Abstract
Background and Objective
Maximizing the durability of viral suppression is a key goal of antiretroviral therapy. The objective of AIDS Clinical Trials Group Study 372A was to determine whether the intensification strategy of adding abacavir to an effective indinavir-dual nucleoside regimen would delay the time to virologic failure.
Methods
Zidovudine-experienced subjects (n=229) on therapy with indinavir + zidovudine + lamivudine with plasma HIV-1 RNA levels <500 copies/mL were randomized to abacavir 300 mg twice daily or placebo. The primary endpoint was the time to treatment failure, defined as a composite of confirmed virologic failure (2 consecutive HIV-1 RNAs >200 copies/mL) and treatment discontinuation.
Results
At baseline, the study population was 88% male with a median age of 41 years and median CD4 cell count of 250/mm3. Median follow-up was 4.4 years. The primary endpoint was reached in 61/116 of abacavir versus 62/113 of placebo recipients (P = .77); virologic failure occurred in 34/116 and 42/113 patients, respectively (P = .22). There were no differences in the proportions of subjects with plasma HIV-1 RNA levels below 50 copies/mL, in CD4 cell count increases, nor adverse events between the arms. In the study, 17% of subjects developed nephrolithiasis, 2% experienced abacavir hypersensitivity, and 4.8% experienced at least 1 serious cardiovascular event (7 [6%] in the abacavir arm, 4 [3.5%] in the placebo arm). In additional secondary and post hoc analyses, rates of intermittent viremia, suppression below a plasma HIV-1 RNA level of 6 copies/mL, and HIV-1 proviral DNA levels in peripheral blood mononuclear cells were not significantly different in the 2 arms.
Conclusions
The strategy of intensification with abacavir in patients who are virologically suppressed on a stable antiretroviral regimen does not confer a clinical or virologic benefit. As antiretroviral regimens have become more potent since this trial was completed, it will be even more difficult to prove that late intensification of already virologically suppressed patients will add benefit. However, studies are warranted with drugs with new mechanisms of action to determine whether the level of persistent viremia below 50 copies/mL can be further reduced and what influence this may have on latent HIV reservoirs.
Keywords: abacavir, antiretroviral therapy, intensification
Effective antiretroviral therapy is dependent upon the ability of combination drug therapy to suppress viral replication, as measured by the plasma HIV-1 RNA, to levels below the detection limit of the most sensitive assays commercially available.1,2 In practical terms, suppression of viremia is associated with durability of the virologic response, prevention of the emergence of drug resistance mutations, and immunologic and clinical benefit.3 The sustainability of a therapeutic response to a particular antiretroviral regimen is important with respect to maintenance of future treatment options given the long-term management of HIV-1 disease that can now span decades.4 One of the strategies proposed to achieve this goal is treatment intensification, that is, the addition of one or more drugs to an already existing regimen. Intensification of regimens in the setting of moderate levels of viremia with drugs such as abacavir or tenofovir has proven to be effective,5–9 but the efficacy of intensification in the setting of already successful plasma viral suppression has not been demonstrated in a randomized trial. AIDS Clinical Trials Group Study 372A was designed to answer the question of whether intensification with abacavir in this setting would delay the time to treatment failure.
METHODS
Study Design and Patients
ACTG 372A was a randomized, double-blind, placebo-controlled trial of abacavir intensification in patients with plasma HIV-1 RNA levels below 500 copies/mL on a regimen of indinavir, zidovudine (or stavudine), and lamivudine. The study was originally designed as a rollover study to ACTG 320, which proved the superiority of the combination of indinavir, zidovudine (or stavudine), and lamivudine over the dual nucleoside combination of zidovudine (or stavudine) and lamivudine.10 Subjects who were originally randomized to the indinavir-containing arm, or who received indinavir during the course of the ACTG 320 trial after reaching a study endpoint, and whose plasma HIV-1 RNA level was <500 copies/mL at the end of the study were eligible for screening for ACTG 372A. During the enrollment period, patients meeting similar criteria as the original ACTG 320 study population were also eligible for screening.
The primary objectives of the study were (a) to compare the time to the composite endpoint of virologic failure or permanent treatment discontinuation between the study arms and (b) to evaluate the safety and tolerability of the treatment arms. Virologic failure was initially defined as 2 consecutive plasma HIV-1 RNA values >500 copies/mL as determined by the standard Roche assay (Branchburg, New Jersey, USA). Early during the course of the trial, the virologic failure definition was revised to 2 consecutive plasma HIV-1 RNA values >200 copies/mL as measured by the ultrasensitive Roche Amplicor HIV-1 Monitor assay. Permanent treatment discontinuation was defined as discontinuation of one or more components of the regimen except for the substitution of stavudine for zidovudine. The secondary objectives were to compare the following between the treatment arms: time to virologic failure; CD4 cell count responses; AIDS-defining events and death; drug resistance profile of virus at the time of virologic failure; and, in subsets of subjects, plasma virus suppression using an assay with a level of detection of 6 RNA copies/mL, quantitative proviral DNA in peripheral blood mononuclear cells (PBMCs), and the frequency of intermittent viremia.
The patients were recruited from 31 adult AIDS Clinical Trials Units in the United States. For prior participants in ACTG 320, the inclusion criteria were original randomization to indinavir, zidovudine (or stavudine), and lamivudine; receipt of indinavir during the course of the trial after reaching a study endpoint (ie, an AIDS-defining illness); a plasma HIV-1 RNA level <500 copies/mL at the end of ACTG 320; and maintenance of the ACTG 320 study regimen until entry into ACTG 372A. For non-ACTG 320 volunteers, the inclusion criteria were documented HIV-1 infection; documentation of a CD4 cell count ≤200/mm3 at the time of initiation of indinavir, zidovudine (or stavudine), and lamivudine; at least 3 months of therapy with this regimen prior to entry into ACTG 372A; a Karnofsky performance ≥70; and age ≥16 years. The study was approved by the institutional review boards of the participating institutions, and all patients gave written, informed consent. ACTG 320 and non–ACTG 320 patients were excluded if they had an AST or ALT >5 times the upper limit of normal or a serum creatinine >3 times the upper limit of normal. Non–ACTG 320 volunteers were also excluded if the hemoglobin was <9.1 g/dL for men or 8.9 g/dL for women, the absolute neutrophil count was <750/mm3, the platelet count was <55,000/mm3, the total bilirubin was >1.5 times the upper limit of normal, or the total serum amylase was >1.5 times the upper limit of normal.
