Abstract
Objective
To determine the prevalence of mild cognitive impairment (MCI), dementia and subtypes among oldest old women.
Design
Prospective cohort study
Setting
Women, Cognitive Impairment Study of Exceptional Aging
Participants
1299 oldest old (≥ 85 years) women
Main Outcome Measures
All women completed a neuropsychological test battery. Those who screened positive for possible cognitive impairment (n=634) were further assessed for a diagnosis of dementia, MCI, or normal by an expert panel. Remaining women were considered cognitively normal. Dementia and MCI subtypes were determined using standard criteria.
Results
The women had a mean age of 88.2 years and 27.0% were ≥90 years; 231 women (17.8%) were diagnosed with dementia and 301 (23.2%) with MCI for a combined cognitive impairment prevalence of 41.0%. Clinical features consistent with Alzheimer’s disease and mixed dementia were most common, each accounting for 40% of dementia cases. Amnestic multiple domain and non-amnestic single domain were the most common MCI types, accounting for 33.9% and 28.9% of cases respectively. Cognitive impairment was more frequent among women ≥90 years compared to those 85–89 years (dementia 28.2% vs. 13.9%, p<0.0001, and MCI 24.5% v. 22.7%, p=0.02) and more common among women with less education, history of stroke, and prevalent depression.
Conclusions
In this large sample of oldest old women, approximately 40% had clinically adjudicated cognitive impairment. Subtypes of dementia and MCI were similar to younger populations. Our results suggest that women in the fastest growing demographic, the oldest old, should be carefully screened for cognitive disorders, especially high risk groups.
INTRODUCTION
People aged 85 years and older are often referred to as the oldest old. This group is the fastest growing segment of the US population and is expected to increase in number by 40% over the next decade alone.1 Since the oldest old account for a significant portion of health care needs and expenditures, the expected rise in this population will have important societal impacts on health care costs and caregiving.
Initial evidence suggests that the incidence of all-cause dementia nearly doubles with every five years of age2 and that the prevalence of dementia rises from approximately 2–3% in those aged 65 to 75 years to 35% in those 85 years or older.3 However, cognitive impairment among the oldest old is not well characterized.4 In particular, few studies have examined mild cognitive impairment (MCI) in the oldest old5, 6 and the prevalence of MCI and its subtypes has not been well characterized. The prevalence of MCI and dementia by subtypes has important public health implications because the prognosis, symptoms, and treatment vary according to type.7, 8 It is also possible that classic risk factors for MCI and dementia among the young-old—such as low education, cardiovascular disease, or having an Apolipoprotein E (APOE) e4 allele9–12 — may not pertain to the oldest old due to differential survival or differences in coexisting comorbidities and neuropathologic features.
The objective of this study was to characterize the prevalence of MCI, dementia, and their subtypes among oldest old women. A secondary objective of this study was to examine whether some groups of oldest old women were more likely to have cognitive impairment. Our hypothesis was that the prevalence of cognitive impairment in our oldest old cohort would be higher than that reported for young-old populations but that the proportion with specific dementia and MCI subtypes would be similar.
METHODS
Our participants were women enrolled in the ongoing Study of Osteoporotic Fractures (SOF), a multi-center, prospective, observational study of women 65 years and older at baseline.13 In brief, 9,704 primarily white women were recruited to SOF between September 1986 and October 1988 from four areas in the United States: Baltimore, Maryland; Minneapolis, Minnesota; Portland, Oregon; and Monongahela Valley, Pennsylvania. The women attended clinic visits every 2 to 4 years.
At Visit 9 (November 2006 to August 2008), three of the four SOF sites participated in an ancillary study regarding clinical cognitive status called the Women, Cognitive Impairment Study of Exceptional Aging (WISE). The 1338 women from the original SOF cohort who completed an expanded cognitive battery as part of the Visit 9 protocol and who were based at one of the participating sites were part of WISE. Of the remaining 8366 original participants, 5463 had died, 1137 withdrew from the study or were lost to follow-up, 948 were from the non-participating site (Baltimore), 35 did not complete Visit 9, and 783 were still followed but only by self-administered questionnaire. Of the 1338 women, the 1299 who were 85 years or older constituted the current study sample.
