Abstract
Emphysema is largely an under-diagnosed medical condition that can exist in smokers in the absence of airway obstruction. We aimed to determine the sensitivity and specificity of pulmonary function tests (PFTs) in assessing emphysema using quantitative CT scans as the reference standard. We enrolled 224 ever-smokers (current or former) over the age of 40. CT of thorax was used to quantify the low attenuation area (% emphysema), and to measure the standardized airway wall thickness. PFTs were used individually and in combination to predict their ability to discriminate radiographic emphysema. Significant emphysema (>7%) was detected in 122 (54%) subjects. Twenty six (21%) emphysema subjects had no evidence of airflow obstruction (FEV1/FVC ratio <70%), while all subjects with >23% emphysema showed airflow obstruction. The sensitivity and specificity of spirometry for detecting radiographic emphysema were 79% and 75%, respectively. Standardized airway wall thickness was increased in subjects with airflow obstruction, but did not correlate with emphysema severity. In this cohort of lifetime ever-smokers, PFTs alone were inadequate for diagnosing emphysema. Airway wall thickness quantified by CT morphometry was associated with airflow limitation, but not with emphysema indicating that the heterogeneous nature of lung disease in smokers may represent distinct phenotypes.
Keywords: airflow limitation, chronic obstructive pulmonary disease, CT morphometry, emphysema, airway wall thickness, pulmonary function test
1. Introduction
The prevalence of cigarette smoking continues to rise in most developing countries around the world, but in the United States, former smokers now outnumber current smokers [1–3]. Despite smoking cessation, recent demographic data suggest that many ever-smokers (herein defined as former or current smokers) develop chronic obstructive pulmonary disease (COPD) [4,5]. A complete phenotypic characterization of lung disease in smokers is often delayed until the onset of self-reported clinical symptoms and findings of airflow obstruction on pulmonary function tests (PFTs), which further obscures the true prevalence and extent of tissue damage in the smoking population. While newer studies increasingly document the genetic and immune factors critical to the underlying pathogenesis of COPD, a comprehensive understanding of lung parenchymal destruction in ever-smokers remains elusive [6–8].
An exaggerated reduction in Forced Expiratory Volume in 1 second (FEV1), either absolute or expressed as a trend over time, has been shown to have an inverse association with life expectancy [9] and previous studies have indicated that airflow limitation correlated directly with qualitative measurements of emphysema in ever-smokers [10–12]. However the degree of airflow obstruction, if any, is highly variable even among ever-smokers with equal pack year histories [13].
The American Thoracic Society (ATS) and European Respiratory Society (ERS) have advocated the use of the lower limit of normal (LLN) based on the National Health and Nutrition Examination Survey (NHANES) data on lung function in a healthy population [14,15] to diagnose COPD. Alternatively, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) has in the last decade promoted the use of a fixed FEV1/FVC ratio of less than 70% to diagnose COPD [16]. This latter guideline is now widely used to classify smokers with and without COPD. Based on lung function and symptoms, smokers are categorized into one of four GOLD stages: mild, moderate, severe and very severe [16]. There is a lack of knowledge of the prevalence and natural history of lung parenchymal destruction as seen in emphysema. The importance of determining the presence of emphysema in ever smokers, independent of airway obstruction, has recently become more recognized [17]. In particular, emphysema has been found to be an independent risk factor for cardiovascular diseases [18] and is an under-detected clinical condition in ever-smokers. Epidemiological studies have shown that lung cancer risk is strongly related to radiographic emphysema, independent of airflow obstruction [19–22]. Recently released data from the National Lung Screening Trial show that CT scan-based screening of over 53,000 smokers reduced lung cancer deaths by 20%; however obtaining CT scans may not be a feasible option for all smokers given the risk of radiation exposure and cost concerns [23,24]. Therefore, evaluation of currently available tests (such as PFT and spirometry) that may discriminate smokers with emphysema are much needed to better identify those who are at a higher risk for lung cancer, and who would benefit most from CT scan-based cancer surveillance.
Past studies have demonstrated that CT diagnosis by visual assessment is closely correlated with pathologic emphysema grade [25,26] and allows for accurate diagnosis of emphysema. Further, qualitative assessment of emphysema severity has been shown to relate to FEV1 impairment and airway wall thickness (AWT) [27]. AWT can also be quantified by CT imaging, and has recently been shown to correlate with symptoms in COPD patients [28,29]. To gain further insight into the relationships between airflow obstruction and emphysema, we designed a study to determine accuracy of pulmonary function testing in the diagnosis of emphysema, as measured by CT morphometry, in a cohort of ever-smokers [30,31]. A second aim of the study was designed to evaluate the relationship of airway wall thickness (AWT), with airflow obstruction and with the degree of (%) emphysema. A portion of this work was recently reported as an abstract [32].
2. Materials and Methods
2.1. Clinical and Demographic Characteristic of the Study Participants
We prospectively recruited 224 ever-smokers as part of the Longitudinal Exacerbation Study of COPD (LES-COPD). Enrollment criteria included age over 40, no history of concurrent lung cancer, chest surgery, or chronic lung diseases other than COPD (e.g., sarcoidosis, fibrosis, etc.). Random participants that met our inclusion/exclusion criteria were enrolled from local newspaper advertisements and at three clinics within the Texas Medical Center, in Houston, Texas: the Ben Taub General Hospital, Baylor Clinic, and Michael E. DeBakey Veterans Administration (VA) hospital. All studies were approved by the Institutional Review Board at Baylor College of Medicine (H-18029; Longitudinal Exacerbation Study of COPD) and written informed consent was obtained from all study participants.