The patients received open-label indinavir 800 mg every 8 hours, zidovudine 300 mg twice daily (or stavudine 40 mg twice daily [30 mg twice daily if the patient’s weight was <60 kg]), and lamivudine 150 mg twice daily and were randomized in a double-blind fashion to abacavir 300 mg twice daily or matching placebo.
Monitoring and Enrollment
The patients were monitored at weeks 2, 4, and 8 and every 8 weeks thereafter until the closure of the study for clinical symptoms and signs, routine hematology, serum chemistries, liver enzymes, CD4 cell counts, and plasma HIV-1 RNA levels; formal adherence monitoring was not assessed. Enrollment began on October 9, 1997, and closed on January 20, 1998. The status of the study was reviewed 4 times by an independent Interim Review Committee in January 1999, January 2000, February 2001, and February 2002. Each time, the Committee’s recommendation was for the study to continue with no substantive changes.
Genotype analysis of plasma virus was performed using the Viroseq kit (Celera, Alameda, California, USA). In those patients who remained on original study treatment and exhibited sustained virologic suppression (HIV-1 RNA <50 copies/mL) throughout the study, plasma HIV-1 RNA levels were determined by a modification of the Roche ultrasensitive assay that permitted HIV-1 RNA quantitation down to a level of 6 copies/mL. Proviral DNA in PBMCs was quantitated by a polymerase chain reaction method previously described.11
Statistical Analysis
The distributions of all time-to-event endpoints were estimated using the method of Kaplan and Meier and were compared using a log-rank test. Patients without events were censored at the time of their last available HIV-1 RNA measurement. All P values and confidence intervals are nominal and unadjusted for multiple comparisons and interim analyses. All reported P values are 2-sided. All safety analyses were as-treated with data censored 8 weeks after permanent discontinuation of treatment.
Analyses of virologic responses included time-to-event analyses of virologic failure at HIV-1 RNA level >200 copies/mL and >50 copies/mL and proportions of patients with HIV-1 RNA levels <50 copies/mL. The impact of intermittent viremia during the first 48 weeks of follow-up (single HIV-1 RNA level >50 copies/mL) on subsequent risk of virologic failure (>200 copies/mL) was evaluated using Cox proportional hazards models in the subgroup of patients at risk for virologic failure at 48 weeks. A chi-square test compared the number of patients with at least one intermittent viremia episode by treatment arm.
In post hoc analyses, the proportions of patients with plasma HIV-1 RNA levels <6 copies/mL and quantitative proviral HIV-1 DNA levels in PBMCs in the 2 study arms were compared in a subset of patients who remained on initial study treatment with plasma HIV-1 RNA levels consistently <50 copies/mL throughout the course of the study.
CD4 cell counts are presented using mean changes from baseline; Wilcoxon rank sum tests were used to compare the CD4 distributions by treatment arm at yearly intervals. Unless otherwise stated, HIV-1 RNA and CD4 analyses presented were intent to treat.
SAS Versions 6 and 9 (SAS Institute Inc, Cary, North Carolina, USA) were used for all analyses.
RESULTS
Accrual and Characteristics of the Patients
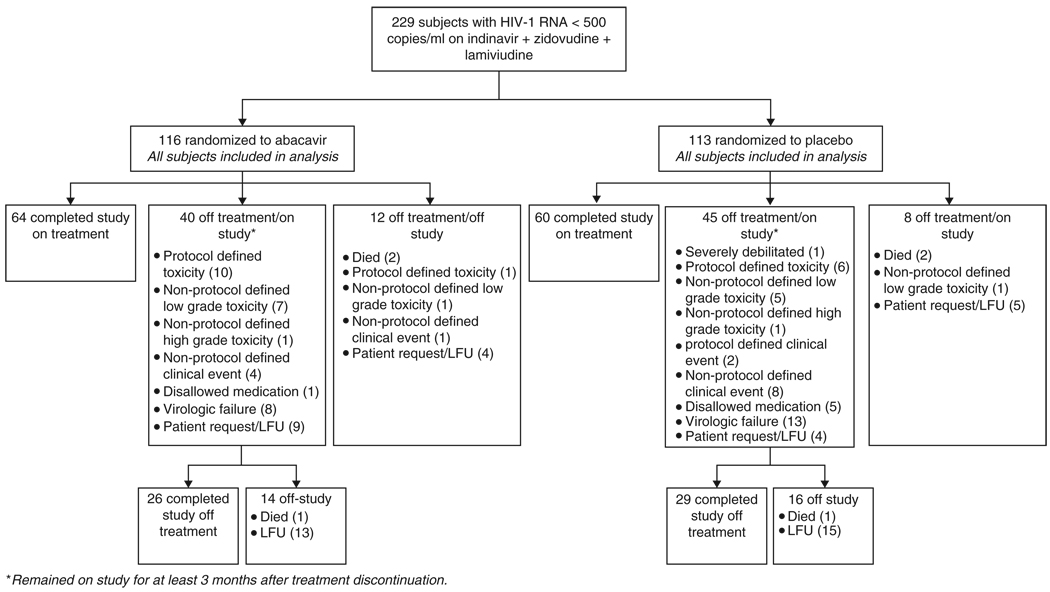
There were 229 patients randomized to the study (Figure 1). Of these, 207 (90%) had received indinavir on enrollment into ACTG 320, 18 (8%) received indinavir in ACTG 320 after reaching a study endpoint, and 4 (2%) were non–ACTG 320 patients. Eighty-eight percent were male; 64% white, non-Hispanic; 15% black, non-Hispanic; 17% Hispanic; and 3% Asian Pacific Islander, American Indian, or Alaskan. The median age was 41 years and the median CD4 cell count was 250/mm3. The baseline characteristics were well balanced across the study arms (Table 1). A total of 36 patients were receiving stavudine in place of zidovudine at the start of the study (18 in each arm).
