Abstract
Adoxorubicin (DOX)-carrier micellar system consisting of poly(histidine)(5K)-b-poly(ethylene glycol)(2K) and poly(l-lactic acid)(3K)-b-PEG(2K)-folate has been developed targeting the early endosomal pH and it have been convincingly proved that intracellular high dose strategy using such micelles is effective in overcoming multidrug resistance (MDR) of cancer cells. Due to the low DOX concentrations in the micelle solution obtained by dialysis and the lack of long-term stability of the micelles, stable and lyophilized micelle formulations were the subject of investigation reported here by using excipients of sucrose, PEG or Pluronic. The reconstituted micelle solutions were examined by particle size, pH sensitivity, and cytotoxicity for MDR cells and the results were compared with the non-lyophilized micelles. Among tested excipients, Pluronic F127 (33 wt%) added to the polymer/drug solution prior to dialysis resulted in a reconstituted product stable for a week and presented equivalent benefits as the fresh micelle formulation. The blank micelles did not present any apparent systemic toxicity in mice up to 2400 mg/kg i.v. injection (800 mg/(kg day) for 3 days). The brief toxicity of reconstituted DOX loaded micelles was examined by the maximum tolerated dose (MTD), which was approximately 7.5-fold higher than free DOX and guaranteeing further animal toxicity and efficacy study.
Keywords: Poly(l-histidine)-b-PEG, pH-sensitive micelle, Multidrug resistance, Reconstitution, Pluronic F127, Maximum tolerated dose
1. Introduction
In most cases, chemotherapy is one of the major therapeutic modalities after cancer surgery. However, there are various challenges for anticancer agent formulations often associated with their solubility, stability, sensitivity and toxicity (Allen et al., 2006). Furthermore, the multidrug resistance (MDR) of cancer cells is a significant impediment to the efficacy of anticancer drugs (Simon and Schindler, 1994; Links and Brown, 1999; Gottesman et al., 2002; Szakacs et al., 2006). Recently, novel drug delivery approaches have been investigated to obtain higher antitumor efficiency with reduced toxicity by altering the biodistribution of anticancer drugs (Oh et al., 2007). Several independent groups have also studied to overcome the MDR using various nanocarrier systems (Alakhov et al., 1999; Vinogradov et al., 1999; Goren et al., 2000; Rapoport, 2004; Advani et al., 2005; Chavanpatil et al., 2006; Li et al., 2006; Pakunlu et al., 2006; Wang et al., 2006, 2007). Among these, polymeric micelles have received much attention due to their favorable characteristics of high drug loading capacity, small size, controlled drug release, flexibility in surface modification, and imparting of various functionalities for effective targeting (Kataoka et al., 2001; Lavasanifar et al., 2002). Especially, the environmentally responsive polymers were investigated as smart drug delivery systems for targeted high-dose cancer therapy and the reversal of MDR (Lee et al., 2003a, 2005; Mohajer et al., 2007).
Bae’s research group has developed a pH-sensitive doxorubicin (DOX) carrier micelle formulation that underwent structural destabilization at acidic pH (Lee et al., 2003b). The DOX-loaded pH-sensitive micelle (DPHSM) systems composed of 75wt% poly(histidine)-b-poly(ethylene glycol) (PHis-PEG,Mn: 5K–2K) and 25 wt%poly(l-lactic acid) (PLLA)-b-PEG (Mn: 3K–2K) showed destabilization of micelles at pH 6.8 and enhanced anticancer efficacy. Furthermore, folate modified micelles showed high cytotoxicity against MDR tumor cells in vitro and in vivo (Lee et al., 2003a, 2005; Mohajer et al., 2007). However, from a pharmaceutical point of view, the DPHSM system exhibits a few aspects that need optimization of the formulation. For example, an aqueous formulation may cause the degradation of DOX and polymers during storage. The DPHSM solution prepared via dialysis has difficulties in adjusting a target final drug concentration, which should be able to provide a therapeutic dose when necessary. A powder form of micelles which can be reconstituted to a colloidal dispersion by simply adding water or a buffer, will be extremely useful for in vivo applications, large scale preparation of the product, as well as for concentration adjustments in final doses (Miyata et al., 2005).