2.2. Phenotypic Characterization of Former and Current Smokers
All pulmonary function tests were performed in diagnostic laboratories (Michael E. DeBakey VA and BCM clinics) that use standardized equipments and follow ATS/ERS guidelines; if the FEV1 was reduced below 80% of predicted or FEV1/FVC was below 70%, the participants received two doses of bronchodilator (albuterol 180 microgram) and a repeat measurement of spirometry was performed according to the ATS/ERS guidelines [14]. Calculation of all pulmonary function tests, in relation to reference values, were performed using computer programs and reviewed by trained physicians, without knowledge of the CT morphometry results. Normative values for spirometry were based on NHANES III data and absolute lung volumes were measured using plethysmograph [33]. Lung volume normative values were based on the equations endorsed by the ERS [15], with the upper limits of normal (ULN) calculated based on the 95% confidence interval. The current ATS/ERS guidelines [14], do not recommend a single reference equation for the DLCO, due to measurement variability among laboratories; we calculated our normal values based on three representative equations: Crapo [34], Cotes [35], and Miller [36], and used the latter equation as the most optimal predictive values with the lower limit of normal (LLN) calculated based on the 95% confidence interval.
2.3. Quantitative CT Morphometry Assessment of Emphysema
CT scans of the lung were acquired with the subject in supine position during end-inspiratory breath holding using a Siemens Cardiac Sensation Cardiac scanner (Siemens Medical Solutions). The subjects were coached to take a full breath, with imaging parameters of 120 kVp, 130 100 mAs and a pitch of 1.75. The images were reconstructed at 1 and 5 mm thickness using both an intermediate (b35f) and a high (b65f) spatial frequency reconstruction algorithm. The de-identified CT images were archived on to CDs, sent to the University of British Columbia (UBC), and were analyzed using the previously validated EmphylxJ custom software [30,37]. Briefly, emphysema was assessed by segmenting the lung parenchyma from the chest wall and large central blood vessels using a modified border tracing with a prior position-knowledge algorithm. A value of greater than 7% Low Attenuation Area (%LAA) below −950 HU was used as the upper limit of non-emphysematous lung; this value has been carefully validated with macroscopic and microscopic evaluation of lung tissue, which found that 6.8% LAA represents the upper limit of non-emphysematous lung [25,26,37,38].
2.4. Measurement of Airway Wall Thickness
The airway wall thickness (AWT) was determined using the algorithm as previously devised at iCAPTURE center at UBC [39]. Briefly, airways that were cut in an acceptable cross section (long/short internal diameter 2.2 or less) were identified and AWT analysis was performed using the 1 mm thick CT images. Every 10th image was chosen using the spatial frequency reconstruction algorithm CT (b65f), to sample unique airways; on average 39 ± 23 airways per subject were measured (minimum = 5, maximum = 118). Briefly, a seed point was placed in the lumen of the airway, and radial lines were drawn to find the internal and external points at which the X-ray attenuation value was half the maximum value within the wall as we have described [40,41]. A standardized measurement of AWT was obtained to minimize sampling bias; the measurements of internal perimeter (Pi) was correlated with the square root of airway wall area for each subject as described [40,41].
2.5. Statistical Analysis
Since the data were not normally distributed, the degree of linear association between two continuous measures was assessed using Pearson’s correlation coefficient. Comparisons between unmatched pairs were performed using the Mann-Whitney U non-parametric test. Test characteristics for PFT (sensitivity, specificity, likelihood ratios, and predictive values) were calculated using previously described formulas [15,42] and using CT quantified emphysema (%LAA) with ≥7% as the reference standard [25,26,37,38]. Test characteristics were also calculated for combinations of PFTs, whereas if any component of the combination is positive, the combination is considered positive. Each combination includes FEV1/FVC < 70%, as this is the current standard for diagnosis of COPD. Positive (PPV) and negative (NPV) predictive values were also calculated based on this study population, as the exact prevalence of anatomic emphysema in ever-smokers has not yet been defined in the U.S. or worldwide populations. Statistical significance was defined as a p value less than 0.05.
3. Results
3.1. Demographics Distributed by GOLD Classification and CT Evidence of Emphysema
In all, 235 ever smokers were enrolled, of which five were excluded or withdrew consent. Six subjects performed PFT, but failed to obtain CT scan, therefore the analyses included data collected from 224 subjects that completed both PFT and CT (Figure 1A). The clinical and demographic characteristics of study subjects, categorized by GOLD criteria, are shown in Table 1.
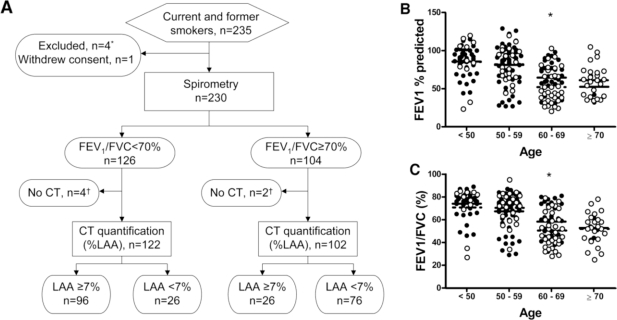
Figure 1.
Schematic overview of the study design and FEV1% predicted and FEV1/FVC ratio among ever-smokers. (A) Flowchart of the recruitment, exclusions, dropouts, measurements, and results in evaluating the diagnostic accuracy of spirometry (FEV1/FVC < 70%) in the detection of emphysema, as defined by CT quantification (Low Attenuation Area; LAA ≥ 7%) is shown. The number of participants recruited, excluded, and measured is shown within the respective boxes. # Four subjects were excluded (three for concomitant lung disease; one for α-1-antitrypsin deficiency). ¶ Six total subjects did not have CT performed (two expired; three lost to follow-up; one withdrew consent). (B) FEV1% predicted was plotted by age group among current (N = 133, solid circles) and former (N = 91, open circles) smokers. (C) FEV1/FVC ratio was plotted by age group among current (N = 133, solid circles) and former (N = 91, closed circles) smokers. The group means are depicted by solid (current smokers) and dashed (former smokers) lines. * P < 0.05 current smokers versus former smokers.
Table 1.