Figure 1.
Disposition of study subjects. LFU = loss to follow-up.
Table 1.
Baseline characteristics
| Treatment arm |
||||
|---|---|---|---|---|
| All subjects | ZDV/3TC/IDV/ABC | ZDV/3TC/IDV | ||
| Age, years | n | 229 | 116 | 113 |
| Mean (SD) | 42 (9) | 42 (9) | 42 (8) | |
| Gender | Male | 202 (88%) | 101 (87%) | 101 (89%) |
| Female | 27 (12%) | 15 (13%) | 12 (11%) | |
| Race/ethnicity | White, non-Hispanic | 148 (65%) | 76 (66%) | 72 (64%) |
| Black, non-Hispanic | 35 (15%) | 16 (14%) | 19 (17%) | |
| Hispanic | 38 (17%) | 20 (17%) | 18 (16%) | |
| Other | 8 (3%) | 4 (3%) | 4 (4%) | |
| ACTG 320 | In 320 | 225 (98%) | 114 (98%) | 111 (98%) |
| Not in 320 | 4 (2%) | 2 (2%) | 2 (2%) | |
| HIV-1 RNA, copies/mL | <500 | 220 (96%) | 111 (96%) | 109 (96%) |
| 500–1000 | 3 (1%) | 2 (2%) | 1 (1%) | |
| >1000 | 6 (3%) | 3 (3%) | 3 (3%) | |
| CD4 cell count, cells/mm3 | Mean (SD) | 273 (130) | 258 (112) | 289 (145) |
| Median (Q1, Q3) | 250 (185, 341) | 245 (180, 336) | 252 (191, 359) | |
| Min, Max | 53, 767 | 53, 659 | 62, 767 | |
| 51–200 | 71 (31%) | 35 (30%) | 36 (32%) | |
| 201–500 | 145 (63%) | 78 (67%) | 67 (59%) | |
| >500 | 13 (6%) | 3 (3%) | 10 (9%) | |
Note: Values are expressed as n (%), unless otherwise indicated. ZDV = zidovudine; 3TC = lamivudine; IDV = indinavir; ABC = abacavir.
Duration of Follow-Up and Study Treatment
The median duration of follow-up was 277 weeks (range, 30–285). One hundred seventy-nine patients (78%) completed the study, and 124 (54%) completed the study on their original treatment assignment. The median duration of randomized treatment was 272 weeks (range, 3–283). Of the 105 patients prematurely discontinuing study treatment prior to study completion or discontinuation, 17 discontinued for protocol-defined toxicity. Low-grade toxicities (14 patients), virologic failure (21 patients), and non–protocol-defined clinical events (n=13) were the other predominant reasons for patients or clinicians deciding to prematurely discontinue study treatment among those patients completing follow-up (Figure 1). Three subjects switched from zidovudine to stavudine during the course of the study for zidovudine-related toxicity. There were no differences between the arms with respect to study follow-up or duration of study treatment (P = .82).
Primary and Virologic Efficacy Endpoints
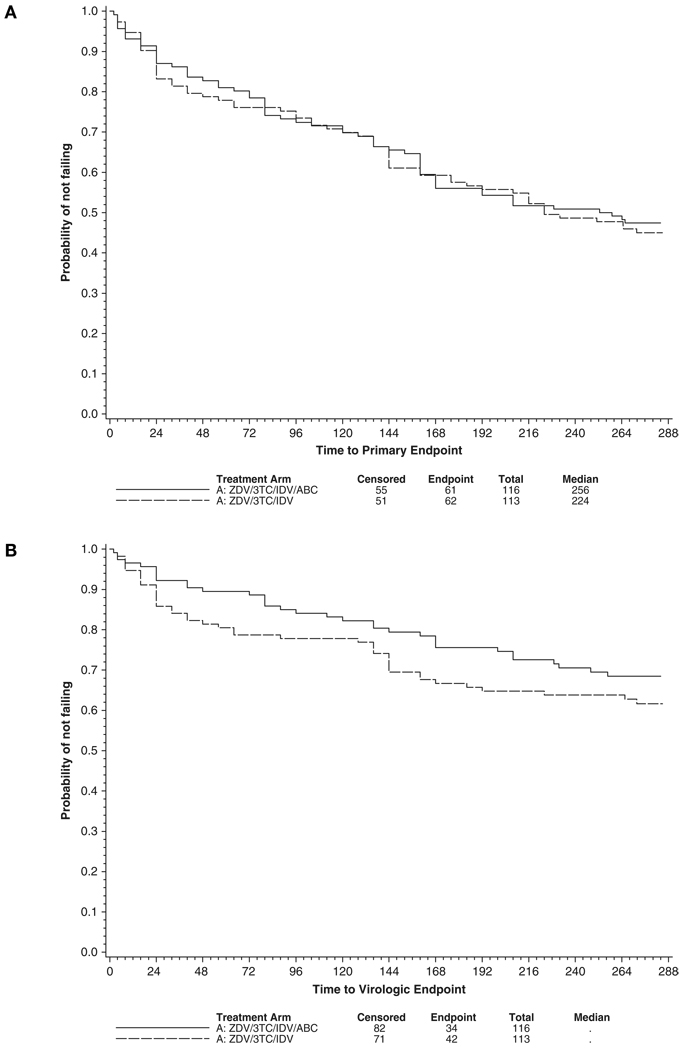
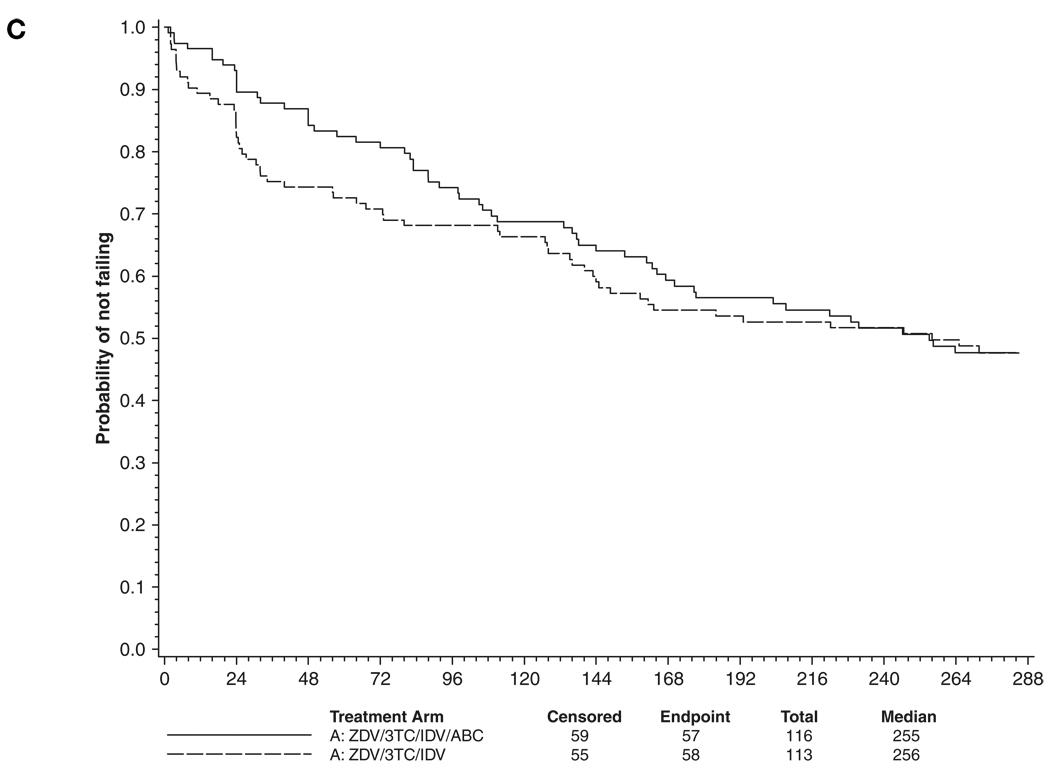
Sixty-one (53%) of 116 patients and 62 (55%) of 113 patients in the abacavir and placebo arms, respectively, reached the primary study endpoint (Figure 2A; P = .77). Thirty-four (29%) of 116 patients and 42 (37%) of 113 patients in the abacavir and placebo arms, respectively, reached the virologic failure endpoint of 2 consecutive plasma HIV-1 RNA levels >200 copies/mL (Figure 2B; P = .22). There was also no significant difference in the distribution of time to virologic failure at the HIV-1 RNA level >50 copies/mL (Figure 2C; P = .65). At week 240, the estimated probability of continued plasma HIV-1 RNA levels <50 copies/mL was 52% (95% confidence interval [95% CI], 42–61) in both the abacavir and the placebo arms.
Figure 2.
Primary and secondary virologic time to event distributions (in weeks). (A) Primary endpoint (first of confirmed HIV-1 RNA level >200 copies/mL or treatment discontinuation) (P = .77). (B) Virologic failure (confirmed HIV-1 RNA level >200 copies/mL) (P = .22). (C) Virologic failure (confirmed HIV-1 RNA level >50 copies/mL) (P = .65). Treatment arms were compared using log rank tests. ZDV = zidovudine; 3TC = lamivudine; IDV = indinavir; ABC = abacavir.