Baseline characteristics included self-reported age, education, and race. APOE phenotype was determined using standard procedures for the women enrolled at one clinic site.14 At Visit 9, the women had their blood pressure, height, and weight measured, and body mass index (BMI) was calculated. Participants reported their type of residence (community or nursing home). Self-reported medication use over the prior 30 days was recorded and confirmed by examination of pill bottles (medication and dosage). Participants also reported whether a doctor had ever diagnosed them with a variety of medical conditions, including stroke or transient ischemic attack (TIA), dementia or Alzheimer’s disease (AD), diabetes, and Parkinson’s disease. Women who reported a clinician-identified heart attack or coronary disease or that they had undergone angioplasty or stenting were classified as having coronary artery disease (CAD). As part of the medical history, women were also asked to report on the occurrence of poor memory symptoms in the past week.
Depressive symptoms were evaluated using the 15-item Geriatric Depression Scale. Those with a score of 6 or higher were considered to have symptoms consistent with depression.15 Functional status was evaluated at Visit 9 by caregivers or proxies using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)16 and by a scale based assessment of self-reported ability to perform activities of daily living (ADLs) and instrumental activities of daily living (IADLs) with or without difficulty.
Neuropsychological Test Battery
Women were administered either a slightly shortened (26-point)14 or standard (30-point) Mini-Mental State Examination17 (mMMSE or MMSE) as well as the Trails B every 2 to 4 years over the 20-year study period. The MMSE is a brief test of global cognitive function that evaluates orientation, concentration, language, praxis, and memory.17 Trails B is a timed test of executive function in which participants connect a series of alternating numbers and letters.18
At Visit 9, centrally trained clinic staff administered an expanded neuropsychological test battery to participants. The battery included the Trails B and the Modified Mini-Mental State Examination (3MS),19 a 100-point extended version of the MMSE that is more sensitive, the California Verbal Learning Test, Second Edition (CVLT-II) Short Form, Digit Span, and category and verbal fluency. The CVLT-II is a test of verbal episodic memory with immediate and 10-minute delay scores.20 Digit Span is a test of attention with forward and backward scores.21 Category and verbal fluency measure semantic memory, requiring the participant to say as many words as possible that fit into a given category (for the WISE examination, “vegetables” and words that begin with the letter “F”) in a minute.22
Clinical Cognitive Status Evaluation
Cognitive impairment was determined in a two-step process. First, women were screened at Visit 9 for the following criteria: 1) score <88 on the 3MS;23 2) score <4 on the CVLT delayed recall;20 3) score ≥3.6 on the IQCODE;16 4) previous dementia diagnosis; or 5) nursing home residence. The 634 women who screened positive for one or more criteria as well as a random sample of 20 who screened negative were adjudicated for clinical cognitive status. The remaining women who screened negative were considered cognitively normal.
A randomly selected member of a panel of clinical experts, which included a neurologist, two neuropsychologists, and a geropsychologist, adjudicated the cognitive status of each woman. Information considered for the adjudication included the Visit 9 neuropsychological test scores, depression score, functional status, medications, prior cognitive test scores, and medical history. To test inter-rater reliability, all four adjudicators evaluated 20 participants who screened positive for cognitive impairment. The average weighted kappa for inter-rater reliability of diagnoses was 0.77 (95% CI: 0.71 – 0.84), indicating substantial strength of agreement.24
A diagnosis of dementia was made based on DSM-IV criteria—that is, the development of multiple cognitive deficits that include memory impairment and impairment in at least one other cognitive domain that is a decline from previous level of functioning and is sufficiently severe to cause impairment in function.25 Impairment in functional status was determined primarily by the IQCODE. If women did not have an informant, functional status was informed by self-reported difficulty to perform ADLs and IADLs. The likely dementia etiology (AD, vascular dementia, dementia due to multiple etiologies (mixed), or other) was also determined. AD diagnosis was made in accordance with National Institute of Neurological Disorders and Stroke (NINDS) criteria.26 Vascular dementia was based on DSM-IV criteria.25 Dementia was classified as “other” if the participant had a history consistent with other neuropsychiatric conditions such as head trauma, Parkinson’s disease, or active major depression. Finally, adjudicators diagnosed mixed dementia if there was evidence of multiple etiologies. If the criteria for dementia were met but the type of dementia could not be established, the participant was classified as having an indeterminate type of dementia.