The clinical and demographic characteristics of study subjects, categorized by GOLD criteria.
| Stage | NGC (previously Stage 0) | COPD (GOLD) | |||
|---|---|---|---|---|---|
| (I) | (II) | (III) | (IV) | ||
| Number | 102 | 18 | 55 | 39 | 10 |
| Age, mean (±s.d.) | 53 ± 8 | 55 ± 9 | 64 ± 9 | 64 ± 9 | 59 ± 7 |
| Height, cm (±s.d.) | 170 ± 9 | 174 ± 11 | 174 ± 9 | 175 ± 8 | 173 ± 8 |
| Weight, kg (±s.d.) | 88 ± 21 | 79 ± 12 | 88 ± 18 | 80 ± 19 | 74 ± 16 |
| Male, no. (%) | 42 (41%) | 14 (78%) | 46 (84%) | 35 (90%) | 8 (80%) |
| Ethnicity, no. (%) | |||||
| Black | 60 (59%) | 7 (39%) | 12 (22%) | 13 (33%) | 2 (20%) |
| Hispanic | 4 (4%) | 1 (6%) | 1 (2%) | 1 (3%) | 1 (10%) |
| White | 35 (34%) | 13 (56%) | 42 (76%) | 25 (64%) | 7 (70%) |
| Other | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Smoking status, no. (%) | |||||
| Current | 72 (71%) | 14 (78%) | 29 (53%) | 14 (36%) | 4 (40%) |
| Former | 30 (29%) | 4 (22%) | 26 (47%) | 25 (64%) | 6 (60%) |
| Pack-years, mean (±s.d.) | 33 ± 30 | 40 ± 18 | 69 ± 41 | 66 ± 35 | 62 ± 24 |
| Quitting years, mean (±s.d.) | 14 ± 11 | 21 ± 11 | 12 ± 11 | 9 ± 10 | 9 ± 6 |
| Lung Function | |||||
| % FEV1 (±s.d.) | 92 ± 15 | 90 ± 7 | 64 ± 8 | 39 ± 5 | 25 ± 3 |
| FEV1/FVC ratio (±s.d.) | 78 ± 5 | 66 ± 4 | 56 ± 8 | 42 ± 8 | 34 ± 5 |
| % DLCO (±s.d.) | 78 ± 16 | 63 ± 20 | 59 ± 14 | 42 ± 15 | 35 ± 13 |
NGC: “Not GOLD Classified,” current and former smokers without airflow obstruction; s.d.: standard deviation; cm: centimeters; kg: kilograms; no.: number; %FEV1: forced expiratory volume in 1 second, percent of predicted (post-bronchodilator); FVC: forced vital capacity; %DLCO: diffusing capacity for carbon monoxide, percent of predicted; pack-years: number of years smoked multiplied by packs per day; quitting years: number of years since last reported smoking activity.
A total of 102 participants did not meet GOLD criteria for COPD (i.e., FEV1/FVC < 70%) and were thus labeled as not GOLD classified (NGC). When FEV1% predicted and FEV1/FVC ratio (%) were plotted by age group [Figures 1(B,C)], we found that individuals >60 years of age had more severe disease than the younger individuals, though each group is well represented in our study population.
The demographic and clinical characteristics of the study subjects, categorized by presence or absence of CT-based emphysema using low attenuation area (LAA) are shown in Table 2. The stratification that distinguishes patients with significant emphysema defined as ≥7% in ever-smokers is based on published studies using a healthy cohort as control subjects where CT images were analyzed using custom software (EmphylxJ) as previously described [43,44]. Slightly older subjects and predominantly former smokers were over-represented in the emphysema group, compared with the group without emphysema. Those with emphysema ≥7% had significantly lower FEV1% of predicted, FEV1/FVC ratios and DLCO% of predicted than those without significant emphysema.
Table 2.
Clinical and demographic information of the study participants when separated by CT quantification.
| CT Quantification | LAA < 7% No emphysema | LAA ≥ 7% Emphysema |
|---|---|---|
| Number | 102 | 122 |
| Age, mean (±s.d.) | 53 ± 8 | 62 ± 10 |
| Height, cm (±s.d.) | 170 ± 10 | 174 ± 9 |
| Weight, kg (±s.d.) | 85 ± 20 | 86 ± 19 |
| Male, no. (%) | 45 (44%) | 100 (82%) |
| Ethnicity, no. (%) | ||
| Black | 59 (58%) | 35 (29%) |
| Hispanic | 4 (4%) | 4 (3%) |
| White | 37 (36%) | 82 (67%) |
| Other | 2 (2%) | 1 (1%) |
| Smoking status, no. (%) | ||
| Current | 84 (82%) | 49 (40%) |
| Former | 18 (18%) | 73 (60%) |
| Pack-years, mean (±s.d.) | 41 ± 36 | 56 ± 36 |
| Quitting years, mean (±s.d.) | 13 ± 13 | 12 ± 10 |
| Lung function | ||
| %FEV1 (±s.d.) | 87 ± 18 | 62 ± 26 |
| FEV1/FVC (±s.d.) | 74 ± 10 | 55 ± 16 |
| %DLCO (±s.d.) | 74 ± 16 | 57 ± 22 |
CT: computerized tomography; LAA: low attenuation area (% emphysema) by CT quantification; s.d.: standard deviation; cm: centimeters; kg: kilograms; no.: number; %FEV1: forced expiratory volume in 1 second, percent of predicted (post-bronchodilator); %DLCO: diffusing capacity for carbon monoxide, percent of predicted; pack-years: years smoked x packs per day; quitting years: number of years since last reported smoking activity.