A freeze-drying process is a useful process to obtain a dry powder form for long-term stability and preserving the original properties of pharmaceutical and biological products. However, when the pH-sensitive micelle powder without additives was prepared, the reconstitution by adding a buffer solution resulted in the formation of micron-sized aggregates or phase-separation. For many lyophilized pharmaceutical formulations, the addition of saccharides as a lyoprotectant is demonstrated to prevent the formulations from aggregation after freeze-drying (Talsma et al., 1997; Abdelwahed et al., 2006; Li et al., 2008). However, adding low molecular weight saccharides to our micellar formulations did not help improve colloidal stability after reconstitution. This paper reports on the lyophilized DPHSM which can be reconstituted and concentrated for dose adjustment. Furthermore, the reconstituted DPHSM was investigated for preservation of the original properties of the freshly made micellar formulation preserves in terms of the particle size, pH-sensitivity, and cytotoxicity against MDR cells. Finally, the toxicity of the reconstituted DPHSM was briefly tested in a mouse model using maximum tolerance dose (MTD) determination.
2. Materials and experimental design
2.1. Materials
1-Benzyl-N-carbobenzoxy-l-histidine (Z-His(Bzl)-OH), thionyl chloride, isopropylamine (IPA), triethylamine (TEA), poly(ethylene glycol) (PEG) (MW 400 and 2000), potassium tetraborate, ammonium bicarbonate, N-hydroxysuccinimide (NHS), N,N′-dicyclohexylcarbodiimide (DCC), folate, diethylaminoethyl (DEAE) Sephadex A-25, DOX·HCl, anhydrous diethyl ether, phosphate buffer saline (PBS), anhydrous 1,4-Dioxane, N,N-dimethylformamide (DMF), methylene chloride (MC), chloroform, toluene, dimethyl sulfoxide (DMSO), and DMSO-d6 with tetramethylsilane (TMS) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Penicillin–streptomycin, Tris–HCl (pH 8.4), fetal bovine serum (FBS), 0.25% (w/v) trypsin–0.03% (w/v) EDTA solution, and RPMI1640 medium were purchased from Gibco (Uxbridge, UK). Pluronic P85 and F127 (abbreviated as P85 and F127 hereafter) were obtained from BASF (New Jersey). PHis (Mn: 5K)-PEG (Mn: 2K) was synthesized as described in detail in previous reports (Lee et al., 2003b, 2007; Kim et al., 2005). PLLA (Mn: 3K)-PEG(2K) was prepared by conventional method (Han et al., 2003; Lee et al., 2003b). The synthesis of PLLA-PEG-folate was prepared as described in previous reports (Lee et al., 2003a).
2.2. Preparation of mixed micelles and DPHSM
The pH-sensitive mixed micelles were prepared by blending of PHis-PEG and PLLA-PEG in 75/25% (w/w). The polymers dissolved in DMSO were transferred to a preswollen dialysis membrane (MWCO 1000) and dialyzed against HCl (or NaOH)–Na2B4O7 buffer solution (pH 8.0, 10mM) for 24 h with several buffer replacements. DPHSM were also prepared as detailed in previous reports (Lee et al., 2003a, 2005). HCl from DOX·HCl was removed by TEA (two molar ratio of DOX·HCl) in DMSO to obtain the DOX base. Polymers (PHis-PEG and PLLA-PEG-folate, 75/25,w/w)and DOX were dissolved in DMSO and dialyzed against NaOH–Na2B4O7 buffer solution (pH 9.0) for 24 h at 4 °C. The micelle solution obtained after dialysis was lyophilized and the amount of entrapped DOX was determined by measuring UV absorbance at 481 nm of the drug-loaded polymeric micelles after dissolution in DMSO.