Forty-nine of the 192 patients at risk for virologic failure at week 48 experienced at least one episode of intermittent viremia during the first 48 weeks of the study, with no significant differences between the study arms (P = .13). Compared to patients with HIV-1 RNA levels continually <50 copies/mL, intermittent viremia was not significantly associated with subsequent virologic failure (hazard ratio [HR], 1.06; 95% CI, 0.38–3.01; P = .91).
Samples in the 98 patients who remained on initial study treatment and whose plasma HIV-1 RNA levels were consistently <50 copies/mL throughout the course of the study were reassayed at baseline and at weeks 2, 4, and 48 using a modification of the Roche ultrasensitive plasma HIV-1 RNA assay that lowered the limit of detection from 50 to 6 copies/mL. In the abacavir arm, 51% (23/45), 56% (19/34), 47% (17/36), and 49% (22/45) of samples were below 6 copies/mL at baseline and weeks 2, 4, and 48, respectively. In the placebo arm, 51% (20/39), 62% (21/34), 50% (17/34), and 52% (24/46) were suppressed below 6 copies/mL. In this same subset of patients, 82 had results for quantitation of proviral HIV-1 DNA in PBMCs. After a median of 252 and 246 weeks on study, the median (1st, 3rd quartile) log10 DNA copies per 107 PBMCs in the abacavir arm was 3.72 (3.48, 3.95) and in the placebo arm it was 3.78 (3.60, 4.03), respectively.
Genotype Analysis of Virologic Failure Samples for Drug Resistance
Sequencing of the HIV-1 reverse transcriptase and protease genes from plasma was successful in 69 of the 76 instances of virologic failure (28 of 34 failures in the abacavir arm, 41 of 42 failures in the placebo arm). Table 2 lists the amino acid substitutions in reverse transcriptase and protease by codon site for the abacavir and placebo arms using the mutations listed in the International AIDS Society–USA table (www.iasusa.org). There were no differences in the types or frequencies of amino acid substitutions seen. The M184V/I lamivudine-associated mutation was detected in 46% (13/28) and 45% (18/41) of the plasma samples derived from virologic failures in the abacavir and placebo arms, respectively. Thymidine analog (zidovudine)–related mutations (TAMs) were present at codons 41, 67, 70, 210, 215, and 219 at comparable rates in both arms. Their detection was expected because all original ACTG 320 subjects were required to be zidovudine experienced for inclusion into that study. Supportive data for this were provided by genotyping baseline ACTG 320 samples that were available for 58 patients who subsequently enrolled in ACTG 372A (23 in the abacavir arm, 35 in the placebo arm) and experienced virologic failure. In patients for whom paired samples existed, a high proportion of the patients with TAMs detected at the time of virologic failure in ACTG 372A were found to have had the same mutations at entry into ACTG 320. For each of the following mutations, the number of patients with that mutation at ACTG 320 baseline divided by the number of patients with the same mutation detected at the time of virologic failure in ACTG 372A, grouped across study arms, were as follows: M41L (20/23 [87%]), D67N (23/27 [85%]), K70R (22/23 [96%]), L210W (12/14 [86%]), T215Y/F (32/35 [91%]), and K219Q/E (10/12 [83%]). In contrast, M184V/I was not detected in any of the ACTG 320 baseline samples sequenced.
