activity in addition to decreased proapoptotic Smad signaling in the MCT model of PAH. Combination sildenafil and simvastatin treatment prevented the MCT-induced increases in right ventricular systolic pressure (RVSP) and right ventricular hypertrophy (RVH), exerted an antiproliferative effect on pulmonary artery smooth muscle cells (PASMC). Our results indicate that combination therapy with sildenafil and simvastatin attenuated the development of pulmonary hypertension more than either treatment alone.
Keywords: monocrotaline, pulmonary arterial hypertension, vascular remodeling, simvastatin, sildenafil
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by elevated pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP). If left untreated, PAH leads to right heart failure and death. Although there is no single cause of PAH, it is widely believed to involve a combination of both genetic and environmental factors that result in an imbalance between both vasoconstrictive and proliferative signals and competing vasodilatory and apoptotic factors. Recently, PAH has become increasingly recognized as a chronic proliferative disease, particularly because of the extensive vascular remodeling of the small pulmonary arteries [1–2]. Pathologically, PAH is characterized by dramatic changes in the structure and function of the pulmonary microcirculation, particularly at the level of the distal arteriolar bed. A common pathological finding of PAH is hypertrophy of the medial smooth muscle layers. Obstruction of the arteriolar lumen, which causes the rise in PVR and PAP, occurs through a combination of endothelial cell dysfunction, chronic vasoconstriction, thrombosis, fibrosis, and pulmonary arteriolar remodeling [1–2].
At the molecular level, defects in bone morphogenetic protein (BMP) signaling have been linked to PAH [3–7]. The two main pathways downstream of BMP signaling are the Smad-dependent pathway, which uses Smad signaling proteins, and the Smad-independent pathway which involves p38, MAPK, ERK, and JNK proteins [7, 8]. In PASMC, Smad signaling, activated by TGF-β and BMP ligands for example, is proapoptotic, whereas signaling through MAPK is mitogenic [9, 10]. The balance between the two pathways normally maintains homeostasis in the lung vasculature and prevents overproliferation of PASMC; however it is thought that in PAH patients, defective Smad signaling allows for the unopposed influence of the MAPK pathway and thus an overproliferation of PASMC.
Current treatments target three pathways that, through their dysfunction, lead to an increase in pulmonary vascular tone: endothelin receptors, nitric oxide (NO) and prostacyclin. In recent years, several treatments, including prostanoids such as oral beraprost and inhaled iloprost, endothelin receptor antagonists such as bosentan and ambrisentan, and the phosphodiesterase (PDE)-5 inhibitor sildenafil (Revatio) have been shown to improve exercise capacity and hemodynamics of PAH patients over short term use [11–14]. However, over the long term, many PAH patients fail to maintain clinical stability using a single drug while others remain refractory to single treatments; these patients deteriorate rapidly. Furthermore, current treatments improve patients’ symptoms without curing the disease.
Combination therapy of two or more conventional PAH drugs has been proposed in treatment algorithms for idiopathic PAH (IPAH) [15]. Approximately 43% PAH patients need combination therapy with a two-drug regimen and ~16% require a combination of three drugs to achieve the desired clinical stability [16]. Various combination therapeutic approaches have been reported and show great promise in clinical trials, case reports and animal studies [17–23]. However, these combinations may be prohibitively expensive for individual patients, and some patients fail to improve on combination treatment. It is therefore important to find a combination approach that is both effective and accessible.
Molecularly, sildenafil prolongs the vasodilator effects of NO by inhibiting PDE-5 and thus stabilizes cyclic guanosine monophosphate (cGMP), the second messenger of nitric oxide (NO). Clinically, it improves PAH patients’ symptoms and exercise capacity [14]. An overcirculation-induced animal model of pulmonary hypertension has shown that sildenafil can increase BMPR-Ia levels [24]. Although statins are not included in traditional PAH therapy, simvastatin has been shown to decrease monocrotaline (MCT)-induced pulmonary hypertension in rats and to improve their survival rates [25]. Simvastatin has also been shown to enhance the expression of BMPR-II in cultured human cells [26]. Here we determine that the combination use of sildenafil and simvastatin is more effective at preventing the development of MCT-induced PAH than either of the drugs used alone in the rat MCT model of pulmonary hypertension.
Materials and Methods
Experimental protocols and treatments
All experiments were approved by the Animal Policy and Welfare Committee of the Capital Medical University of China. Forty adult male Sprague-Dawley rats (220–250 g) were randomized to MCT (60 mg/kg) or 0.9% saline subcutaneous injection and assigned to be orally administered distilled water, sildenafil, simvastatin, or both of these drugs. There was a 0% mortality rate in both the 50 mg/kg and the 60 mg/kg MCT groups over a time course of 4 weeks. We therefore used the higher dosage (60 mg/kg) to study the effects of treatment on MCT-induced pulmonary hypertension as these effects were more apparent with the more severe pulmonary hypertension induced by the higher MCT dosage. This protocol resulted in the creation of five groups (each group, n=8): saline-injected rats (control group) and MCT-injected rats given distilled water (sham-treated group), MCT rats treated with oral sildenafil (sildenafil group), MCT rats treated with oral simvastatin (simvastatin group), and MCT rats treated with both sildenafil and simvastatin (combination group). After the subcutaneous injections on the first day of the experiment, distilled water, sildenafil (20 mg/kg/day; Pfizer Pharmaceuticals Limited), simvastatin (7 mg/kg/day, Hangzhou MSD Pharmaceutical Co., Ltd.), or both of these drugs were administered orally once a day for 3 weeks. All animals were fed standard rat chow.
