Capsule summary
This is the first study to examine measured folate levels in pregnancy in relation to respiratory outcomes in the children. Higher pregnancy levels of folate were associated with increased risk of asthma at age 3.
Keywords: Asthma, Case-Control Studies, Child, preschool, Cohort Studies, folate, folic acid, Norway, Plasma, Pregnancy
To the Editor
Women are advised to increase their folate intake during early pregnancy to lower the risk of neural tube defects in their children.1–3 Folate is involved in nucleotide synthesis, cell division, cell differentiation, and DNA methylation and is important in fetal development. Experimental data in mice and observational data in humans have suggested possible adverse effects of folic acid supplement use in pregnancy on respiratory and allergic outcomes in offspring. 4–6 We previously reported that use of folic acid supplements in the first trimester of pregnancy was associated with an increased risk of respiratory tract infections and wheeze up to 18 months of age in children.4 A recent study from Australia reported folic acid supplementation during late pregnancy to be associated with increased risk of asthma at age 3.5 years and with persistent asthma up to age 5.5 However, none of these studies had biochemical measures of maternal folate status. In the present study, we examined the risk of asthma at age three years in relation to measured maternal plasma folate levels in pregnancy.
We conducted a case-control study nested within the Norwegian Mother and Child Cohort (MoBa), a large population based pregnancy cohort following more than 100,000 pregnant women and their offspring.7 The current study included 1455 control children and 507 case children. Cases were children whose mothers reported that their child had asthma and had used inhalant medication in the past year on the three year questionnaire. Blood plasma folate was measured during the second trimester of pregnancy (median 18 weeks) Details on the recruitment of the study population, folate measurements and the statistical methods are contained in the methods section and Figure 1 in this article’s Online Repository at www.jacionline.org. The MoBa study has been approved by the Regional Committee for Ethics in Medical Research, the Norwegian Data Inspectorate and the Institution Review Board of the National Institute of Environment Health Sciences, USA. This substudy was approved by the Regional Committee for Ethics in Medical Research.
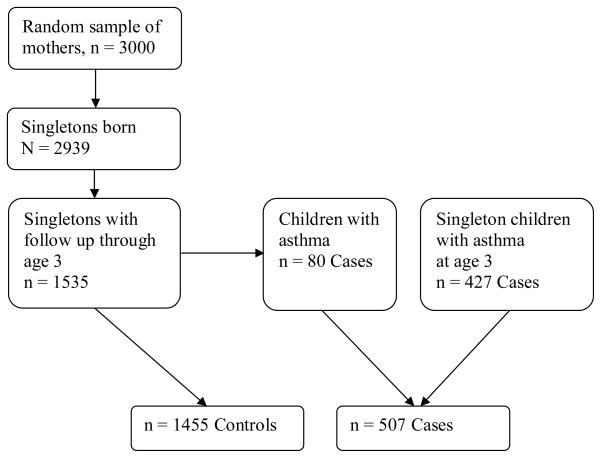
Figure 1.
Flow chart illustrating the inclusion of cases and controls in the Norwegian Mother and Child Cohort Study (MoBa).
Some baseline characteristics differed between mothers of asthma cases and controls (Table 1) for example, maternal atopy was more prevalent among mothers of asthmatic children. Therefore, we adjusted for potential confounding factors and present both crude and adjusted odds ratios in Table 2.
TABLE 1.