Table 2.
| Table 2A. Amino acid substitutions in reverse transcriptase determined at the time of virologic failure. | ||
|---|---|---|
| Amino acid substitution | Abacavir arm (n=28) | Placebo arm (n=41) |
| M41L | 10/28 (36%) | 17/41 (41%) |
| E44D | 1/28 (4%) | 1/41 (2%) |
| K65R | 0/28 (0%) | 0/41 (0%) |
| D67N | 12/28 (43%) | 20/41 (49%) |
| K70R | 11/28 (39%) | 16/41 (39%) |
| L74V | 0/28 (0%) | 3/41 (7%) |
| V118I | 1/28 (4%) | 8/41 (20%) |
| M184V | 13/28 (46%) | 18/41 (44%) |
| L210W | 6/28 (21%) | 11/41 (27%) |
| T215F/Y | 16/28 (57%) | 24/41 (59%) |
| K219Q/E | 4/28 (14%) | 10/41 (24%) |
| Table 2B. Amino acid substitutions in protease determined at the time of virologic failure. | ||
|---|---|---|
| Amino acid substitution | Abacavir arm (n=28) | Placebo arm (n=41) |
| L10I/F/V/C | 6/28 (21%) | 5/41 (12%) |
| K20R/M/I/T/V | 1/28 (4%) | 2/41 (5%) |
| L24I | 1/28 (4%) | 1/41 (2%) |
| V32I | 0/28 (0%) | 1/41 (2%) |
| M36I/L/V | 5/28 (18%) | 4/41 (10%) |
| M46I/L | 3/28 (11%) | 5/41 (12%) |
| I54L/V/M/T/A | 1/28 (4%) | 1/41 (2%) |
| A71V/I/T/L | 6/28 (21%) | 5/41 (12%) |
| G73C/S/T/A | 0/28 (0%) | 1/41 (2%) |
| V77I | 10/28 (36%) | 13/41 (32%) |
| V82A/T/F/I | 4/28 (14%) | 4/41 (10%) |
| I84V | 0/28 (0%) | 0/41 (0%) |
| L90M | 1/28 (4%) | 0/41 (0%) |
CD4 Cell Changes
The mean increases in CD4 cell counts from baseline at week 240 in the abacavir and placebo arms were 189/mm3 (range, −124 to 648/mm3) and 177/mm3 (range, −446 to 720/mm3), respectively, with no significant difference in the distributions between treatment arms (P = .37).
Clinical Events
Two AIDS-defining events (1 in each arm) and 10 deaths (6 in abacavir arm, 4 in placebo arm), of which 2 reflected HIV disease progression, occurred during the course of the study.
Adverse Events
The overall proportion of patients with at least one new grade 3 and 4 sign and symptom in the study population over the course of the study was 32%, with general body, gastrointestinal, and neurologic complaints predominating. The overall proportion of patients with at least one new grade 3 and 4 laboratory abnormality was 64%, with elevations in creatine phosphokinase, triglycerides, bilirubin, aspartate aminotransferase, alanine aminotransferase, and amylase predominating. There were no significant differences between the study arms in the overall distribution of the time to first adverse event (P = .48, signs and symptoms; P = .43, laboratory events).
The overall rate of nephrolithiasis was 17%, with 14 events seen in the abacavir arm and 26 in the placebo arm (P = .037). Abacavir hypersensitivity was identified retrospectively as the syndrome had not been fully defined at the initiation of the study. Two cases, yielding a rate of 2%, were identified in the abacavir arm; this rate is lower than that seen in prospective studies before the advent of HLA-B*5701 testing and may relate to the retrospective nature of the case finding.
Eleven patients experienced serious cardiovascular events during study follow-up: 7 (6%) in the abacavir arm and 4 (3.5%) in the placebo arm. The median age at study entry of these 11 patients was 49 years, and 100% were male. Myocardial infarction occurred in 4 and 3 patients, unstable angina in 1 and 0, and cerebrovascular accident in 2 and 1 in the abacavir and placebo arms, respectively. One subject had discontinued study treatment 2 years prior to the event. For the remaining subjects, the time on study (and study treatment) at the time of the event was 99 and 163 weeks in the 2 arms, respectively.
DISCUSSION
Current guidelines for the treatment of HIV-1 infection emphasize the importance of maximal viral suppression to achieve durability of the immunologic and clinical responses and to prevent the emergence of drug resistance.1,2 Operationally this has translated into achieving suppression of plasma HIV-1 RNA to <48 to 75 copies/mL as this is the lower limit of detection of commercially available assays. However, in persons whose plasma HIV-1 RNA levels are consistently suppressed below this level, residual viremia can be detected in most, and replication-competent HIV-1 remains in the memory, resting CD4+ lymphocyte pool. In addition, other viral reservoirs may exist, and viral evolution, albeit limited, may continue.12–21 This raises the question of whether the strategy of intensifying an already successful antiretroviral regimen would yield a clinical benefit in terms of reducing the likelihood of subsequent treatment failure. ACTG 372A was designed to answer this question.
Abacavir was chosen as the drug with which to intensify the indinavir, zidovudine, and lamivudine regimen because efficacy had been demonstrated in other intensification studies in which abacavir had been added to patients with persistent viremia.5,7 The use of a non–ritonavir- boosted, indinavir-containing regimen is outdated by current standards but may have provided a greater likelihood of seeing a benefit from intensification given the superior antiviral potency of ritonavirboosted versus nonboosted protease inhibitors.22
This study is notable for its substantial follow-up (median 4.4 years) and relatively high patient retention (79%). The study was event- rather than time-driven, with the original event target being 80 virologic failures. The study duration, well over 4 years during which 76 virologic failure endpoints accrued, demonstrates how well this originally advanced disease population did on a potent antiretroviral drug combination over the course of several years.