Measurements of hemodynamics and plasma levels of NO, cGMP, BNP, t-PA, PAI-1, MMP-2 and MMP-9
Rats were anesthetized with intraperitoneal pentobarbital (30 mg/kg). A polyethylene tube (outside diameter = 0.99 mm, Scientific Commodities, Inc.) was inserted through the right jugular vein into the right ventricle (RV) for measurement of RV systolic pressure (RVSP); these measurements were made with BIOPAC MP150 and the Acqknowledge 3.81 data analysis system. For the treatment experiments, hemodynamic studies were performed 22 days after MCT (or saline) injection. After RVSP was measured, blood was collected from the animals for determination of plasma NO, cGMP, brain natriuretic peptide (BNP), tissue plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), and matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9). Blood samples for the determination of these substances were collected in the following conditions and measurements made using the kits indicated: NO (1% heparin; human NO kit, Nanjing Institute of Juli Biomedical Engineering); cGMP (5% EDTA; cGMP direct enzyme immunoassay kit, Sigma); BNP (5% EDTA; enzyme immunoassay kit of peptides, Phoenix Peptide); t-PA and PAI-1 (1/10 volume 10.9 mM sodium citrate; t-PA and PAI-1 test kits, Sunbiote Inc.); and MMP-2 and MMP-9 (1/10 volume 10.9mM sodium citrate; MMP2 kit, Calbiochem; MMP9 kit, R&D Systems, Inc.). Cervical dislocation was then performed and hearts and lungs harvested using DEPC-treated tools. For RVH determination, the ratio of the wet weight of RV to left ventricle plus septum (RV/LV+S) was calculated.
Histology
Isolated right lungs were inflated with 10% formalin, fixed and then embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (HE) and Weigert elastic stain. At least 2 separate lung sections from each rat were examined and pulmonary arterioles [external diameter (ED): 50–150 μm] were randomly chosen for analysis with the IDA-2000 digital image analysis system (Konghai Co.). Parameters, including total area, luminal area, ED and inner diameter (ID) were measured from 8–10 arterioles per animal. Medial thickness (MT) was calculated as (ED-ID)/2 and wall area as total area-luminal area. Percent of MT relative to total wall area was calculated as 2×MT×100/ED and percent wall area as (total area-luminal area)×100/total area. PASMC proliferation was measured by proliferating cell nuclear antigen (PCNA) stain. At least 2 separate lung sections from each rat were examined and 8–10 resistance pulmonary arterioles were randomly chosen for analysis. The PCNA index, calculated as the number of cells staining positive divided by the total number of PASMC in the resistance pulmonary arteriole, was used as an indicator of cell proliferation.
Immunoblot analysis for p-Smad1 and total ERK protein levels
Left lung tissue samples were lysed in a buffer composed of 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM α-Glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF at 4°C. Protein concentrations were quantified by Bradford protein assay using bovine serum albumin as a standard. The protein was then subjected to SDS-PAGE and electrophoretically transferred to nitrocellulose membranes (Bio-Rad). The blot was incubated for 1 hr at 4°C in blocking buffer (10 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Tween 20, and 5% nonfat milk) and then incubated overnight with anti-rabbit phosphorylated Smad1 (p-Smad1) antibody (Santa Cruz, 1:1000) or anti-goat ERK (ERK1/ERK2) antibody (Cell Signaling, 1:1000). Detection was performed using the enhanced chemiluminescence kit (Amersham) after incubation with a horseradish peroxidase-conjugated anti-goat IgG (Santa Cruz, 1:2,000) or anti-rabbit IgG (Santa Cruz, 1:2,000). The relative expression of p-Smad1 and ERK proteins were normalized relative to a-tubulin (Santa Cruz, 1:1,000).
Statistical Analysis
The statistical software SPSS 11.5 for Windows was used for data analysis. Data are presented as mean ± SD. Differences between groups were analyzed by one way ANOVA and the Student-Newman-Keul post-hoc test for multiple comparisons, with a probability value P<0.05 regarded as significant and P<0.01 regarded as very significant.
Results
MCT induces vascular remodeling and pulmonary hypertension 3 weeks after injection
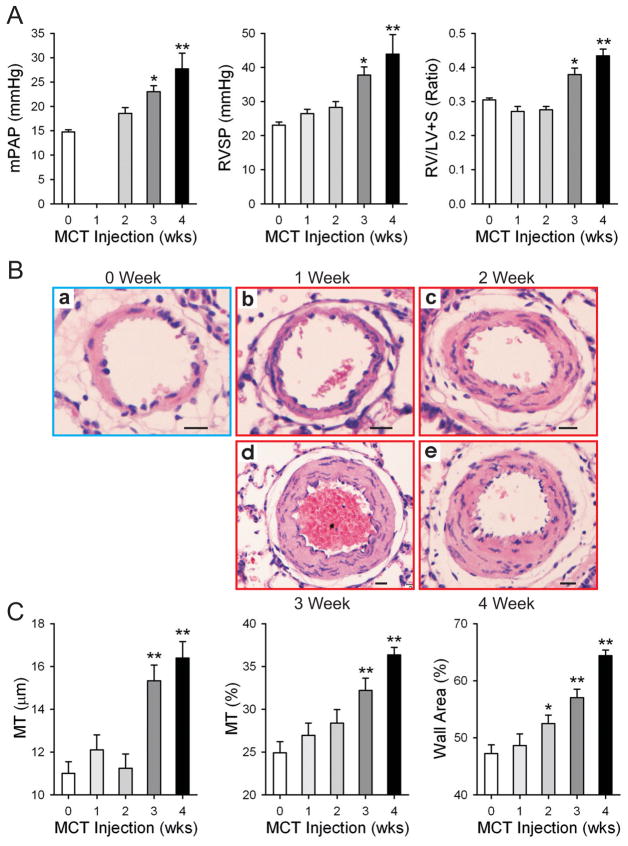
In order to establish a time course of MCT-induced pulmonary hypertension, we examined rats 1–4 weeks after injection with MCT and compared them to saline injected control animals which were examined on the first day of the experiment. Compared to these controls, MCT-injected rats had increased RVSP, and mean PAP (mPAP), as early as 1 week post-injection, with a significant increase first observed 3 weeks after injection that continued into week 4 (MCT: 26.5±3.6 mmHg, 28.3±4.8 mmHg, 37.8±6.7 mmHg, 43.9±16.2 mmHg for weeks 1–4 respectively vs. Control: 23.1±3.0 mmHg) (Fig. 1A and B). Both RV hypertrophy (RVH) (as measured by the RV:LV+S weight ratio) and mPAP followed a trend similar to that observed for RVSP, with a significant increase first observed 3 weeks after MCT injection and a further increase at 4 weeks (Fig. 1B and C). In addition to the increases in RVSP, RVH and mPAP, we also observed an increase in the muscularization of resistance pulmonary arterioles at 3 weeks (Fig. 1B), marked by significant increases in medial thickness (16.4±2.2 μm for the 4 week MCT group vs. 11.0±1.7 μm for the control group), percent medial thickness (36.3±2.4% for 4 week MCT group vs. 24.9±4.2% for control), and percent of wall area (64.4±2.8% for 4 week MCT group vs. 47.2±5.0%) which continued to worsen into week 4 (Fig. 1C).