3.2. Diagnostic Value of Pulmonary Function Tests in Emphysema
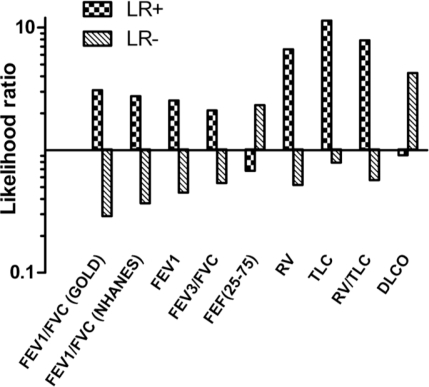
We next measured the sensitivity and specificity of individual pulmonary function tests for predicting emphysema (LAA ≥ 7%) (Table 3). Positive and negative likelihood ratios (LR+, LR− respectively), and positive and negative predictive values (PPV and NPV respectively) for each pulmonary function test were also determined (Figure 2).
Table 3.
Individual pulmonary function tests as predictors of radiographic emphysema.
| Criteria | Sensitivity % (95% CI) | Specificity % (95% CI) | LR+ | LR− | PPV % | NPV % |
|---|---|---|---|---|---|---|
| FEV1/FVC < 70% | 79 (71–85) | 75 (65–82) | 3.09 | 0.29 | 79 | 75 |
| FEV1/FVC < LLN (NHANES) | 73 (65–80) | 74 (64–81) | 2.76 | 0.37 | 77 | 68 |
| FEV1< LLN | 67 (59–75) | 74 (64–81) | 2.54 | 0.45 | 75 | 65 |
| FEV3/FVC < LLN | 62 (53–70) | 71 (62–79) | 2.11 | 0.54 | 71 | 61 |
| FEF25–75 < LLN | 68 (59–76) | 43 (34–53) | 0.68 | 2.32 | 59 | 54 |
| RV > ULN | 52 (43–61) | 92 (86–96) | 6.64 | 0.52 | 89 | 62 |
| TLC > ULN | 22 (16–30) | 98 (94–100) | 11.38 | 0.79 | 93 | 52 |
| RV/TLC > ULN | 46 (38–55) | 94 (88–98) | 7.87 | 0.57 | 90 | 60 |
| DLCO < LLN | 91 (84–95) | 23 (16–33) | 0.91 | 4.26 | 59 | 68 |
LLN: lower limit of normal; ULN: upper limit of normal; CI: confidence interval; LR+: positive likelihood ratio; LR−: negative likelihood ratio; PPV: positive predictive value; NPV: negative predictive value; %FEV1: forced expiratory volume in 1 second, % of predicted (post-bronchodilator); FVC: forced vital capacity; FEV3: forced expiratory volume in 3 seconds; FEF25–75: forced expiratory flow between 25% and 75% of expired FVC; RV: residual volume; TLC: total lung capacity, % of predicted; %DLCO: diffusing capacity for carbon monoxide, % of predicted.
Figure 2.
Likelihood ratios (LR) for detection of emphysema from pulmonary function tests. The positive (+) and negative (−) likelihood ratios for detection of emphysema as assessed by individual PFTs are shown where a LR+ of >10, or LR− of <0.1 represent conclusive increase in the likelihood of the presence, or absence of emphysema respectively.
The sensitivity and specificity of FEV1/FVC < 70% were 79% and 75%, respectively for detecting emphysema. Similar sensitivity and specificity were found when using NHANES reference values (73% and 74% respectively), indicating that both cutoff values are similar for the detection of significant emphysema in our cohort. The PPV and NPV for FEV1/FVC < 70% were 79% and 75%, respectively. The only pulmonary function test that met the threshold (LR+ > 10; LR− < 0.1) for a conclusive test in identifying emphysema was, TLC > upper limit of normal (ULN) (Figure 2).
However in the absence of airflow obstruction, the TLC was abnormal only three times, yielding a specificity of 99% but a sensitivity of only 8%. Combining pulmonary function tests proved no better than individual tests in discriminating emphysema (Table 4). As expected, increasing the number of abnormal tests increased the sensitivity of discriminating emphysema but resulted in drastically reduced specificity. For example, the combination of airflow obstruction (FEV1/FVC < 70%), elevated lung volumes (RV > ULN and TLC > ULN), and reduced DLCO (<LLN) resulted in 95% sensitivity. However, the specificity for emphysema using this combination dropped to 21%. None of the combinations met likelihood ratio criteria for a definitive test and none of the combinations out-performed an FEV1/FVC < 70% with regards to LR+.
Table 4.
Combined pulmonary function tests as predictors of radiographic emphysema.