2.3. Preparation of reconstituted micelle formulation
The stock solution of 20 wt% sucrose, 20 wt% PEG (MW 400 and 2000), 1 wt% P85, and 10% F127 were prepared with distilled water and used as excipients for reconstitution of DPHSM. The excipient solutions were mixed with DOX (10 mg) and polymers (90 mg) in DMSO before dialysis (pre-adding), or DPHSM after dialysis (post-adding) in the proportions indicated further. In the first method, the amount of water in the polymer/drug-mixed solutions was not over at most 30% (v/v) of DMSO. Lyophilization was carried out in a lab freeze-dryer, FreeZone 6 (Labconco Corp., Kansas City,MO,USA). The freeze-dried samples were sealed with Parafilm® and stored in vacuum chamber at −20 °C until further analysis.
2.4. Reconstitution of freeze-dried products
After freeze-drying, the products can be reconstituted by addition of buffer solution (PBS or Na2B4O7 buffer). The rehydration process was observed visually as the vials were gently agitated by hand, leading to the formation of the aqueous liquid micelles for further characterization.
2.5. Particle size determination of the polymeric micelles by dynamic light scattering (DLS)
The effective hydrodynamic diameter (Deff) of the particles was measured by photon correlation spectroscopy using a Zetasizer 3000HS (Malvern Instrument, USA) equipped with a He–Ne Laser beam at a wavelength of 633 nm. The sizing measurements were performed in a thermostatic cell at a scattering angle of 90°. Software (Zetasizer 3000HS-Easy. ver. 1.61) provided by the manufacturer, which employed cumulant analysis, was used to calculate the size of the nanoparticles and the polydispersity index. The average diameters values were calculated from at least 10 measurements performed on each samples.
2.6. Cytotoxicity test
Ovarian A2780 DOX resistant cells (A2780/DOX) grown in flasks were treated with 0.2% (w/v) trypsin–0.1% (w/v) EDTA solution in order to detach the cells. The detached cells (5 × 104 cells/ml) were seeded in a 96 well plate and incubated for 1 day. The equivalent DOX concentrations of each formulation were prepared by diluting with RPMI 1640 cell culture medium. After 24 h treatments, 20 µl of MTT solution (500 µg/l) was added to each well and incubated for 4 h. The medium was removed and then DMSO (100 µl) was added to each well for 10min incubation. The absorbance of each well was read with a microplate reader (SpectraMax M2®, Molecular Devices, Sunnyvale, CA,USA) using a test wavelength of 570 nm and a reference wavelength 630 nm. Animal toxicity study experiments were performed using Balb/c mice (Simonsen Lab Inc., Gilroy, CA) with bodyweights of approximately 21–23 g (10–12weeks old). To briefly test the polymer toxicity, a polymer stock solution (80 mg/ml PHis-PEG) was prepared in saline (0.9% NaCl and pH adjusted to 6.0 for higher polymer solubility). Five mice per group were used to monitor weight loss and visually observe the mice for polymer toxicity. The polymer solution was injected intravenously (i.v.). Due to limited polymer solubility and injection volume, the doses of 1600 and 2400 mg/kg were administered by injecting the dose of 800mg/kg two times or three times on a daily basis. The maximum tolerated dose (MTD) of the reconstituted DPHSM administered i.v. was determined in healthy Balb/C mice (n = 5). The freeze-dried DPHSM prepared by the above method was redispersed in PBS buffer (pH 8.0) immediately before use. The reconstituted DPHSM with different concentrations (6.5–12.5 mg/ml) was delivered i.v. through the lateral tail vein in mice. Animals were monitored for 2 weeks after the first injection of different dose levels and euthanized when they lost more than 20% of their initial body weight. The MTD was defined as one dose step below a dose where more than one animal in the test group had to be sacrificed.