Characteristics in cases (507 children with asthma) and controls (1455 children without asthma) in the Norwegian Mother and Child cohort
| Characteristic | n | Controls n = 1455 %* | Cases n= 507 %* |
|---|---|---|---|
| Maternal age | |||
| <25 | 248 | 12.1 | 14.2 |
| 25–20 | 945 | 47.2 | 50.9 |
| >30 | 769 | 40.7 | 34.9 |
| Maternal educational level (years) | |||
| <=12 | 733 | 37.7 | 36.3 |
| 13–16 | 849 | 41.8 | 47.5 |
| 17+ | 324 | 17.3 | 14.2 |
| Missing | 56 | 3.2 | 2.0 |
| Maternal smoking in pregnancy | |||
| No | 1752 | 88.5 | 91.7 |
| Yes | 194 | 10.5 | 8.1 |
| Missing | 16 | 1.0 | 0.2 |
| Parity** | |||
| 0 | 849 | 44.2 | 40.6 |
| 1 | 758 | 36.5 | 44.8 |
| >1 | 355 | 19.3 | 14.6 |
| Maternal prepregnancy BMI | |||
| <18.5 | 62 | 3.0 | 3.6 |
| 18.5 – 24.9 | 1233 | 63.4 | 61.1 |
| 25 – 29.9 | 412 | 20.6 | 22.3 |
| 30+ | 194 | 9.8 | 10.3 |
| Missing | 61 | 3.2 | 2.8 |
| Maternal atopy** | |||
| No | 1320 | 70.7 | 57.6 |
| Yes | 642 | 29.4 | 42.4 |
| Maternal smoking when the child is age 3 | |||
| No | 1578 | 80.0 | 81.7 |
| Yes | 342 | 17.4 | 17.6 |
| Missing | 42 | 2.6 | 0.8 |
| Child’s use of vitamin supplements age 3 | |||
| No | 1064 | 55.5 | 50.5 |
| Yes | 847 | 42.0 | 46.6 |
| Missing | 51 | 2.5 | 3.0 |
| Child’s use of cod liver oil supplements age 3 | |||
| No | 881 | 44.1 | 47.1 |
| Yes | 1030 | 53.4 | 49.9 |
| Missing | 51 | 2.5 | 3.0 |
Percentages may not add to 100 due to rounding
p < 0.05
TABLE 2.
Crude and adjusted* odds ratios for asthma at three years of age according to maternal levels of plasma folate in the second trimester of pregnancy in 507 children with asthma (cases) and 1455 controls
| Maternal plasma folate** in pregnancy (nmol/L) | Controls | Cases | Asthma at age 3 years
|
||||
|---|---|---|---|---|---|---|---|
| Crude OR | (95% CI) | Adjusted OR | (95% CI) | p-value | |||
| <5.54 | 293 | 83 | 1 | 1 | |||
| 5.54 – 7.68 | 294 | 98 | 1.18 | (0.84 to 1.64) | 1.16 | (0.80 to 1.66) | 0.44 |
| 7.68 – 10.60 | 283 | 105 | 1.31 | (0.94 to 1.82) | 1.48 | (1.03 to 2.11) | 0.03 |
| 10.60 – 17.84 | 292 | 96 | 1.16 | (0.83 to 1.62) | 1.28 | (0.89 to 1.85) | 0.18 |
| >17.84 | 293 | 125 | 1.51 | (1.09 to 2.08) | 1.66 | (1.16 to 2.37) | < 0.01 |
| P-trend | 0.03 | 0.006 | |||||
OR: Odds Ratio
CI: Confidence Interval
nmol/L: nanomolar per Liter
Adjusted for maternal educational level, maternal age, parity, maternal atopy, maternal BMI, maternal smoking in pregnancy and maternal smoking at age 3 years, supplement use at age 3 years..
Cutoffs based on quintiles of plasma folate levels in a random sample of 1535 women.
Median maternal plasma folate levels was 9.1 nmol/L, the 25th percentile was 6.2 nmol/L and 75th percentile was 16.1 nmol/L. Median maternal plasma folate level was 9.1 nmol/L for controls and 9.4 nmol/L for cases. As expected, plasma folate levels were substantially higher among women who reported use of folic acid supplements in pregnancy (median 10.9 nmol/L) compared to non users (median 5.8 nmol/L). The Spearman correlation was 0.46 between plasma folate levels in second trimester and questionnaire reports of folic acid supplement use after pregnancy week 13. Plasma folate levels were higher in non-smokers than smokers, nulliparous than parous women, in women older than 30 years, normal weight women, and in women with higher educational level (data not shown). Table 2 presents the crude and adjusted odds ratios with 95% confidence intervals (CI) for asthma at three years across quintiles of maternal plasma folate levels. There was an increased risk of asthma at age three for children with maternal plasma folate levels in pregnancy in the highest compared with the lowest quintile (adjusted odds ratio: 1.66, 95% confidence interval: 1.16 to 2.37). There was a trend of increasing risk across quintiles of plasma folate (P-trend = 0.006).