| Criteria | Sensitivity % (95% CI) | Specificity % (95% CI) | LR+ | LR− |
|---|---|---|---|---|
| FEV1/FVC < 70% and FEV1/FVC < LLN (NHANES) | 79 (71–85) | 74 (64–81) | 2.97 | 0.29 |
| FEV1/FVC < 70% and RV/TLC > ULN | 79 (72–87) | 72 (62–80) | 2.79 | 0.29 |
| FEV1/FVC <70% and FEV1 < LLN | 80 (73–87) | 63 (53–72) | 2.16 | 0.31 |
| FEV1/FVC < 70% and FEV3/FVC < LLN | 80 (72–87) | 65 (54–73) | 2.27 | 0.31 |
| FEV1/FVC < 70% and TLC > ULN | 80 (72–87) | 74 (64–81) | 3.03 | 0.27 |
| FEV1/FVC < 70% and RV > ULN | 81 (73–87) | 70 (60–78) | 2.66 | 0.27 |
| FEV1/FVC < 70% and RV > ULN and TLC > ULN | 82 (74–88) | 70 (60–78) | 2.69 | 0.26 |
| FEV1/FVC < 70% and FEF25–75 < LLN | 84 (77–90) | 38 (29–48) | 1.36 | 0.41 |
| FEV1/FVC < 70% and FEV1 < LLN and FEV3/FVC < LLN and FEF25–75 < LLN | 87 (80–92) | 27 (19–37) | 1.20 | 0.48 |
| FEV1/FVC < 70% and FEV3/FVC < LLN and DLCO < LLN | 93 (87–97) | 16 (10–25) | 1.11 | 0.42 |
| FEV1/FVC<70% and DLCO<LLN | 93 (87–97) | 21 (14–30) | 1.19 | 0.32 |
| FEV1/FVC < 70% and DLCO < LLN and RV/TLC > ULN | 93 (87–97) | 21 (14–30) | 1.19 | 0.32 |
| FEV1/FVC < 70% and DLCO < LLN and RV > ULN | 94 (89–97) | 21 (14–30) | 1.20 | 0.28 |
| FEV1/FVC < 70% and FEF25–75 < LLN and DLCO < LLN | 95 (90–98) | 13 (8–21) | 1.09 | 0.39 |
| FEV1/FVC < 70% and DLCO < LLN and TLC > ULN | 95 (90–98) | 21 (14–30) | 1.21 | 0.24 |
| FEV1/FVC < 70% and DLCO < LLN and RV > ULN and TLC > ULN | 95 (90–98) | 21 (14–30) | 1.21 | 0.24 |
| FEV1/FVC < 70% and FEF25–75 < LLN and DLCO < LLN and RV > ULN and TLC > ULN | 97 (92–99) | 13 (8–21) | 1.11 | 0.26 |
LR+ = positive likelihood ratio; LR− = negative likelihood ratio; LLN = lower limit of normal; ULN = upper limit of normal; FEV1 = forced expiratory volume in 1 second (post-bronchodilator); FVC = forced vital capacity; FEV3 = forced expiratory volume in 3 seconds; FEF25–75 = forced expiratory flow between 25% and 75% of expired FVC; RV = residual volume; TLC = total lung capacity; DLCO = diffusing capacity for carbon monoxide.
The predictive value of FEV1/FVC < 70% and TLC>ULN were only 21% sensitive, but was 99% specific for emphysema with LR+ of 21.07 and PPV of 96%. Combining FEV1/FVC < 70% with other lung volume measurements, such as RV > ULN (LR+ 16.86, PPV 95%) or RV/TLC > ULN (LR+ 15.45, PPV 95%) produced similar results. The combination of all spirometry measures (FEV1/FVC, FEV1, FEV3/FVC, and FEF25–75) produced a sensitivity of 48% and specificity of 88%, while combining FEV1/FVC < 70% and DLCO < LLN produced a sensitivity of 74% and specificity of 80%.
3.3. Correlation between CT Emphysema and Pulmonary Function Tests
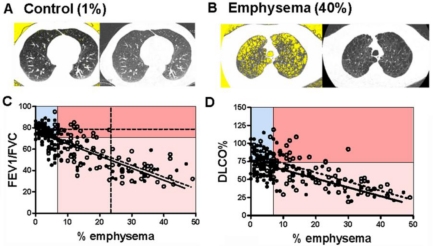
We found a significant negative correlation between the severity of emphysema, as quantified by CT morphometry (%LAA, representative samples are shown in [Figures 3(A,B)]) with FEV1/FVC (Figure 3C), and DLCO% predicted (Figure 3D). The negative correlation between % emphysema and FEV1/FVC was virtually identical in current and former smokers but there was a significant difference in the Y intercept of the correlation line in current when compared to former smokers.
Figure 3.
Correlation between PFTs and % emphysema in ever-smokers. Representative quantitative CT images of lung (left images) are matched to conventional CT images of the same lung (right images) from individuals without (A) and with (B) emphysema. Yellow background within lung margins (left images) was quantified as a percent of whole lung to determine the percent emphysema (1% and 40%, respectively; see Methods). (C) FEV1/FVC ratio was plotted against % emphysema, with regression lines in current (N = 133; solid line) and former (N = 91; dashed line) smokers. P < 0.0001; r = −0.7136 and r = −0.7574 Goodness of Fit for current and former smokers respectively. The lines are similar in slope (P = 0.78) and elevation (P = 0.17). The graph is divided into quadrants based on cutoff values for FEV1/FVC (70%) and % emphysema (7%). Additional dashed lines identify FEV1/FVC 78% (horizontal) and 23% emphysema (vertical). (D) DLCO% predicted plotted against % emphysema with regression lines in current (N =126; solid line) and former (N =89; dotted line) smokers. P < 0.0001; r = −0.4690 and r = −0.7074 Goodness of Fit for current and former smokers respectively. The lines are significantly different in elevation (p = 0.0003) but not slope (P = 0.79). The graph is separated into quadrants based on cutoffs for DLCO% (75%) and % emphysema (7%).
The FEV1/FVC ratio demonstrated a stronger correlation with % emphysema in current (r = −0.71, P < 0.0001) and former smokers (r = −0.76, P < 0.0001) than either FEV1% or DLCO%. All subjects with LAA > 23% (vertical dashed line) also demonstrated FEV1/FVC < 70%, with 26 subjects with FEV1/FVC ≥ 70% demonstrating significant radiographic emphysema (LAA range 7–23%). The mean age of this group was 52 ± 7 indicating that emphysema in these ever-smokers is not due to lung parenchymal senescence reported in the aging population [45]. While no reasonable FEV1/FVC cutoff excluded emphysema, a cutoff of 78% excluded 94% of ever smokers with emphysema (horizontal dashed line).
3.4. Airway Wall Thickness Is Associated with Airway Obstruction but Not Emphysema
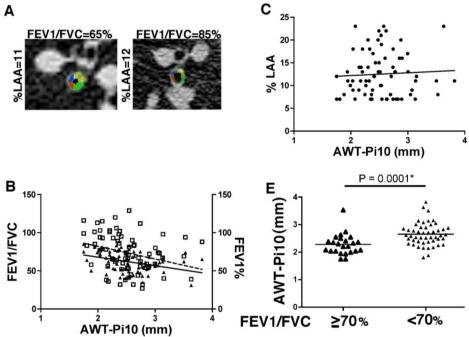
In this cohort, we determined that all ever-smokers with >23% emphysema had concurrent airflow obstruction. Therefore, we next analyzed the contribution of airway wall thickness (AWT-Pi10) to airflow obstruction and emphysema, in a subgroup of ever-smokers with >7% and < or equal to 23% emphysema (representative images shown in (Figure 4A).