MCI was diagnosed using a modified Petersen Criteria,27 which requires cognitive impairment that is insufficient to be dementia and generally intact function. Women with MCI were classified into amnestic or non-amnestic and single or multiple domains based on the cognitive domain(s) with impairment, defined as 1.5 standard deviations poorer than age appropriate norms.28 Diagnosis of amenestic MCI was primarily informed by CVLT scores, and diagnosis of non-amenstic MCI was informed by scores on Trails B, 3MS, Digit Span, and category and verbal fluency test. If the criteria for MCI were met but the type of MCI could not be established, the participant was classified as having indeterminate MCI. Those who did not meet criteria for MCI or dementia were classified as cognitively normal. Of the 20 women screened normal but were adjudicated, 19 were diagnosed with normal cognition and 1 was diagnosed with MCI.
Statistical Analysis
Differences in baseline demographics and cognitive scores between WISE participants and non-participants were first compared using analysis of variance (ANOVA), Kruskal-Wallis (skewed data), or chi-squared (χ2) tests, as appropriate. Then, the prevalence of dementia, MCI, and their subtypes were calculated by strata of age (85–89 years, ≥90 years). Group differences in characteristics by cognitive status were described in bivariable analyses using ANOVA, Kruskal-Wallis (skewed data), or χ2 tests. In addition, neuropsychological test scores were compared by cognitive diagnosis using ANOVA. Post hoc t-tests were performed as necessary. Finally, we compared the prevalence of cognitive impairment (combined dementia and MCI) across strata of women including education (<high school, ≥high school), presence/absence of comorbidities (stroke, CAD, depression), and presence/absence APOE e4 using χ2. All analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
All women provided written informed consent, and the study was approved by the committees on human research at each study site and at the coordinating center, the University of California, San Francisco.
RESULTS
The 1299 women had a mean age of 88.2 years, a mean education of 12.8 years, and a mean baseline mMMSE score of 24.9. Among this oldest old cohort, 231 (17.8%) were diagnosed with dementia and 301 (23.2%) with MCI, for a combined total of 41.0% with clinical cognitive impairment; the remaining 767 (59.0%) women were cognitively normal (639 from negative screening and 128 from adjudication). Compared to the WISE participants, the 1012 SOF surviving non-participants were slightly older, less educated and had lower baseline cognitive score (p<0.05 for all).
Prevalence of Dementia and MCI and Subtypes by Age Group
The prevalence of dementia among women 90 years or older was approximately double that among those 85–89 years (28.2% versus 13.9% respectively, p<0.0001) (Table 1). Clinical features consistent with AD and mixed dementia were most common (accounting for nearly 40% of dementia cases each). Features consistent with vascular dementia and ‘other’ dementia were less common (12.1% and 0.9% of dementia respectively) and 7.4% were indeterminate. Although the overall prevalence of dementia was higher in those ≥90 years, the distribution of dementia subtypes was similar across age groups (p=0.94 for difference by age group). Of the women diagnosed with dementia, approximately one-quarter reported a previous dementia diagnosis and about 20% reported that they currently took dementia medications.
Table 1.