Figure 1. MCT induces pulmonary hypertension and vascular remodeling 3 weeks after injection.
mPAP, RVSP and RVH (RV/LV+S wet weight ratio) were measured in rats sacrificed at the times indicated after MCT injection (A). The week 0 group was injected with saline and measurements taken the subsequent day. Representative hematoxylin and eosin (H&E) staining of cross sections of resistance pulmonary arterioles is shown in (B). Scale bas = 10 μm. Based on digital analysis of these images, medial thickness (MT), MT percent (MT/2%) and percent wall area were calculated; the summarized data for the different groups are presented in (C). Data are presented as mean ± SD; n=8/group; * P<0.05 vs. 0 week control group; ** P<0.01 vs. 0 week control group.
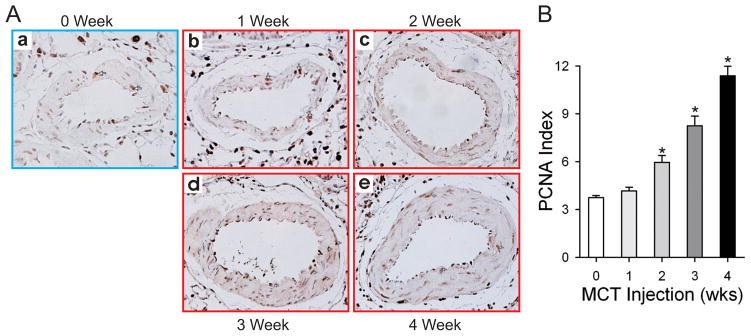
Evidence of vascular remodeling is readily apparent in representative histological sections prepared from rats sacrificed at different time points after MCT injection (Fig. 1B and Fig, 2A). Compared to the control group, rats at 2 weeks after injection also had a significantly increased pulmonary artery PCNA index; this trend continued into weeks 3 and 4 (6.0±1.2%, 8.2±1.7%, 11.4±1.7% for MCT 2–4 week groups respectively vs. 3.7±0.4% for saline injected control) (Figure 2). Thus, MCT induces pulmonary hypertension 3 weeks after injection.
Figure 2. MCT-induced PH is characterized by increased pulmonary artery smooth muscle cell proliferation.
Lung sections from each rat in the different groups were fixed in formalin and embedded in paraffin. PCNA staining was used to indicate pulmonary artery smooth muscle cell proliferation over the time course indicated. Representative sections are shown in (A) and the summarized data in (B). Data are presented as mean ± SD; n=8/group; * P<0.05 vs. 0 week group.
Changes in lung tissue levels of Smad1 and ERK1 in MCT-induced pulmonary hypertension
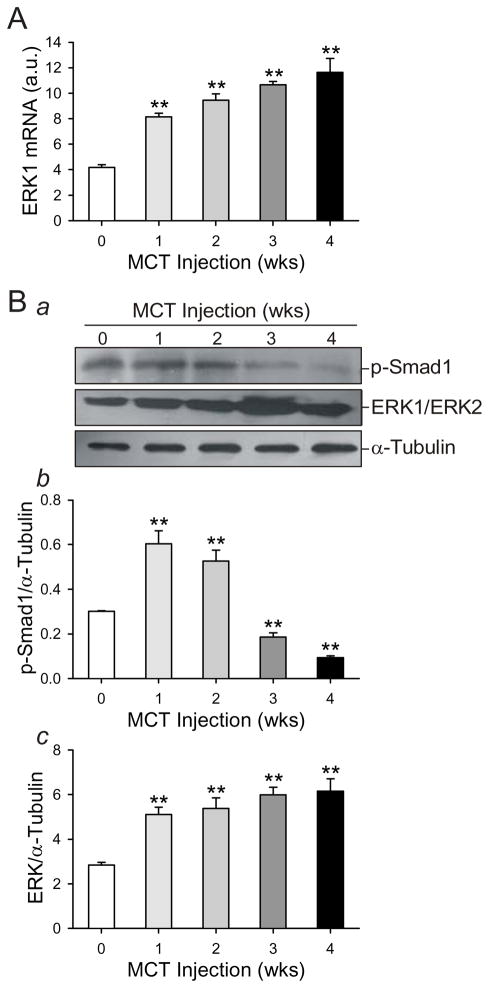
Proapoptotic BMP signaling results in the phosphorylation of Smad1. We were therefore interested in examining levels of phosphorylated Smad1 (p-Smad1) protein in our rat model. Levels of p-Smad1 in lung tissue were significantly higher in both the week 1 and week 2 groups compared to control (0.60±0.15, 0.52±0.13; 0.30±0.01, respectively) (Fig. 3Ba and Bb). Animals from the week 3 and week 4 groups had significantly decreased p-Smad1 levels compared to the control group (0.18±0.05 for MCT week 3 group, 0.09±0.02 for MCT week 4 group vs. 0.30±0.01 for control) (Fig. 3Ba and Bb). Our observations from the MCT model of pulmonary hypertension in the rat accord with previously published studies [27, 28]. We also examined ERK1 mRNA levels in lung tissues from rats treated with MCT. Compared with the rats in 0 week group, MCT induced a significant and nearly two fold increase in ERK1 mRNA as early as one week after MCT injection (8.15±0.79 MCT week 1 group vs. 4.18±0.63 control), and ERK1 mRNA levels continued to rise throughout the entire time course (Fig. 3A). Total ERK protein (ERK1/ERK2) levels paralleled this pattern (Fig. 3Ba and Bc). Thus, the effects of MCT on both hemodynamic and molecular indicators of PAH are evident 3 weeks after injection.