Figure 4.
Airway wall thickness is increased in ever smokers with emphysema and airway obstruction. (A) A representative images of the internal perimeter of CT identified airway in subjects with similar degree of CT emphysema (11 and 12%) and with (left panel) or without (right panel) airflow obstruction. (B) Linear regression correlation of airway wall thickness (AWT-Pi10) was versus FEV1/FVC ratio (solid line; P = 0.0005; r = −0.3981) and FEV1% predicted (dashed line; P = 0.003; r = −0.3457) in 73 subjects with % emphysema between 7–23%. (C) AWT was plotted against % emphysema, with linear regression in the same group of 73 subjects. The correlation was not significant (P = 0.81, r = 0.0284). (D) Airway wall thickness (AWT-Pi10) measurements were assessed in 73 ever smokers with similar degree of emphysema (range 7 to 23%) comparing those with no airflow limitation (FEV1/FVC ≥ 70; N = 24) and with airflow obstruction (FEV1/FVC < 70; N = 49).
This subgroup was similar to the overall study participants in terms of age (60 ± 11), pack-years (52 ± 36), FEV1/FVC (62 ± 13), FEV1 % predicted (73 ± 25). Analyses of AWT-Pi10 showed significant correlation between FEV1 % predicted (r = −0.40, P = 0.0005) and FEV1/FVC (r = −0.35, P = 0.0027), shown in Figure 4B, but not with %LAA (r = 0.03, P = 0.81, Figure 4C). When smoking status was taken into account, former smokers showed a significant correlation between AWT-Pi10 and FEV1 % predicted (r = −0.40, P = 0.009) and FEV1/FVC (r = −0.56, P = 0.0001), though these findings were not seen in current smokers (data not shown). Further, significantly higher AWT-Pi10 values were found in smokers with airflow obstruction (2.62 mm ± 0.42) compared to smokers without airflow obstruction (2.29 mm ± 0.40; P-value = 0.0001) (Figure 4D).
4. Discussion and Conclusions
In this study we determined the performance characteristics of standard PFTs in discriminating emphysema in a well-defined cohort of current and former smokers. We further evaluated the correlations between airway wall thickness, airflow obstruction, and emphysema in the same population. We found that airflow obstruction, as defined by an FEV1/FVC < 70%, was 79% sensitive and 75% specific in discriminating significant emphysema. Combining FEV1/FVC < 70% with FEV1 < LLN increased the sensitivity of emphysema detection to 80% but reduced specificity to 63%, while other combinations of PFTs decreased specificity further, and failed to improve predictive values. Measurement of lung volumes, specifically TLC, was the only method found to meet criteria for a definitive test, i.e., a test in which a positive result would rule-in the presence of emphysema. However, an abnormally high TLC, in the absence of airflow obstruction, occurred rarely in our population, and therefore does not appear to have a practical application in clinical setting for detecting emphysema. Conversely, a reduced diffusion capacity was found to be quite sensitive for emphysema, however, this test is very non-specific as evidenced by a low LR+ < 1. Further current smokers had significantly reduced DLCO when compared to former smokers with the same degree of emphysema. This finding is consistent with the previously tested hypothesis that active smoking acutely reduces DLCO independent of emphysema by decreasing subjects’ lung capillary blood volume [46].
We show here that measurement of the FEV1/FVC ratio from simple spirometry demonstrated the best combination of sensitivity and specificity for discriminating emphysema and spirometry could be performed readily in clinics [15]. In a subset of subjects with less severe emphysema (LAA 7–23%), increased airway thickness was associated with airflow obstruction, suggesting that an increase in airway wall thickness significantly contributed to airflow obstruction. Further, the failure of emphysema severity to correlate with airway wall thickness in this subset suggests that airway disease and anatomical emphysema from smoking, at least in part, may represent distinct pathophysiological processes.
Our findings are of clinical importance to the study of COPD for many reasons. Currently, the exact prevalence of emphysema in smokers is unknown and we show here that, consistent with prior studies, emphysema remains an under diagnosed medical condition [47,48]. Further spirometry based classification of ever-smokers with smoking-related lung disease may fail to include those with emphysema who do not show reduced FEV1. Our findings here, together with those of others [48,49], highlight the importance of additional diagnostic tests that could provide an accurate estimate of concurrent emphysema in ever smokers. We show here that while standard pulmonary function tests correlate well with airway wall thickening, they fail to identify a substantial number of ever smokers with emphysema. These findings have important implications for the design of future clinical studies that should consider a better phenotypic classification of ever-smokers with radiographic assessment of emphysema.
Individuals with distinct patterns of anatomical emphysema may present with different degrees of pulmonary function abnormalities [50]. In this study we found no correlation between airway wall thickness and severity of emphysema, with the caveat that we did not measure the transpulmonary pressure to control for the elastic recoil pressure. Our findings thus represent an in vivo demonstration of the dissociation between airflow limitation, airway wall thickness and anatomical emphysema in smokers and suggest that the pathological processes underlying airway obstruction and emphysema are likely distinct. With a growing number of interventions and treatments targeted to patients specifically with emphysema [51,52], and our current findings that more advanced emphysema (LAA > 23%) is always associated with airflow obstruction, it is of increasing clinical importance that these patients are identified properly at an earlier stage of their disease.