Prevalence (95% confidence interval) of mild cognitive impairment (MCI), dementia, and subtypes by age group.
| Prevalence of Dementia | Subtype of Dementia | ||||||
|---|---|---|---|---|---|---|---|
| Age | N | % (95% CI) | Alzheimer’s Disease |
Vascular Dementia |
Mixed | Other | Indeterminate |
| 85–89 years | 132 | 13.9 (11.7, 16.1) | 37.9 (29.6, 46.2) | 12.1 (6.6, 17.7) | 40.9 (32.5, 49.3) | 0.8 (0.0, 2.2) | 8.3 (3.6, 13.0) |
| ≥90 years | 99 | 28.2 (23.5, 32.9) | 42.4 (32.7, 52.2) | 12.1 (5.7, 18.5) | 38.4 (28.8, 48.0) | 1.0 (−1.0, 3.0) | 6.1 (1.4, 10.8) |
| All | 231 | 17.8 (15.7, 19.9) | 39.8 (33.5, 46.1) | 12.1 (7.9, 16.3) | 39.8 (33.5, 46.1) | 0.9 (0.0, 2.1) | 7.4 (4.0, 10.7) |
| Prevalence of MCI | Subtype of MCI | ||||||
| N | % (95% CI) |
Amnestic Single Domain |
Amnestic Multiple Domain |
Non-Amnestic Single Domain |
Non-Amnestic Multiple Domain |
Indeterminate | |
| 85–89 years | 215 | 22.7 (20.0, 25.3) | 22.3 (16.8, 27.9) | 34.0 (27.6, 40.3) | 29.3 (23.2, 35.4) | 7.4 (3.9, 10.9) | 7.0 (3.6, 10.4) |
| ≥90 years | 86 | 24.5 (20.0, 29.0) | 20.9 (12.3, 29.5) | 33.7 (23.7, 43.7) | 27.9 (18.4, 37.4) | 11.6 (4.9, 18.4) | 5.8 (0.9, 10.8) |
| All | 301 | 23.2 (20.9, 25.5) | 21.9 (17.3, 26.6) | 33.9 (28.5, 39.2) | 28.9 (23.8, 34.0) | 8.6 (5.5, 11.8) | 6.6 (3.8, 9.5) |
The prevalence of MCI was also higher among women ≥90 years than among women 85–89 years (24.5% versus 22.7%, p=0.016). Amnestic multiple domain was the most common subtype of MCI followed by non-amnestic single domain and amnestic single domain (33.9%, 28.9%, and 21.9% respectively). Fewer participants were classified as non-amnestic multiple domain or indeterminate type (8.6% and 6.6% each). The proportion of oldest old women with each MCI subtype was similar by age group (p=0.83) (Table 1).
Participant Characteristics by Cognitive Status
Compared to women with normal cognition, those with dementia were, on average, older, less likely to have completed high school, and more likely to live in a nursing home. In addition, women with dementia were more likely than women with normal cognition to be depressed, have a history of stroke, and have an APOE e4 allele (p<0.05 for all pairwise comparisons). Compared to women with normal cognition, women who were diagnosed with MCI were older, more likely to be depressed, live in a nursing home, and have a history of stroke but less likely to have completed high school. (p<0.05 for all pairwise comparisons)
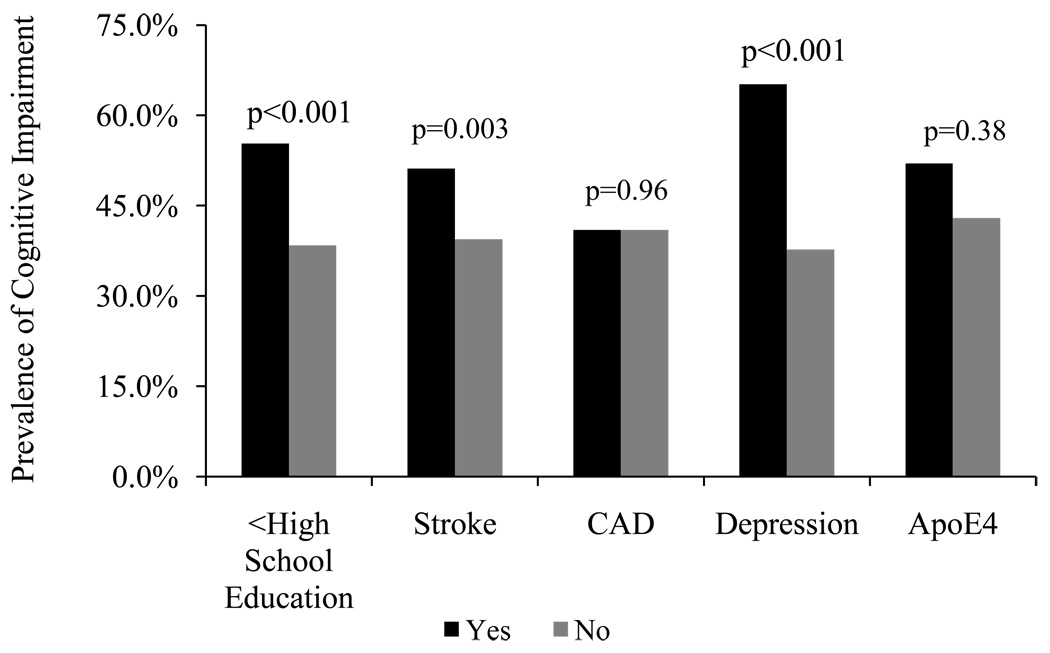