Figure 3. MCT induces an increase in ERK protein levels and a decrease in activated (or phosphorylated) Smad1 protein in whole rat lung.
mRNA and protein were collected from whole lung from rats injected with MCT at the indicated timepoints after injection. The week 0 group was injected with saline, and protein and mRNA was collected the next day. mRNA was used in RT-PCR to quantify ERK1 mRNA levels (A) using the primer listed in Table 1. This signal was normalized against GAPDH mRNA levels. Protein lysate resolved electrophoretically was transferred to a nitrocellulose membrane which was immunoblotted for phosphorylated Smad1 (p-Smad1) and ERK. Representative gels are shown in (Ba) and summarized data in (Bb and Bc). Protein signals were normalized to a-tubulin protein levels. Data are presented in arbitrary units (a.u.) as mean ± SD; n=5/group; ** P<0.01 vs. 0 week control group.
Comparison of time courses of MCT-mediated changes in Smad/ERK and hemodynamic properties
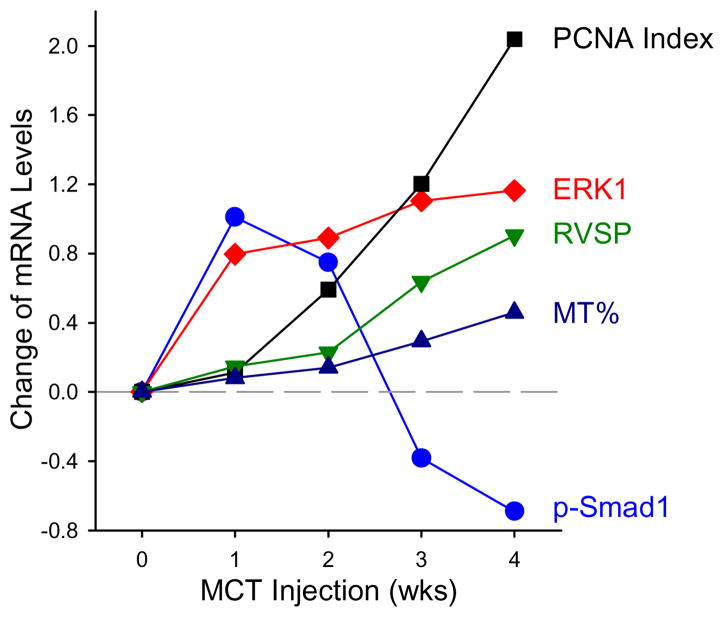
Our observations indicate that MCT injection caused a transient increase in Smad1 phosphorylation (Fig. 3). However, MCT injection caused a sustained increase in the mRNA and protein expression of ERK1 (Fig. 3A, Ba and Bc). The increases in BMP signaling proteins (e.g., phosphorylation of Smad1) and the MAPK signaling protein ERK in rats injected with MCT both preceded the increases in RVSP and the development of pulmonary arterial medial hypertrophy (Fig. 4), implying that initial increases in BMP and ERK signaling may play an important role in the development of pulmonary hypertension in MCT-injected rats.
Figure 4. Time course of hemodynamic and molecular changes induced by MCT injection.
Summarized data from Figures 1–3, including changes in pulmonary artery smooth muscle cell proliferation, ERK and p-Smad1 protein, right ventricular systolic pressure and remodeling, are presented over the 4 week time course after MCT injection. Dashed grey line represents control levels in normotensive saline-injected rats.