Our findings underscore the need to develop improved diagnostic and prognostic methods for evaluating ever smokers who will eventually develop irreversible lung parenchymal disease. Chest CT evaluation is clearly the standard for detecting early emphysema in vivo, but is limited by cost and the long-term consequences of exposure to ionizing radiation [53]. Improved diagnostic methods are essential for answering critical questions regarding the natural history of smoking-related lung disease, specifically regarding the relationship of obstructive to destructive lung diseases and whether these processes represent inexorably progressive or self-limited conditions. A proposed strategy in the evaluation of ever smokers may be to measure full pulmonary function tests, including spirometry and lung volumes, and diffusion capacity; if spirometry is normal but hyperinflation, air trapping, or reduced gas exchange are noted, a CT should be considered to evaluate for isolated emphysema. Detection of emphysema without airflow obstruction may also provide an explanation of symptoms such as dyspnea or exercise intolerance in ever smokers with normal spirometry, who might otherwise be identified as normal. Future studies are necessary to identify emphysema at an early-stage, and smokers who are at risk of developing lung disease where intervention may be more efficacious. In summary, our data supports the notion that emphysema is a pathologic process that occurs in a substantial proportion of smokers independent of airflow obstruction, and thus highlighting the need for a modern approach to the evaluation of this large population of patients.
Acknowledgments
Sean E. Hesselbacher, Robert Ross, Manoj J. Mammen, David B. Corry, and Farrah Kheradmand designed the study, and analyzed data. Matthew B. Schabath and E. O’Brian Smith assisted with statistical analyses. Sarah Perusich, Nadia Barrow, Pamela Smithwick recruited subjects and assisted in PFT measurement and data retrieval. Harvey Coxson, Natasha Krowchuk analyzed CT scans and measured airway wall thickness. Supported by grants to F.K (HL082487; HL72419; CX000104-02). Harvey Coxson is supported by a GSK Clinical Scientist Award and, in part, by the University of Pittsburgh COPD SCCOR NIH 1P50 HL084948 and R01 HL085096 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD to the University of Pittsburgh. Harvey Coxson was a CIHR/BC Lung Association New Investigator during the time of this research. We thank all the subjects who participated in this study and Li-Zhen Song for technical support.
References
- 1.Centers for Disease Control and Prevention (CDC) State-specific prevalence and trends in adult cigarette smoking—United States, 1998–2007. MMWR. 2009;58:221–226. [PubMed] [Google Scholar]
- 2.Shafey O, Eriksen M, Ross H, Mackay J. The Tobacco Atlas. 3rd ed. American Cancer Society; Atlanta, GA, USA: 2009. [Google Scholar]
- 3.Schubert C. Anti-tobacco efforts going up in smoke. Nature Med. 2006;12:866. [Google Scholar]
- 4.Rennard SI, Vestbo J. COPD: The dangerous underestimate of 15% Lancet. 2006;367:1216–1219. doi: 10.1016/S0140-6736(06)68516-4. [DOI] [PubMed] [Google Scholar]
- 5.Rennard SI, Vestbo J. Natural histories of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008;5:878–883. doi: 10.1513/pats.200804-035QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, et al. Lung myeloid dendritic cells coordinately induce Th1 and Th17 responses in human emphysema. Sci. Transl. Med. 2009;4:132–140. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 7.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, Arenberg DA, Meldrum CA, Getty C, McCloskey L, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman EK, Spira A, Pare PD. Genetics and genomics of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009;6:539–542. doi: 10.1513/pats.200904-021DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stavem K, Aaser E, Sandvik L, Bjornholt J, Erikssen G, Thaulow E, Erikssen J. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur. Resp. J. 2005;4:618–625. doi: 10.1183/09031936.05.00008504. [DOI] [PubMed] [Google Scholar]
- 10.Kim WJ, Silverman EK, Hoffman E, Criner GJ, Mosenifar Z, Sciurba FC, Make BJ, Carey V, San Jose Estepar R, Diaz A, et al. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136:396–404. doi: 10.1378/chest.08-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han MK, Bartholmai B, Liu LX, Murray S, Curtis JL, Sciurba FC, Kazerooni EA, Thompson B, Frederick M, Li D, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6:459–467. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 12.Pescarolo M, Sverzellati N, Verduri A, Chetta A, Marangio E, De Filippo M, Olivieri D, Zompatori M. How much do GOLD stages reflect CT abnormalities in COPD patients? Radiol. Med. 2008;113:817–829. doi: 10.1007/s11547-008-0284-3. [DOI] [PubMed] [Google Scholar]
- 13.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am. J. Respir. Crit. Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 15.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity—ATS workshop on lung volume measurements. Official statement of the european respiratory society. Eur. Respir. J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 16.GOLD Executive Committee Global Strategy for the Diagnosis, Management, and Prevention of COPD. Available online: http://www.goldcopd.com/GuidelinesResources.asp?l1=2&l2=0.2008 (accessed on 21 May 2009).