Cognitive impairment (dementia and MCI combined) was more common among women with less than high school education (55.3% versus 38.4%, p<0.001). The prevalence of cognitive impairment was also higher in women with a history of stroke (51.2% versus 39.4%, p=0.003) and with depression (65.2% versus 37.7%, p<0.001), but did not differ among those with and without CAD (p=0.96) or an APOE e4 allele (p=0.38) (Fig).
Figure 1.
Prevalence of cognitive impairment (MCI and dementia) among sub-groups of oldest old women
Neuropsychological Test Scores by Cognitive Status
As expected, women with dementia had the worst scores and women with normal cognition had the best scores on all Visit 9 neuropsychological tests (Table 3). These differences across cognitive diagnoses were most noticeable on tests of global cognition, executive function, and memory. For example, the mean score for the 3MS was 92.6, 84.6 and 72.7 for normal, MCI and dementia groups respectively (p<0.001 across all groups).
Table 3.
Neuropsychological test score (mean (sd)) by cognitive status among 1299 oldest old women.
| Neuropsychological Test | Normal N = 767 |
MCI N = 301 |
Dementia N = 231 |
p-valuea |
|---|---|---|---|---|
| 3MSd | 92.6 (4.5) | 84.6 (6.4)c | 72.7 (13.6)c | <0.0001 |
| Trails B | 148 (76) | 239 (183)c | 285 (206)c | <0.0001 |
| CVLT Immediate Recalld | 25.7 (4.2) | 20.8 (4.2)c | 16.9 (5.0)c | <0.0001 |
| CVLT Delayed Recalld | 6.6 (1.6) | 3.7 (2.4)c | 1.3 (1.7)c | <0.0001 |
| Forward Digit Span | 7.5 (2.1) | 7.2 (2.1)b | 7.0 (2.2)b | 0.003 |
| Backward Digit Span | 6.0 (2.0) | 5.0 (1.8)c | 4.7 (1.8)c | <0.0001 |
| Verbal Fluency | 11.6 (4.0) | 9.4 (3.8)c | 8.4 (3.6)c | <0.0001 |
| Category Fluency | 11.7 (3.1) | 9.5 (3.0)c | 6.9 (3.1)c | <0.0001 |
P-value by ANOVA across all groups
P-values for pairwise comparisons with normal cognitive function are bp<0.05 or cp<0.001
3MS = Modified Mini-Mental Examination, CVLT = California Verbal Learning Test
DISCUSSION
Our study is among the few to characterize the prevalence of dementia, MCI, and their subtypes in the oldest old. In our cohort of oldest old women, 41% met criteria for clinically significant cognitive impairment. Cognitive impairment, and particularly dementia, was more common among those ≥90 years than among those 85–89 years of age. However, the distribution of subtypes appeared to be similar across age groups, with AD and mixed dementia being the most common types of dementia and amnestic multiple domain and non-amnestic single domain being the most common forms of MCI.