The primary efficacy endpoint of this study was a composite of virologic failure and premature treatment discontinuation in order to reflect clinical practice. A total of 123 endpoints were accrued during the course of the trial, with no significant difference between the abacavir and placebo arms. Similarly, there was no significant difference between the study arms when the virologic endpoints of a confirmed rise in plasma HIV-1 RNA to above 200 copies/mL and virologic suppression below 50 copies/mL during the course of the study were analyzed.
The negative results of the first-order analyses led us to explore other virologic parameters to determine whether a more subtle difference between treatment arms could be detected. Four approaches were taken. These included analyzing the proportion of subjects suppressed to below 6 HIV-1 RNA copies/mL by a modification of the Roche ultrasensitive assay through 48 weeks, quantification of proviral HIV-1 DNA in PBMC after long-term suppression (median 246–252 weeks), the frequency of intermittent viremia (“blips”), and the patterns of drug resistance mutations appearing at the time of virologic failure. None of these analyses revealed differences between the 2 treatment arms, providing additional data in support of the primary analysis.
CD4 cell count increases were substantial and similar across both study arms. No differences in clinical endpoints (AIDS-defining events and deaths) were seen between the arms, but the study was not powered for this comparison.
Overall rates of grade 3 and 4 signs and symptoms and laboratory abnormalities were not significantly different between the 2 treatment arms and no unexpected toxicities were seen. Nephrolithiasis is a well-recognized complication of indinavir therapy. Fewer nephrolithiasis events were seen in the abacavir arm, but there is no evidence for a pharmacokinetic interaction between indinavir and abacavir.23
Subsequent to the closure of ACTG 372A, an association between abacavir and risk for myocardial infarction was reported,24 although this association and any causal relationship remain under debate.25 The placebo-controlled addition of abacavir to a prior regimen, in combination with the long-follow-up in this study, provided an opportunity to examine this question in a retrospective, post hoc fashion. Eleven patients were reported to have experienced serious cardiovascular events during study follow-up: 7 in the abacavir arm and 4 in the placebo arm. These data are presented descriptively; although there were more of these events in the abacavir arm, the numbers are too small to draw comparative conclusions. Overall, the incidence of serious cardiovascular events was low.
Our virologic results differ from the reports of Havlir et al26 and Ramratnam et al.27 In the Havlir et al report, 14 patients treated with indinavir and efavirenz who had plasma HIV-1 RNA levels <50 copies/mL for more than 5 years were studied. Of these 14 patients, 5 were intensified with abacavir. Four of 5 patients with detectable plasma HIV-1 RNA who intensified with abacavir demonstrated a decline in residual viremia with an estimated infected cell half-life of 6.7 days. No differences in CD4 cell count changes were seen. In the Ramratnam et al study, 5 patients on long-term protease inhibitor–containing combinations had their regimens intensified with abacavir or abacavir plus efavirenz. Three of these 5 patients were noted to have a decrease in the size of the memory, resting CD4+ cell latent reservoir. The frequency of intermittent viremia decreased in 4 of 5 of these patients. Both of these studies were small, and there are notable differences from our study in design, drug regimen, assays performed, and duration of follow-up.
In contrast, our findings are supported by the report of Maldarelli et al that the viral set point below 50 copies of HIV-1 RNA/mL in patients on antiretroviral therapy is related to the level of pretherapy viremia and not the potency of the treatment regimen.28 Our results are also consistent with a number of well-powered studies that failed to show that initial therapy with 4 versus 3 drugs provides additional benefit.29–33 Prolonged release of virus from chronically infected cells has been proposed as the origin of residual viremia below 50 HIV-1 RNA copies/mL despite reverse transcriptase inhibitor– and protease inhibitor–based therapies.14 Current therapies, including abacavir, block new cycles of viral replication but do not affect virus production from long-lived chronically infected cells. The lack of effect of abacavir on persistent viremia in our study is consistent with this paradigm.
Interest in intensification strategies has recently been renewed in parallel with the approval of antiretroviral agents with mechanisms of action different from reverse transcriptase and protease inhibitors, specifically, raltegravir, an integrase strand transfer inhibitor, and maraviroc, a CCR5 antagonist. Single-arm and randomized trials of raltegravir intensification ranging from 4 to 48 weeks in individuals virologically suppressed on potent antiretroviral regimens have consistently shown that low-level plasma viremia is not reduced,34–39 a finding compatible with the absence of ongoing viral replication. However, in 2 of these studies, Yukl et al37 reported a decrease in HIV RNA and immune activation in the ileum and Buzon et al38 reported an increase in 2-LTR HIV DNA circles in PBMCs in 29% of patients in the raltegravir arm, suggesting that some individuals may have ongoing viral replication in cell or tissue reservoirs.
Studies of intensification with maraviroc have yielded consistent results: no demonstrable decrease in low-level plasma viremia but some decrease in markers of immune activation in PBMCs and gastrointestinal tract tissue.39–41
Thus, novel strategies will be needed to reach the cellular reservoirs responsible for the irreducible level of detectable viremia.42 Approaches being considered include pharmaco- or immunotherapeutic interventions targeted to disrupt HIV proviral DNA latency in cellular reservoirs, including histone deacetylase inhibitors (more potent than valproic acid43), kinase antagonists (eg, hexamethylbisacetamide), phorbol esters (eg, prostratin), and interleukin-7 (see ref. 42 for review).
Despite its limitations, ACTG 372A is important in that it tested the strategy of late intensification in a virologically suppressed population, and it is the largest and longest randomized trial of this strategy reported to date. This study lends strong support to the current practice of using a potent regimen capable of suppressing plasma HIV-1 RNA levels to <50 copies/mL, but that once this goal is achieved, further intensification of the regimen provides no demonstrable benefit.
ACKNOWLEDGMENTS
Acknowledgments for ACTG 372A
The authors have received the following support: Clinical Trials Unit (CTU) grant AI069470 and Clinical and Translational Science Award (CTSA) RR024156 (Dr. Hammer); CTU grant AI069494-03S1 (Dr. Mellors); CTU grant AI068636 (Dr. Morse); ACTG Pharmacology Specialty Laboratory grant BRS-ACURE-Q-06-00140-T001 (Social and Scientific Systems).