Combination treatment of sildenafil and simvastatin is more effective in preventing MCT-induced pulmonary hypertension and medial thickening than either drug alone
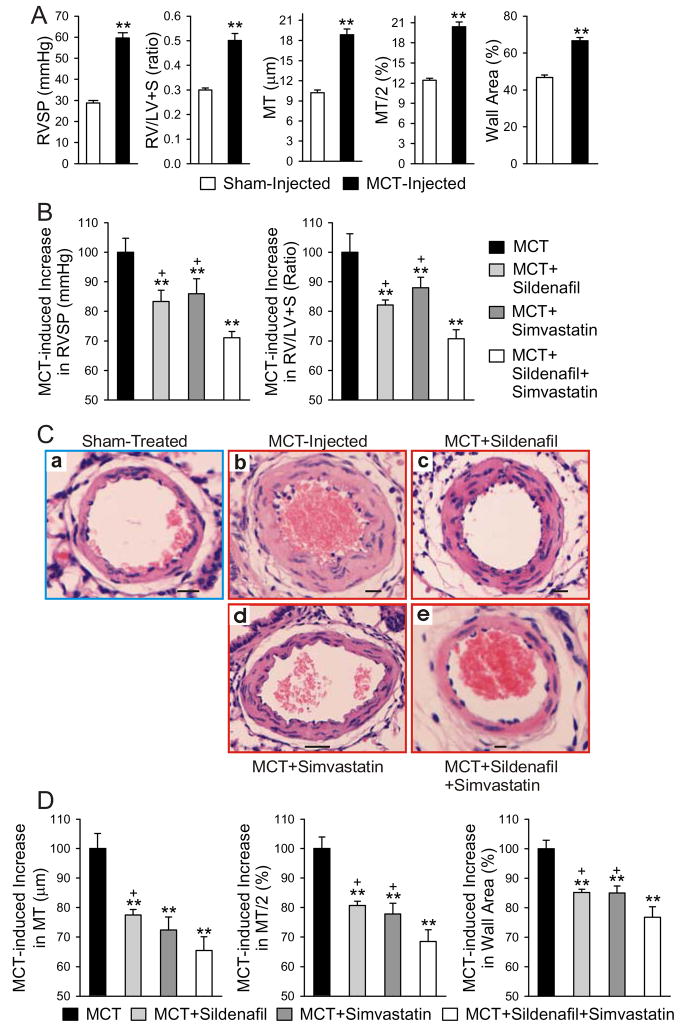
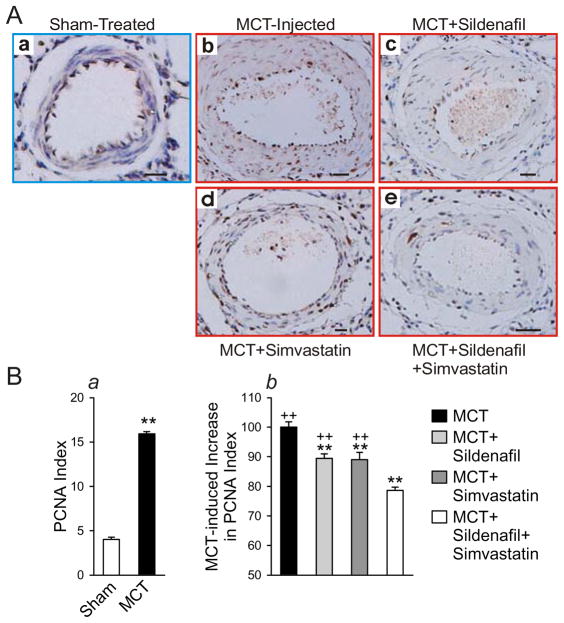
The MCT-injected animals developed severe pulmonary hypertension within 3 weeks, as is evident from the significant increases in both RVSP (60±8 vs. 29±3 mmHg) and RV\LV+S weight ratio (0.50±0.09 and 0.30±0.02) in the untreated control MCT-injected group compared to the sham group (saline injected) (Fig. 5A). Pulmonary vascular remodeling was also evident from increases in medial thickness and wall area (Fig. 5A). While treatment with sildenafil (20 mg/kg/day) or simvastatin (7 mg/kg/day) alone was able to partly prevent the increase of RVSP and RVH induced by MCT injection (approximately 12–17% decrease compared to untreated animals), combination treatment with sildenafil and simvastatin was most effective at preventing the increase in RVSP (29% decrease; P<0.05 vs. sildenafil or simvastatin alone) and RVH, as measured by the RV/LV+S ratio (29.14% decrease; P<0.05 vs. sildenafil or simvastatin alone) (Fig. 5B). Combination treatment also prevented the increases in MCT-induced arteriolar thickening, as measured by increased medial thickness, MT% and wall area% in small pulmonary arterioles (Fig. 5C and D). Arteriolar thickening was at least partially due to an increase in PASMC proliferation, as indicated by an increase in PCNA index in MCT-injected control animals compared to saline-injected animals (15.9±0.8% vs. 4.0± 0.7%; Fig. 7A and Ba). Either sildenafil or simvastatin alone attenuated the increase in PASMC proliferation, but their combination use was more effective at preventing the MCT-induced increase in PASMC proliferation (Fig. 6A and Bb).
Figure 5. Combination Sildenafil and Simvastatin treatment is more effective at preventing MCT-induced pulmonary hypertension than either Sildenafil or Simvastatin alone.
MCT induces pulmonary hypertension 4 weeks after injection as indicated by the increases in right ventricular systolic pressure (RVSP), RV/LV+S weight ratio, medial thickness (MT), MT/2% and wall area parameters in MCT-injected animals compared to sham (saline)-injected animals (A). MCT-injected rats were treated with sildenafil, simvastatin or combination sildenafil + simvastatin therapy and sacrificed after 4 weeks. Summarized data of RVSP and RV/LV+S weight ratio for the MCT-injected controls and the three treatment groups are shown in (B). Representative hematoxylin and eosin (H&E) staining of cross sections of resistance pulmonary arterioles are shown in (C). Scale bars = 10 μm. Based on digital analysis of these images, MT, MT/2% and percent wall area were calculated; the summarized data for the different groups are presented in (D). Data are presented as mean ± SD; n=8/group; ** P<0.01 vs. sham injected group (A) or vs. MCT-injected control group (B, D); +P<0.05 vs. combination group.
Figure 6.
Combination Sildenafil and Simvastatin treatment is more effective than single therapy at attenuating the MCT-induced increase in PASMC proliferation in pulmonary arterioles. Lung sections taken from each rat in each treatment group 4 weeks after MCT injection were fixed in formalin and embedded in paraffin. PCNA staining was used to indicate pulmonary artery smooth muscle cell proliferation in resistance pulmonary arterioles [external diameter: 50–150 μm] from sham-treated saline-injected, MCT-injected control, MCT+sildenafil, MCT+simvastatin and MCT+sildenafil+simvastatin groups. Representative histological sections are shown in (A) and the summarized data in (B). Treatment group data are presented as a percent of the MCT-injected control animals which was set at 100%. Scale bars = 10 μm. Data are presented as mean ± SD; n=8/group; ** P<0.01 vs. sham-injected (Ba) or vs. MCT-injected control (Bb); ++ P<0.01 vs. combination group (Bb).