Our observed prevalence estimate for dementia was lower than that in most previous studies,3, 29–32 but not all33 which could be due to the relative young age of our population compared to other oldest old studies. The mean age of all WISE participants was 88 years and among those ≥90 years, it was 92 years whereas the mean age of the 90+ Study was 94 years.29 In addition, it is probable that the women who survived to SOF Visit 9 but did not participate in WISE were more likely to have dementia than WISE participants. However, this is likely to be true for previous longitudinal, population-based studies of the oldest old. Finally, while many studies followed a two-stage protocol for dementia ascertainment similar to the WISE protocol, most prior studies did not include MCI diagnoses.29–31 As a result, participants with MCI may have been included among demented cases, resulting in higher estimates of dementia, especially where the diagnosis was not formed by clinical evaluation.29, 30
The distribution of dementia subtypes is vital for public health planning because the treatment and course of dementia differs by type. Among our sample of oldest old women, AD and mixed dementia were the most common types, accounting for nearly 80% of dementia cases combined and vascular dementia accounted for 12.1% of cases. This distribution is similar to the distribution found in a meta-analysis of European studies that stratified by age; AD accounted for 76.6% and vascular dementia accounted for 18.8% of dementia cases among the oldest old, totaling 95.5%.30
MCI has been poorly characterized among the oldest old and we are unaware of any previous large studies that characterized the prevalence of MCI and subtypes in this age group. Among the WISE cohort, the prevalence of MCI was 23.2% and MCI was slightly more common among those ≥90 years. In order, amnestic multiple domain, non-amnestic single domain, and amnestic single domain were the most common types of MCI, each accounting for more than 20% of cases. These results are similar to those from the younger Women’s Health Initiative cohort (women ≥65 years), which also found that amnestic multiple domain MCI was most common;34 however, in that cohort, non-amnestic multidomain MCI was the next most common type followed by amnestic single domain MCI. It is important to characterize MCI prevalence by subtype as rate of progression to dementia may vary by MCI type. Some studies have suggested that amnestic, single-domain MCI is most likely to progress to dementia while multiple domain and non-amnestic single domain MCI were least likely,8, 35 although this is controversial.36
Women with low education, a history of stroke, and depression were more likely to have cognitive impairment, similar to prior findings in young-old populations.37 Although having an APOE e4 allele did not carry an excess risk of MCI, women with an e4 allele were more likely to be diagnosed with dementia, confirming earlier results in the oldest old.9, 38 However, we found no association between APOE e2 and preserved cognitive function, unlike in the 90+ Study.38 Of note, our observed prevalence of e2 was high, possibly due to enhanced survival among those with an e2 allele as previously reported.39 While most studies of young-old adults report that diabetes is associated with increased likelihood of dementia or MCI,10 we did not observe that in the WISE cohort, possibly due to differential survival. That is, people with diabetes and cognitive impairment may have been less likely to survive to 85 years of age.
Our study has several strengths. Most importantly, we studied a very large cohort of oldest old women with careful cognitive evaluation. In addition, the women are well-characterized and have been closely followed for 20 years. However, our study also has some important limitations. The cognitive diagnoses were made without standard neuroimaging or confirmatory autopsy; and while we gave preference to informant based variables such as the IQCODE, we relied on participants’ self report of medical conditions and, for women who did not have an informant, self report of functional limitations. Nevertheless, extensive demographics, medical history, and neuropsychological test scores were considered for diagnoses. Surviving women with cognitive impairment may also have been more likely to drop out of SOF over the 20-year follow-up period. As a result of these limitations, it is likely that our prevalence estimates are conservative. Finally, most of our participants were white women and our prevalence estimates should not be generalized to men or to more diverse populations.