- 17.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: The future of COPD. Am. J. Respir. Crit. Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu D, Kelly TN, Wu X, Chen J, Samet JM, Huang JF, Zhu ML, Chen JC, Chen CS, Duan XF, et al. Mortality attributable to smoking in China. N. Engl. J. Med. 2009;360:150–159. doi: 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 19.Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, Shete S, Etzel CJ. A risk model for prediction of lung cancer. J. Nat. Cancer Inst. 2007;99:715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD. Association of radiographic emphysema and airflow obstruction with lung cancer. Am. J. Respir. Crit. Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nature Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 22.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: Needs and opportunities for integrated research. J. Nat. Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Klaveren RJ, de Koning HJ, Mulshine J, Hirsch FR. Lung cancer screening by spiral CT. What is the optimal target population for screening trials? Lung Cancer. 2002;38:243–252. doi: 10.1016/s0169-5002(02)00222-2. [DOI] [PubMed] [Google Scholar]
- 24.Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, Hirai T, Niimi A, Nishimura K, Chin K, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 25.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am. J. Respir. Crit. Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 26.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am. J. Respir. Crit. Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 27.Patel BD, Coxson HO, Pillai SG, Agusti AGN, Calverley PMA, Donner CF, Make BJ, Müller NL, Rennard SI, Vestbo J, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;178:500–505. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 28.Grydeland TB, Dirksen A, Coxson HO, Eagan TML, Thorsen E, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am. J. Respir. Crit. Care Med. 2010;181:353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 29.Orlandi I, Moroni C, Camiciottoli G, Bartolucci M, Pistolesi M, Villari N, Mascalchi M. Chronic obstructive pulmonary disease: Thin-section CT measurement of airway wall thickness and lung attenuation. Radiology. 2005;234:604–610. doi: 10.1148/radiol.2342040013. [DOI] [PubMed] [Google Scholar]
- 30.Coxson HO, Rogers RM. Quantitative computed tomography of chronic obstructive pulmonary disease. Acad. Radiol. 2005;12:1457–1463. doi: 10.1016/j.acra.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Gelb AF, McKenna RJ, Jr, Brenner M, Fischel R, Baydur A, Zamel N. Contribution of lung and chest wall mechanics following emphysema resection. Chest. 1996;110:11–17. doi: 10.1378/chest.110.1.11. [DOI] [PubMed] [Google Scholar]
- 32.Hesselbacher SE, Perusich S, Barrow N, Smithwick P, Coxson HO, Krowchuk N, Corry D, Ross R, Kheradmand F. Physiological impairment in smokers with emphysema. Am. J. Respir. Crit. Care Med. 2010;181:A5952. [Google Scholar]
- 33.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 34.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Amer. Rev. Resp. Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 35.Cotes JE, Chinn DJ, Reed JW. Body mass, fat percentage, and fat free mass as reference variables for lung function: Effects on terms for age and sex. Thorax. 2001;56:839–844. doi: 10.1136/thorax.56.11.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Amer. Rev. Resp. Dis. 1983;127:270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 37.Yuan R, Hogg JC, Pare PD, Sin DD, Wong JC, Nakano Y, McWilliams AM, Lam S, Coxson HO. Prediction of the rate of decline in FEV(1) in smokers using quantitative computed tomography. Thorax. 2009;64:944–999. doi: 10.1136/thx.2008.112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan R, Mayo JR, Hogg JC, Pare PD, McWilliams AM, Lam S, Coxson HO. The effects of radiation dose and CT manufacturer on measurements of lung densitometry. Chest. 2007;132:617–623. doi: 10.1378/chest.06-2325. [DOI] [PubMed] [Google Scholar]
- 39.Bosken CH, Wiggs BR, Pare PD, Hogg JC. Small airway dimensions in smokers with obstruction to airflow. Am. Rev. Respir. Dis. 1990;142:563–570. doi: 10.1164/ajrccm/142.3.563. [DOI] [PubMed] [Google Scholar]
- 40.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am. J. Respir. Crit. Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 41.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Paré PD. The prediction of small airway dimensions using computed tomography. Am. J. Respir. Crit. Care Med. 2004;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 42.Feinstein AR. Principles of Medical Statistics. Chapman & Hall/CRC; Boca Raton, FL, USA: 2002. [Google Scholar]
- 43.Coxson HO, Rogers RM, Whittall KP, D’Yachkova Y, Pare PD, Sciurba FC, Hogg JC. A quantification of the lung surface area in emphysema using computed tomography. Am. J. Respir. Crit. Care Med. 1999;159:851–856. doi: 10.1164/ajrccm.159.3.9805067. [DOI] [PubMed] [Google Scholar]
- 44.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am. J. Respir. Crit. Care Med. 2004;170:748–752. doi: 10.1164/rccm.200405-651OC. [DOI] [PubMed] [Google Scholar]
- 45.Gillooly M, Lamb D. Airspace size in lungs of lifelong non-smokers: Effect of age and sex. Thorax. 1993;48:39–43. doi: 10.1136/thx.48.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansores RH, Pare PD, Abboud RT. Acute effect of cigarette smoking on the carbon monoxide diffusing capacity of the lung. Am. Rev. Respir. Dis. 1992;146:951–958. doi: 10.1164/ajrccm/146.4.951. [DOI] [PubMed] [Google Scholar]
- 47.Remy-Jardin M, Remy J, Boulenguez C, Sobaszek A, Edme JL, Furon D. Morphologic effects of cigarette smoking on airways and pulmonary parenchyma in healthy adult volunteers: CT evaluation and correlation with pulmonary function tests. Radiology. 1993;186:107–115. doi: 10.1148/radiology.186.1.8416548. [DOI] [PubMed] [Google Scholar]
- 48.Spaggiari E, Zompatori M, Verduri A, Chetta A, Ormitti F, Sverzellati N, Rabaiotti E. Early smoking-induced lung lesions in asymptomatic subjects. Correlations between high resolution dynamic CT and pulmonary function testing. Radiol. Med. 2005;109:27–39. [PubMed] [Google Scholar]
- 49.Sashidhar K, Gulati M, Gupta D, Monga S, Suri S. Emphysema in heavy smokers with normal chest radiography. Detection and quantification by HCRT. Acta Radiol. 2002;43:60–65. doi: 10.1080/028418502127347457. [DOI] [PubMed] [Google Scholar]
- 50.Soejima K, Yamaguchi K, Kohda E, Takeshita K, Ito Y, Mastubara H, Oguma T, Inoue T, Okubo Y, Amakawa K, et al. Longitudinal follow-up study of smoking-induced lung density changes by high-resolution computed tomography. Am. J. Respir. Crit. Care Med. 2000;161:1264–1273. doi: 10.1164/ajrccm.161.4.9905040. [DOI] [PubMed] [Google Scholar]
- 51.Group NETTR A randomized trial comparing lung-volume—reduction surgery with medical therapy for severe emphysema. N. Engl. J. Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 52.Wood DE, McKenna RJ, Jr, Yusen RD, Sterman DH, Ost DE, Springmeyer SC, Gonzalez HX, Mulligan MS, Gildea T, Houck WV, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J. Thorac. Cardiovasc. Surg. 2007;133:65–73. doi: 10.1016/j.jtcvs.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 53.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, de González BA, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]