The effects of combination treatment with sildenafil and simvastatin on plasma concentrations of NO, cGMP, BNP, t-PA, PAI-1, MMP-2, and MMP-9
We also sought to determine the effects of sildenafil, simvastatin and combination therapy on plasma concentrations of NO and cGMP. As expected in MCT-control animals, plasma concentrations of NO and cGMP are significantly lower than in saline-injected (Sham group) animals (MCT-injected controls have 48% NO and 25% of cGMP, expressed as % of Sham levels) (Table 1). Sildenafil or simvastatin alone prevented the decrease in both plasma NO [77% (sildenafil) or 69% (simvastatin), expressed as % of sham] and cGMP [76% (sildenafil) and 69% (simvastatin), expressed as % of sham]. While the increase in NO plasma concentrations with combination treatment were modest compared to either treatment alone (79% compared to sham), the rise in cGMP was significant compared to either treatment alone (87% of sham levels) (Table 1). The data indicate that although the NO level in MCT-injected rats treated with the combination therapy is the same as the level in rats treated with sildenafil or simvastatin alone, combination therapy significantly raises the level of cGMP compared to the single treatment animals.
Table 1.
Effect of sildenafil, simvastatin and combination therapy on plasma NO, cGMP, BNP, t-PA, PAI-1, MMP-2, and MMP-9 concentrations (each group, n=8)# in rats injected with MCT.
| Molecules | Sham-treated | MCT-Control | Sildenafil | Simvastatin | Combination |
|---|---|---|---|---|---|
| NO (μmol/L) | 39.51±9.22 | 18.89±3.87 | 30.45±7.55** | 27.34±7.71* | 31.28±6.08** |
| cGMP (pmol/ml) | 12.19±4.76 | 3.08±1.71 | 9.22±4.63**+ | 8.42±4.77*+ | 10.64±4.77** |
| BNP (ng/ml) | 13.13±2.47 | 33.78±4.91 | 22.62±2.96**+ | 22.71±2.86*+ | 17.87±2.84** |
| t-PA (ng/ml) | 0.56±0.13 | 1.74±0.79 | 1.06±0.46** | 1.11±0.54* | 1.09±0.29* |
| PAI-1 (ng/ml) | 0.21±0.04 | 0.71±0.30 | 0.31±0.13** | 0.23±0.04** | 0.22±0.04** |
| MMP2 (ng/ml) | 9.96±2.82 | 15.82±4.24 | 13.99±1.34+ | 12.49±1.08* | 10.75±1.29** |
| MMP9 (ng/ml) | 79.75±21.86 | 172.25±33.16 | 108.63±36.05** | 103.13±29.19* | 88.13±28.82** |
Data are presented as mean ± SD; Sham-treated, rats injected with vehicle;
MCT-Control, rats injected with MCT; Sildenafil, MCT-injected rats treated with sildenafil alone; Simvastatin, MCT-injected rats treated with simvastatin alone; Combination, MCT-injected rats treated with sildenafil and simvastatin.
P<0.05,
P<0.01 vs. MCT-Control group;
P<0.05 vs. Combination group.
Additionally, all MCT-Control group values were found to be significantly different from the Sham-treated group values.
Circulating levels of BNP may correlate to PAH clinical severity, and matrix metalloproteinases (MMP-2 and MMP-9) as well as fibrinolytic pathway molecules (t-PA and PAI-1) are indicators of vascular remodeling. Plasma BNP levels were found to increase in the MCT injected control group compared to the sham group (34±5 vs. 13±3 ng/ml), whereas plasma levels of BNP were significantly lower in the combination treatment group than in either of the single treatment groups [18±3 (combination) compared to 23±3 (sildenafil) or 23±3 (simvastatin) ng/ml] (Table 1). We observed near doubling or more than doubling of the concentrations of MMP-2, MMP-9, t-PA and PAI-1 in the MCT control group compared to the sham treated group (Table 1). The increase of plasma t-PA, PAI-1, MMP-2, and MMP-9 concentrations were attenuated significantly in the sildenafil, simvastatin and combination groups, but no difference was found among the three treatment groups (Table 1). These observations imply that the preventative effects of the combination therapy, compared with either sildenafil or simvastatin alone, is probably not caused by changes levels of t-PA, PAI-1 and MMPs.
Discussion
The causes of PAH are complex and as yet not completely understood. MCT is known to cause damage to both alveolar lining cells and pulmonary vascular endothelial cells as early as 24 hours after injection [29]. Our results show significant remodeling, marked by an increase in PASMC proliferation and muscularization of arterioles, in the pulmonary vasculature over the course of MCT-induced pulmonary hypertension. PAH, characterized here by significant increases in RVH and RVSP, was fully developed by week 3 and continued into week 4. It was surprising that the medial thickness (MT) of the 2-week group was not significantly different from that of the 0-week group. However, we believe that the MT/2 (%) measurement is a more accurate reflection of the histology sections, as this minimizes the variability among different pulmonary arterial branches as well as individual variations of different animals.
The pulmonary vascular remodeling associated with clinical PAH is believed to result, at least in part, from increased proliferation and decreased apoptosis of PASMC [1, 2, 7] that could be caused by defects in the proapoptotic BMP/Smad signaling pathway and increases in the mitogenic ERK signaling pathway. Our results indicate a role for the ERK pathway in MCT-induced pulmonary hypertension, as the expression of ERK1 mRNA and ERK1/ERK2 protein increased with concomitant increases in RVSP. These changes were similar to the change in vascular remodeling and PCNA index, suggesting a possible mitogenic action of ERK in the development of pulmonary hypertension induced by MCT. Previous studies indicate the existence of crosstalk between the Smad and MAPK pathways [30], but their combined significance in the pulmonary vasculature in PAH needs further investigation.