By 1994, the oldest old already represented nearly 40% of people with dementia,3 despite accounting for just over 1% of the population. The absolute and relative growth of the oldest old population in the coming decades will increase the number and proportion of dementia cases among the oldest old. In this study, we found that over 40% of oldest old women are cognitively impaired and that this rate was higher among those 90 years or older than those 85–89 years. The distributions of MCI and dementia subtypes were similar to young-old populations. Screening for cognitive disorders in the oldest old is of the utmost importance, especially among high risk groups.
Table 2.
Characteristics of the 1299 oldest old women in WISE by cognitive status.
| Characteristic (mean (sd) or %) |
Normal N = 767 |
MCI N = 301 |
Dementia N = 231 |
p-valuea |
|---|---|---|---|---|
| Age, Years | 87.8 (2.5) | 88.5 (2.7)d | 89.4 (3.4) d | < 0.001 |
| Education, < High school | 11.5 | 21.3d | 19.5c | < 0.001 |
| Nursing Home Residence | 2.7 | 6.7c | 19.6d | <0.001 |
| History of Stroke | 10.8 | 15.4b | 17.9c | 0.009 |
| Coronary Artery Disease | 19.2 | 18.7 | 20.0 | 0.93 |
| Diabetes | 13.0 | 12.7 | 13.5 | 0.97 |
| Depression | 7.1 | 18.4d | 19.7d | < 0.001 |
| Body Mass Index, kg/m2 | 26.2 (4.5) | 25.7 (4.3) | 25.7 (5.1) | 0.18 |
| APOE e4 allelee | 6.9 | 4.2 | 15.9b | 0.03 |
| APOE e2 allele | 20.1 | 25.0 | 20.6 | 0.69 |
| Previous diagnosis of dementia | 0.1 | 0.7 | 23.9d | <0.001 |
| Taking dementia medication | 1.1 | 3.7c | 20.3d | <0.001 |
| mMMSE | 24.5 (2.0) | 22.1 (3.0)d | 18.4 (5.0) d | <0.001 |
P-value by ANOVA across all groups for continuous variables and chi-square for categorical variables
P-value for pairwise comparisons with normal cognitive function is bp<0.05, cp<0.01, dp<0.001
Only 309 women were tested for APOE.
ACKNOWLEDGMENTS
The Study of Osteoporotic Fractures (SOF) and SOF-WISE is supported by the National Institutes of Health funding (AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1, 5R01AG026720-04).
Dr. Kristine Yaffe had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. She is supported in part by the National Institute of Aging grant K24 AG 031155 and an Independent Investigator Award from the Alzheimer’s Association.
Footnotes
Dr. Laura Middleton is supported by a Canadian Institute of Health Research (CIHR) fellowship.
Ms. Li-Yung Lui reports no disclosures
Dr. Spira is supported by a Mentored Research Scientist Development Award (1K01AG033195) from the National Institute on Aging.
Dr. Katie Stone reports no disclosures.
Dr. Caroline Racine reports no disclosures.
Dr. Kristine Ensrud reports no disclosures.
Dr. Joel Kramer reports no disclosures.