Analyses with additional adjustments for maternal income, daycare attendance, the child’s sex, birth weight, breastfeeding or use of folic acid supplements did not substantially influence the results (data not shown).
As in any observational study, biases can limit causal interpretation of the associations. Accurate folate status may be difficult to obtain through questionnaires, and in the present study, biochemical measurements of pregnancy folate levels increases the accuracy of the actual fetal exposure. The measurements were conducted on non-fasting plasma samples which may be influenced by recent intake of folate. This may have added preanalytical variation and probably attenuated associations. Although some asthma at age three represents transient wheezing illness that may resolve by school age, asthma at age three with use of inhalant medication in the past year is probably a better proxy for later asthma than wheezing at earlier ages. We limited our asthma case group to children for whom mothers had listed the name of a doctor-prescribed inhalation medication for asthma used within the last year, thereby increasing the validity of the asthma diagnoses. Maternal reports of specific asthma medications have been validated in MoBa against prescription records.8 In Norway, doctor visits and prescribed medications for asthma are free for this age group, which probably decreases confounding by social class. Differential misclassification could also influence associations. If mothers with high folate levels in pregnancy were more health aware and reported more asthma in their children, this could have introduced a positive bias of the association between higher folate levels and childhood asthma. However, proxies for health awareness, like higher maternal education, maternal BMI or cod liver oil in pregnancy, were not associated with asthma at age three in this study, indicating such differential reporting to be unlikely. We adjusted for a number of potential confounders, however, the possibility of residual confounding cannot be excluded. We were also able to address differences in maternal plasma folate levels between subjects followed to age 3 and those who were lost to follow up, and the folate levels in pregnancy were similar, for details see the Methods section in this article’s Online Repository at www.jacionline.org.
The modest associations between second trimester folate levels and early respiratory outcomes in children observed in this study as well as results from the small number of previous studies based on supplement use,4, 5 could be subject to biases inherent of observational data. Even if these associations are confirmed in additional studies, they do not negate the value of folate supplementation in pregnancy. However, should higher folate in pregnancy pose a slight increase risk of respiratory illness in the child, additional studies might delineate levels below which adverse effects are unlikely and help to fine tune public health recommendations to maximize benefits.
Methods
Study population
The Norweigan Mother and Child Cohort Study (MoBa) is a cohort with more than 100,000 pregnant women included between June 1999 and December 2008. The MoBa study is conducted by the Norwegian Institute of Public Health. Women were included around the time of their routine ultrasound examination offered freely to all pregnant women in Norway, and all geographical areas of Norway were represented in the study. Around 44% of invited women agreed to participate, and around 60% of participating women returned the questionnaire at age three years of the child. The questionnaires are available at the MoBa website: http://www.fhi.no/morogbarn.
Based on data from MoBa, we constructed a case-control study. We drew a random sample of 3,000 mothers who gave birth in the MoBa cohort between July 2002 and December 2003. These women had donated a blood sample in the second trimester of pregnancy, were registered in the Medical Birth Registry of Norway and had returned the baseline questionnaire from pregnancy. Of the children born to this random sample of 3000, 1,535 were singletons and had questionnaire follow-up through age three years. Eighty of these children fulfilled our asthma definition at age three and were classified as cases, leaving 1455 children to be controls. Children were defined as asthma cases if the mother reported current asthma at age three and had listed a name of an inhalation medication for asthma when asked to list medications used by their child during the last 12 months. In addition to the eighty asthma case children born to mothers within the random sample, we selected all asthma cases born between July 2002 and June 2004 with maternal samples and similar follow up, giving 427 more case children, resulting in a study population of 1962 children, comprising 507 asthma cases and 1455 controls, see figure 1.