Additional protocol team members: Michael Robertson (Merck Research Laboratories), Steven Schnittman (Bristol-Myers Squibb), Marjorie Dehlinger (Division of AIDS, NIAID), and Ann Walawander (Frontier Science & Technology Research Foundation).
Clinical site personnel and grant support: Judith Feinberg and Sharon Kohrs (University of Cincinnati College of Medicine), CTU grant AI-069513; Michael Para and Kathy Watson (Ohio State University), CTU grant AI069474; Jorge Santana Bagur and Santiago Marrero (University of Puerto Rico), CTU grant AI069415; David Ragan, Cheryl Marcus, and Joseph Eron (University of North Carolina), CTU grant AI069423, GCRC grant RR00046, CTSA grant RR025747, CFAR grant AI50410; Hector Bolivar and Sandra Navarro (University of Miami), CTU grant AI069477 and AI027675; CFAR grant AI073961; Ge-Youl Kim and Michael Klebert (Washington University at St. Louis), CTU grant AI069495; Ilene Wiggins and Andrea Weiss (Johns Hopkins University), CTU grant AI069465, GCRC grant RR023561; Dee Dee Pacheco and Jill Kunkel (University of California San Diego), CTU grant AI 69432; Karen Cavanagh and Janet Forcht (New York University/Bellevue Hospital Center), CTU grant AI027665 and AI069532; Charles Hicks and Joan Riddle (Duke University Medical Center), CTU grant AI069484; Barbara Gripshover and Jane Baum (Case Western University), CTU grant AI069501; Kenneth Fife and Beth Zwickl (Indiana University), CTU grant AI025859; Melinda Robertson and Rebecca Creamer (University of Alabama at Birmingham), CTU grant AI069452; Henry Balfour, Jr and Heather Vezina (University of Minnesota), CTU grant AI2766; William O’Brien and Gerianne Casey (University of Texas, Galveston), CTU grant AI032782; Mary Albrecht and Carol Silver (Beth Israel Deaconess Medical Center); CTU grant AI069472; Sadia Shaik and Mario Guerrero (Harbor-UCLA Medical Center), CTU grant AI069424; Mussolini Africano and Luis M. Mendez (University of Southern California), CTU grant AI069428; Nancy Hanks and Scott Souza (University of Hawaii), CTU grant AI032853; Rob Roy MacGregor and Isabel Matozzo (University of Pennsylvania), CTU grant AI-069467; CFAR grant AI045008; Jane Reid and Carol Greisberger (University of Rochester), CTU grant AI069511, GCRC grant RR 024160; Juan Lertora and Erin Plaia (Tulane University) GCRC grant RR05096; Kim Whitely and Robert Kalayjian (Metrohealth Medical Center, Cleveland) CTU grant AI069501; Lisa Dasnoit and Tim Lane (Moses Cone Memorial Hospital, Greensboro, NC), CTU grant AI069423; Beverly Putnam and Graham Ray (University of Colorado), CTU grant AI69450, GCRC grant RR025780, CFAR grant AI54907; Michael Morgan and Janet Nicotera (Vanderbilt University), CTU grant AI069439; Susan Swindells and Frances Van Meter (University of Nebraska Medical Center), CTU grant AI027661; Sharon Hewitt and James Horton (Carolinas Medical Center, Charlotte); Melody Palmore and Betsy Hall (Emory University), CTU grants AI069418 and AI050409; Valery Hughes and Todd Stroberg (Cornell University), CTU grant AI069419, GCRC grant RR-024996; Jeffery Meier and Jack Stapleton (University of Iowa Hospitals and Clinics), CTU grants AI027661 and AI058740; Donna Mildvan and Gwendolyn Costantini (Beth Israel Medical Center), CTU grant AI046370; Harold Kessler and Ruth Davis (Rush Presbyterian/St. Luke’s Medical Center), CTU grant AI069471; Joseph Pulvirenti (Cook County Hospital); Timothy Cooley and Ruth Haivanis (Boston Medical Center), CTU grant AI069472; Jane Norris and Patricia Cain (Stanford University), CTU grant AI069556; Debbie Slamowitz and Sandra Valle (San Mateo County AIDS Program), CTU grant AI069556; Tamara O’Hara (State University at Buffalo); Robert Murphy and Baiba Berzins (Northwestern University), CTU grant AI069471; Neel French (Louis A. Weiss Memorial Hospital).
Footnotes
Study Sponsor
This study was performed by the AIDS Clinical Trials Group and was sponsored by the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases, National Institutes of Health. Data management and analysis were performed under the auspices of the ACTG, and all authors had access to original datasets. Drugs were supplied by GlaxoSmithKline, Merck Research Laboratories, and Bristol-Myers Squibb through clinical trials agreements with DAIDS. This study is registered as NCT00000885 at ClinicalTrials.gov.