REFERENCES
- 1.Moore A. Older people. We can work it out. Health Serv J. 2007 Jan 11;117(6038):24–26. [PubMed] [Google Scholar]
- 2.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998 Sep;51(3):728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 3.Canadian study of health and aging: study methods and prevalence of dementia. Can Med Assoc J. 1994 Mar 15;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
- 4.Kawas CH. The oldest old and the 90+ Study. Alzheimers Dement. 2008 Jan;4(1) Suppl 1:S56–S59. doi: 10.1016/j.jalz.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeve B, McCormick J, Smith G, et al. Mild cognitive impairment in the oldest old. Neurology. 2003 February 11;60(3):477–480. doi: 10.1212/wnl.60.3.477. 2003. [DOI] [PubMed] [Google Scholar]
- 6.Pioggiosi PP, Berardi D, Ferrari B, Quartesan R, De Ronchi D. Occurrence of cognitive impairment after age 90: MCI and other broadly used concepts. Brain Research Bulletin. 2006;68(4):227–232. doi: 10.1016/j.brainresbull.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines abstracted from the American Academy of Neurology's Dementia Guidelines for Early Detection, Diagnosis, and Management of Dementia. J Am Geriatr Soc. 2003 Jun;51(6):869–873. doi: 10.1046/j.1365-2389.2003.51272.x. [DOI] [PubMed] [Google Scholar]
- 8.Ravaglia G, Forti P, Maioli F, et al. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement Geriatr Cogn Disord. 2006;21(1):51–58. doi: 10.1159/000089515. [DOI] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 10.Decarli C. Vascular factors in dementia: an overview. J Neurol Sci. 2004 Nov 15;226(1–2):19–23. doi: 10.1016/j.jns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994 March 1;151(3):390–396. doi: 10.1176/ajp.151.3.390. 1994. [DOI] [PubMed] [Google Scholar]
- 12.White L, Katzman R, Losonczy K, et al. Association of education with incidence of cognitive impairment in three established populations for epidemiologic studies of the elderly. J Clin Epidemiol. 1994 Apr;47(4):363–374. doi: 10.1016/0895-4356(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Nevitt MC, Browner WS, et al. Risk Factors for Hip Fracture in White Women. New England Journal of Medicine. 1995;332(12):767–774. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K, Cauley J, Sands L, Browner W. Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol. 1997 Sep;54(9):1110–1114. doi: 10.1001/archneur.1997.00550210044011. [DOI] [PubMed] [Google Scholar]
- 15.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997 Feb 24;157(4):449–454. [PubMed] [Google Scholar]
- 16.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989 Nov;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Battery: Theory and Clinical Interpretation. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition (CVLT-II) San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 22.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York: Oxford University Press; 1991. [Google Scholar]
- 23.Espeland MA, Rapp SR, Robertson J, et al. Benchmarks for designing two-stage studies using modified mini-mental state examinations: experience from the Women's Health Initiative Memory Study. Clin Trials. 2006;3(2):99–106. doi: 10.1191/1740774506cn140oa. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001 Dec;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006 Apr 15;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 29.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008 Jul 29;71(5):337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 30.Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11) Suppl 5:S4–S9. [PubMed] [Google Scholar]
- 31.Riedel-Heller SG, Busse A, Aurich C, Matschinger H, Angermeyer MC. Prevalence of dementia according to DSM-III-R and ICD-10: results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Part 1. Br J Psychiatry. 2001 Sep;179:250–254. doi: 10.1192/bjp.179.3.250. [DOI] [PubMed] [Google Scholar]
- 32.von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol. 1999 May;56(5):587–592. doi: 10.1001/archneur.56.5.587. [DOI] [PubMed] [Google Scholar]
- 33.Heeren TJ, Lagaay AM, Hijmans W, Rooymans HG. Prevalence of dementia in the 'oldest old' of a Dutch community. J Am Geriatr Soc. 1991 Aug;39(8):755–759. doi: 10.1111/j.1532-5415.1991.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 34.Rapp SR, Legault C, Henderson VW, et al. Subtypes of Mild Cognitive Impairment in Older Postmenopausal Women: The Women's Health Initiative Memory Study. Alzheimer Disease & Associated Disorders. doi: 10.1097/WAD.0b013e3181d715d5. Epub 2010 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22(4):312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 36.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006 December 26;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. 2006. [DOI] [PubMed] [Google Scholar]
- 37.Middleton LE, Yaffe K. Promising Strategies for the Prevention of Dementia. Arch Neurol. 2009;66(10):1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009 Mar 3;72(9):829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fillenbaum GG, Burchett BM, Lee JH, Blazer DG. Mortality and apolipoprotein E in African-American, and White Elders: An attempted replication. American Journal of Medical Genetics Part A. 2003;119A(2):141–146. doi: 10.1002/ajmg.a.20146. [DOI] [PubMed] [Google Scholar]