Blood sampling and biochemical analyses of plasma folate
The median gestational week for blood sample collection was 18 weeks. Non-fasting blood samples were collected at the hospitals in ethylene diaminetetraacetic acid tubes. The samples were centrifuged within 30 minutes after collection, and placed in the hospitals’ refrigerator at 4°C until shipped overnight to the Biobank of MoBa at the Norwegian Institute of Public Health in Oslo, Norway. On the day of receipt (usually within 1–2 days), plasma were aliquoted onto polypropylene micro-titre plates, and stored at −80°C. Plasma folate concentration was measured by using the Lactobacillus casei microbiological assay.
Folic acid supplement use
Information on use of folic acid supplements in pregnancy were obtained from the baseline pregnancy questionnaire around week 18 of pregnancy, administered around the same time period as the blood samples were drawn. Subjects with no report of folic supplement use were regarded as non-users of folic acid supplements. Folic acid supplement use was reported in 4 week intervals in pregnancy: week 0–4, week 5–8, week 9 – 12, and after week 13. Use of folic acid supplement was coded as a dichotomous variable, with any intake reported in the first pregnancy questionnaire versus no intake.
Categorizations of plasma folate
Plasma folate levels were divided into quintiles based on levels in the sample of 1535 women initially drawn from the cohort, see figure 1.
Covariates
Information on covariates was based on data from the Medical Birth Registry of Norway and MoBa questionnaires in pregnancy and when the child was three years of age. Covariates were selected a priori based on factors assumed to be associated with folate levels in early pregnancy (for example factors that might influence use of folic acid supplements) and also possibly related to respiratory disease in childhood. Covariates included maternal atopy (history of, or current asthma, hay fever, eczema or urticaria), maternal educational level (years of completed education), parity (based on records in the birth registry), maternal prepregnancy body mass index (BMI) calculated from height and prepregnancy weight reported in the first questionnaire, maternal smoking in pregnancy (report of smoking in the questionnaire around week 18), maternal smoking when the child was three years, and the child’s use of vitamin supplements or cod liver oil at three years of age.
Statistical analyses
Data were analyzed using the statistical software STATA. We estimated odds ratios with 95% confidence intervals for asthma at age three across quintiles of maternal plasma folate. We used univariate and multivariate logistic regression analyses, with the lowest quintile as the reference category. P-values for trend were obtained by treating the quintile variable as linear term in the logistic regression analyses. Missing data on covariates were not included in analyses. The inclusion period for cases were 6 months longer (July 2002 – July 2004) than for the controls (July 2002 – Dec 31 2003) to increase the number of cases and the power. However, we also did the analyses without the cases from this added 6 months period (children born in 2004), and the results are similar.
Loss to follow up
The follow-up rate through age three was in this substudy around 55%, and loss to follow-up could bias results if folate levels and asthma prevalence was different in our study population of children with follow-up at age three and children whose mothers did not return the three year questionnaire. Fortunately we have data to evaluate this possibility from random samples of 2939 singleton mothers with folate levels measured in pregnancy. Folate levels were similar between the 1535 mothers who were followed to age three and the 1,404 mothers who were not. For the 1,404 children who were lost to follow-up, the maternal plasma folate quintiles were: 5.2, 7.2, 10.0, and 16.0 nmol/L. For the 1535 children of who were followed to age three, the maternal plasma quintiles were: 5.5, 7.7, 10.6 nmol/L, and 17.8 (see table 2).
Acknowledgments
Funding: The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, NIH/NIEHS (grant no N01-ES-85433), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10), and by the Division of Intramural Research, National Institute of Environmental Health Sciences, National Institute of Health, USA (contract ES044008 and project ZO1 ES 49019).
Abbreviations
- DNA
Deoxyribonucleic acid
- MoBa
the Norwegian Mother and Child Cohort Study
- nmol/L
nanomolar per Liter
- CI
Confidence Interval
Reference List
- 1.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341:1509–19. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 2.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- 3.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 4.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–4. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–93. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 8.Furu K, Karlstad Ø, Skurtveit S, Håberg SE, Nafstad P, London SJ, et al. High validity of mother-reported use of antiasthmatics among children: a comparison with a population-based prescription database. J Clin Epidemiol. 2010 doi: 10.1016/j.jclinepi.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]