Financial Disclosure
Dr. Hammer serves as consultant to Merck and Progenics and served on a Data Monitoring Committee for Bristol-Myers Squibb. Dr. Mellors is a consultant to Merck, Gilead Sciences, and RFS Pharma and is owner of stock options in RFS Pharma. Dr. Demeter’s spouse receives royalty income from GlaxoSmithKline and Merck related to HPV vaccines. Dr. Demeter has no HIV-related conflicts. Dr. Coombs is a consultant to Merck. Dr. Gerber is a consultant to Merck. Dr. Spreen is a GlaxoSmithKline employee and shareholder. Dr. Squires receives grant support from Biocryst, Gilead Sciences, GlaxoSmithKline, Merck, and Tibotec and is consultant to and receives honoraria from Gilead Sciences, GlaxoSmithKline, Merck, Schering-Plough, Tibotec, and Tobira.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed July 23, 2010];Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. 2009 December 1;:1–161. http://www.AIDSinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 2.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 3.Demeter LM, Hughes MD, Coombs RW, et al. Predictors of virologic and clinical outcomes in HIV-1-infected patients receiving concurrent treatment with indinavir, zidovudine and lamivudine. AIDS Clinical Trials Group Protocol 320. Ann Intern Med. 2001;135(11):954–964. doi: 10.7326/0003-4819-135-11-200112040-00007. [DOI] [PubMed] [Google Scholar]
- 4.Fang CT, Chang YY, Hsu HM, et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. Q J Med. 2007;100(2):97–105. doi: 10.1093/qjmed/hcl141. [DOI] [PubMed] [Google Scholar]
- 5.Degen O, van Lunzen J, Stellbrink HJ. Intensification of background antiretroviral therapy with abacavir during low-level failure may restore optimal suppression. Antivir Ther. 2000;5(2):91–94. [PubMed] [Google Scholar]
- 6.Gulick RM, Lalama CM, Ribaudo HJ, et al. Intensification of a triple-nucleoside regimen with tenofovir or efavirenz in HIV-1-infected patients with virological suppression. AIDS. 2007;21(7):813–823. doi: 10.1097/QAD.0b013e32805e8753. [DOI] [PubMed] [Google Scholar]
- 7.Katlama C, Clotet B, Plettenberg A, et al. Intensification of stable background therapy with abacavir in antiretroviral therapy experienced patients: 48-week data from a randomized, double-blind trial. HIV Med. 2001;2(1):27–34. doi: 10.1046/j.1468-1293.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 8.Khanlou H, Guyer B, Farthing C. Efficacy of tenofovir as intensification of zidovudine/lamivudine/abacavir fixed-dose combination in the treatment of HIV-positive patients. J Acquir Immune Defic Syndr. 2005;38(5):627–628. doi: 10.1097/01.qai.0000159322.21133.74. [DOI] [PubMed] [Google Scholar]
- 9.Squires K, Pozniak AL, Pierone G, Jr, et al. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann Intern Med. 2003;139(5 Pt 1):313–320. doi: 10.7326/0003-4819-139-5_part_1-200309020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 11.Krieger JN, Nirapathpongporn A, Chaiyaporn M, et al. Vasectomy and human immunodeficiency virus type 1 in semen. J Urol. 1998;159(3):820–825. discussion 5–6. [PubMed] [Google Scholar]
- 12.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94(24):13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 14.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80(13):6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunthard HF, Frost SD, Leigh-Brown AJ, et al. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J Virol. 1999;73(11):9404–9412. doi: 10.1128/jvi.73.11.9404-9412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkosky J, Sullivan J, Xu Y, et al. Genotypic alteration of HAART-persistent HIV-1 reservoirs in vivo. Virology. 2003;314(2):617–629. doi: 10.1016/s0042-6822(03)00464-1. [DOI] [PubMed] [Google Scholar]
- 17.Lambotte O, Chaix ML, Gubler B, et al. The lymphocyte HIV reservoir in patients on long-term HAART is a memory of virus evolution. AIDS. 2004;18(8):1147–1158. doi: 10.1097/00002030-200405210-00008. [DOI] [PubMed] [Google Scholar]
- 18.Persaud D, Ray SC, Kajdas J, et al. Slow human immunodeficiency virus type 1 evolution in viral reservoirs in infants treated with effective antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23(3):381–390. doi: 10.1089/aid.2006.0175. [DOI] [PubMed] [Google Scholar]
- 19.Siliciano JD, Siliciano RF. A long-term latent reservoir for HIV-1: discovery and clinical implications. J Antimicrob Chemother. 2004;54(1):6–9. doi: 10.1093/jac/dkh292. [DOI] [PubMed] [Google Scholar]
- 20.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340(21):1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346(26):2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 23.DiCenzo R, Forrest A, Squires KE, et al. Indinavir, efavirenz, and abacavir pharmacokinetics in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47:1929–1935. doi: 10.1128/AAC.47.6.1929-1935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D:A:D Study Group. Sabin CA, Worm SW, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371(9622):1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brothers CH, Hernandez JE, Cutrell AG, et al. Risk of myocardial infarction and abacavir therapy: no increased risk acroiss 52 GlaxoSmithKline-sponsored clinical trials in adult subjects. J Acquir Immune Defic Syndr. 2009;51(1):20–28. doi: 10.1097/QAI.0b013e31819ff0e6. [DOI] [PubMed] [Google Scholar]
- 26.Havlir DV, Strain MC, Clerici M, et al. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J Virol. 2003;77(20):11212–11219. doi: 10.1128/JVI.77.20.11212-11219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramratnam B, Ribeiro R, He T, et al. Intensification of antiretroviral therapy accelerates the decay of the HIV-1 latent reservoir and decreases, but does not eliminate, ongoing virus replication. J Acquir Immune Defic Syndr. 2004;35(1):33–37. doi: 10.1097/00126334-200401010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischl MA, Ribaudo HJ, Collier AC, et al. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. J Infect Dis. 2003;188(5):625–634. doi: 10.1086/377311. [DOI] [PubMed] [Google Scholar]
- 30.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296(7):769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 31.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeni P, Cooper DA, Aboulker JP, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet. 2006;368(9532):287–298. doi: 10.1016/S0140-6736(06)69074-0. [DOI] [PubMed] [Google Scholar]
- 34.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e10000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–919. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiegand A, Cossarini F, Poethke C, et al. Raltegravir intensification does not reduce persistent HIV-1 viremia in treatment-experienced patients. Program and abstracts from the 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco. Abstract 280. [Google Scholar]
- 37.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 39.Evering T, Mehandru S, Poles M, et al. Antiviral and immunological effects of intensification of suppressive ART with maraviroc, a CCR5 antagonist. Program and abstracts from the 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco. Abstract 283. [Google Scholar]
- 40.Gutierrez C, Diaz L, Hernandez-Novoa B, et al. Effect of the intensification with a CCR5 antagonist on the decay of the HIV-1 latent reservoir and residual viremia. Program and abstracts from the 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco. Abstract 284. [Google Scholar]
- 41.Wilkin T, Lalama C, Tenorio A, et al. Maraviroc intensification for suboptimal CD4+ cell response despite sustained virologic suppression: ACTG 5256. Program and abstracts from the 17th Conference on Retroviruses and Opportunistic Infections; February 16–19; 2010; San Francisco. Abstract 285. [Google Scholar]
- 42.Richman DD, Margolis DM, Delaney M, et al. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 43.Archin NM, Cheema M, Parker D, et al. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell function. PLoS ONE. 2010;5:e9390. doi: 10.1371/journal.pone.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]