NO, acting through its target of second messenger cGMP, has potent vasodilatory effects. Dysfunctional signaling in the NO pathway has been implemented in PAH pathogenesis [31], and sildenafil acts by prolonging the effects of NO by stabilizing cGMP. Sildenafil is a highly selective PDE-5 inhibitor that is used in PAH treatment. This study confirmed that sildenafil inhibits the increases in RVSP and RVH and attenuates excess PASMC proliferation induced by MCT, confirming previous reports [32]. Simvastatin has strong anti-proliferative and anti-inflammatory effects and can inhibit the effects of endothelial and smooth muscle cell vascular damage. We tried to reverse MCT-induced PH with a two week treatment of intragastrically delivered simvastatin (2 mg/kg/d) given three weeks after MCT-injection. The control group for this study was administered distilled water intragastrically (3 ml/kg/d). However, we observed no differences between the two groups in terms of pulmonary arterial pressure and right ventricular systolic pressure, which fit with a previous report showing that prophylactic simvastatin did not improve MCT-induced PH [33]. Therefore, we changed the design of our experiment by increasing the dose of simvastatin as well as administering the drug immediately after MCT-injection to study the prevention of MCT-induced PH. The higher dose of simvastatin (7 mg/kg/d) was able to prevent MCT-induced increases in RVSP, RVH and PASMC proliferation, as well as vascular remodeling as reported here.
Sildenafil and simvastatin each seem to have an endothelial protective function. PAI-1 and t-PA are synthesized in the endothelium and are key players in fibrinolysis. Decreases of PAI-1 correlate to pulmonary vascular endothelium repair [34]. Either sildenafil or simvastatin alone resulted in lower plasma t-PA and PAI-1 levels compared to the MCT-injected control group, indicating an endothelial protective effect. Moreover, cGMP and NO plasma levels were significantly higher with either drug alone compared to the untreated animals, again indicating that the drugs individually have endothelial protective effects.
In addition to the BMP-signaling pathway, RhoA/Rho kinase (ROCK) signaling has also been implicated in experimental models of PH [35]. This pathway plays a major role in determining vascular tone through its inhibitory effects on myosin light chain phosphatase. Additionally, RhoA activation increases endothelial cell (EC) permeability and proliferation [1]. Thus, overactive RhoA/ROCK signaling may play a role in both the vasoconstriction of vascular smooth muscle cells and the hyperproliferative EC phenotypes observed in PH. Statins have been demonstrated to inhibit RhoA/ROCK signaling through inhibition of the isoprenylation of RhoA [36], whereas sildenafil increases RhoA phosphorylation without affecting ROCK activity [37]. Thus, whether the preventative effects of the combination treatment on MCT-induced PH examined in this study is due to a decrease in RhoA/ROCK signaling remains unclear and needs further study.
Sildenafil and statins may also act through their action on matrix metalloproteinases (MMP), which are involved in remodeling, tissue repair, cell migration, and inflammation. Our finding that MMP expression increased after MCT injection is in accord with previous studies of different PAH models [32, 38], and statins have been shown to alleviate pulmonary hypertension in a rat model of acute pulmonary embolism by attenuating the increase in MMP-9 [39]. The results of this study indicate that single treatment with either sildenafil or simvastatin prevents the MCT-induced increase in plasma levels of MMPs.
In this study, the combination of sildenafil and simvastatin was more effective at preventing MCT-induced pulmonary hypertension than either treatment alone. The effects of combination treatment were seen at both the hemodynamic and molecular levels, closely paralleling the effects of each single treatment. Combination treatment prevented the MCT-induced increases in RVSP, RVH, PASMC proliferation, and vascular remodeling (measured as increases in medial thickness and vessel wall area) to a greater extent than either treatment alone. While combination therapy was no better than either of the single treatments in preventing either the decline in NO or the increases in t-PA or PAI-1, combination therapy did result in significantly elevated plasma cGMP levels, suggesting that the mechanisms of cGMP production are additive between the two drugs. Therefore, the augmented effects of combination treatment on RVSP, RVH, and PASMC proliferation likely act through a mechanism which relies on cGMP (but not on NO). The increased cGMP observed in the combination group could play a large role in the hemodynamic (RVSP, RVH and remodeling) improvement compared to the single treatment groups.
Whatever the mechanism, sildenafil and simvastatin, by preventing the decrease in cGMP and BMP signaling (e.g., phosphorylation of Smad1) in pulmonary hypertension, may prove a useful therapeutic combination. Contraindications have not been found in patients using sildenafil and simvastatin [40, 41]; however, both sildenafil and simvastatin are metabolized through the CYP3A4 system in the liver. It is therefore possible that each drug’s metabolism will be relatively low due to competition, so the safety of their combined use needs to be studied, as an increase of simvastatin in the blood may induce rhabdomyolysis [42]. However, the combination use of sildenafil and statins in cardiovascular injury seems promising. Combination use has been shown to increase the vasodilator effect of sildenafil and has been shown to prevent the damage of ischemia-reperfusion in heart tissue [43, 44]. As our results indicate, the combination of sildenafil and simvastatin may also prove effective at treating PAH. Our studies demonstrate a synergistic improvement in BMP signal transduction, RVH, and RVSP with the combination therapy compared to either of the drugs alone, providing experimental evidence for the clinical efficacy of combination therapy with sildenafil and simvastatin in the treatment of pulmonary arterial hypertension.
Acknowledgments
We would like to thank Dr. Ling Zhu for her helpful comments in preparing this manuscript. This work was supported in part by an International Joint Research Project (NSFC-30810103904), a 973 Project (NSFC-2009CB522107) from the National Natural Science Foundation of China (NSFC), and grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL054043, HL064945 and HL066012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S20–31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26(1):81–4. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 4.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280(26):24443–50. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 5.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68(1):92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, 3rd, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001;345(5):319–24. doi: 10.1056/NEJM200108023450502. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105(14):1672–8. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 8.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277(7):5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, et al. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290(3):L450–8. doi: 10.1152/ajplung.00206.2005. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96(10):1053–63. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 12.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 13.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169(4):441–7. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 15.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131(6):1917–28. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005;26(5):858–63. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 17.Clozel M, Hess P, Rey M, Iglarz M, Binkert C, Qiu C. Bosentan, sildenafil, and their combination in the monocrotaline model of pulmonary hypertension in rats. Exp Biol Med (Maywood) 2006;231(6):967–73. [PubMed] [Google Scholar]
- 18.Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149(8):521–30. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 19.Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest. 2008;134(1):139–45. doi: 10.1378/chest.07-2111. [DOI] [PubMed] [Google Scholar]
- 20.Tawara S, Fukumoto Y, Shimokawa H. Effects of combined therapy with a Rho-kinase inhibitor and prostacyclin on monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;50(2):195–200. doi: 10.1097/FJC.0b013e31806befe6. [DOI] [PubMed] [Google Scholar]
- 21.Mathai SC, Girgis RE, Fisher MR, Champion HC, Housten-Harris T, Zaiman A, et al. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J. 2007;29(3):469–75. doi: 10.1183/09031936.00081706. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174(11):1257–63. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 23.Gomberg-Maitland M, McLaughlin V, Gulati M, Rich S. Efficacy and safety of sildenafil added to treprostinil in pulmonary hypertension. Am J Cardiol. 2005;96(9):1334–6. doi: 10.1016/j.amjcard.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 24.Rondelet B, Kerbaul F, Van Beneden R, Motte S, Fesler P, Hubloue I, et al. Signaling molecules in overcirculation-induced pulmonary hypertension in piglets: effects of sildenafil therapy. Circulation. 2004;110(15):2220–5. doi: 10.1161/01.CIR.0000143836.40431.F5. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108(13):1640–5. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Sung A, Zhao G, Shi L, Qiu D, Nishimura T, et al. Simvastatin enhances bone morphogenetic protein receptor type II expression. Biochem Biophys Res Commun. 2006;339(1):59–64. doi: 10.1016/j.bbrc.2005.10.187. [DOI] [PubMed] [Google Scholar]
- 27.Ramos MF, Lame MW, Segall HJ, Wilson DW. Smad signaling in the rat model of monocrotaline pulmonary hypertension. Toxicol Pathol. 2008;36(2):311–20. doi: 10.1177/0192623307311402. [DOI] [PubMed] [Google Scholar]
- 28.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, et al. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2007;27(5):1072–8. doi: 10.1161/ATVBAHA.107.141200. [DOI] [PubMed] [Google Scholar]
- 29.Valdivia E, Sonnad J, Hayashi Y, Lalich JJ. Experimental interstitial pulmonary edema. Angiology. 1967;18(6):378–83. doi: 10.1177/000331976701800603. [DOI] [PubMed] [Google Scholar]
- 30.Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17(24):2993–7. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 31.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(4):214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 32.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;169(1):39–45. doi: 10.1164/rccm.200302-282OC. [DOI] [PubMed] [Google Scholar]
- 33.McMurtry MS, Bonnet S, Michelakis ED, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293(4):L933–40. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 34.De Monye W, Sanson BJ, Mac Gillavry MR, Pattynama PM, Buller HR, van den Berg-Huysmans AA, et al. Embolus location affects the sensitivity of a rapid quantitative D-dimer assay in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med. 2002;165(3):345–8. doi: 10.1164/ajrccm.165.3.2104099. [DOI] [PubMed] [Google Scholar]
- 35.Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155(4):444–54. doi: 10.1038/bjp.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97(12):1232–5. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guérin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol. 2005;146(7):1010–8. doi: 10.1038/sj.bjp.0706408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–21. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza-Costa DC, Figueiredo-Lopes L, Alves-Filho JC, Semprini MC, Gerlach RF, Cunha FQ, et al. Protective effects of atorvastatin in rat models of acute pulmonary embolism: involvement of matrix metalloproteinase-9. Crit Care Med. 2007;35(1):239–45. doi: 10.1097/01.CCM.0000251638.67104.C3. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338(20):1397–404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 41.Cheitlin MD, Hutter AM, Jr, Brindis RG, Ganz P, Kaul S, Russell RO, Jr, et al. Use of sildenafil (Viagra) in patients with cardiovascular disease. Technology and Practice Executive Committee. Circulation. 1999;99(1):168–77. doi: 10.1161/01.cir.99.1.168. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez CA. Sildenafil-simvastatin interaction: possible cause of rhabdomyolysis? Am Fam Physician. 2001;63(4):636–7. [PubMed] [Google Scholar]
- 43.Castro MM, Rizzi E, Rascado RR, Nagassaki S, Bendhack LM, Tanus-Santos JE. Atorvastatin enhances sildenafil-induced vasodilation through nitric oxide-mediated mechanisms. Eur J Pharmacol. 2004;498(1–3):189–94. doi: 10.1016/j.ejphar.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Rosanio S, Ye Y, Atar S, Rahman AM, Freeberg SY, Huang MH, et al. Enhanced cardioprotection against ischemia-reperfusion injury with combining sildenafil with low-dose atorvastatin. Cardiovasc Drugs Ther. 2006;20(1):27–36. doi: 10.1007/s10557-005-5203-4. [DOI] [PubMed] [Google Scholar]