3. Results and discussion
3.1. DOX incorporated mixed micelles
DOX loaded pH-sensitive micelle (DPHSM) composed of PHis-PEG and PLLA-PEG-folate was obtained by the dialysis method (membrane MWCO, 1000) against borate buffer (10mM, pH 8.0). The maximum DOX loading capacity obtained was approximately 18 wt% and the drug loading efficiency was about 85%, which is consistent with our previous results (Lee et al., 2003a). Furthermore, DPHSM adjusted to 10 wt% drug loading content showed more than 98% loading efficiency. The hydrodynamic size of fresh drug loaded micelles was about 200 nm and when filtered through a syringe filter (0.8 µm), the size of micelles decreased to 70–150 nm with a unimodal distribution and the recovery yield after filtration was approximately 80–98%.
A favorable size and recovery yield of DPHSM could be obtained by controlling the diafiltration conditions, syringe filter size, and the DOX contents in DPHSM (unpublished data). For example, when the water content in drug and polymer stock solution in DMSO was controlled at 30% (v/v), the condition resulted in the formation of DPHSM with favorable properties in terms of particle size, drug loading efficiency, and stability. The borated buffer (pH 9.0) as a dialysis buffer is preferable over phosphate buffer (pH 8.0) for higher drug loading efficiency and recovery yield. Ten to 12wt% drugs loading in DPHSM may be an optimal drug content taking into account of the particle size, the stability, and the further application.
3.2. Effects of excipients on reconstitution of lyophilized micelles
As mentioned above, two methods were tested for adding cryoprotectants during micelle fabrication and freeze-drying processes: ‘pre-adding’ and ‘post-adding.’ The latter resulted in the formation of a dapple-colored cake after lyophilization due to heterogeneously distributed cryoprotectant in the dried micellar cake. Consequently, the redispersed micelles did not show a reproducible particle size of the reconstituted micelles in different batches. The homogeneity in dried micellar cake is a critical factor for the scale-up process and quality control in development. Therefore, the method of post-adding of the cryoprotectants was further excluded from the study.
Table 1 summarizes the results of reconstitution study of powders obtained by employing the ‘pre-adding’ method. Sucrose as a conventional lyoprotectant (Talsma et al., 1997; Abdelwahed et al., 2006; Li et al., 2008),PEGas a solubilizer (Meyuhas and Lichtenberg, 1996; Seo and Choi, 2003), and Pluronic (Donini et al., 2002) as a surfactant were selected as a reconstitution formulations for DPHSM. Adding sucrose did not provide any benefits over the absence of the lyoprotectant, which may be due to the lack of its solubility capacity for hydrophobic agents (data not shown). The drug-loaded micelles with the additives had 160–370 nm in size before filtering. After freeze-drying, 5mg of total dried micelle cake was redispersed by adding 1 ml of the borate buffer.
Table 1.
List of excipients and the size, concentration, recovery yield, and stability of DPHSM including the excipients before freeze-drying (lyophilizer) and after reconstitution
| Before lyophilizer |
Reconstitution |
Recovery yielda (%) | Stabilityb | |||
|---|---|---|---|---|---|---|
| Concentration (µg/ml) | size (nm) | Concentrationc (µg/ml) | Size (nm) | |||
| Control micelle | 234.5 | 214 | 414.5 | P.S. | N.D. | N.A. |
| 50 wt% PEG (2K) | 162.8 | 255 | 230.6 | 558 | 89.4±0.25 | × |
| 50 wt% PEG (400) | 170.6 | 216 | 444.1 | 294 | 91.1±0.68 | Δ |
| 10 wt% P85 | 188.6 | 235 | 424.1 | 208 | 84.5±0.16 | × |
| 10 wt% F127 | 195.1 | 297 | 409.9 | 250 | 86.2±0.40 | Δ |
| 33 wt% F127 | 188.6 | 174 | 479.0 | 149 | 94.9±0.20 | ○ |
| 50 wt% F127 | 741.0 | 367 | 228.0 | 316 | 93.5±0.54 | ○ |
P.S., phase separation; N.D., not determination; N.A., not available.
Recovery yield indicates the ratio of DOX concentration of reconstitution micelle after filtering (0.8 µm) to before filtering (n = 3).
(×) Phase separation at 1 day; (Δ) phase separation at 2–4 days; (○) stable without separation for more than 1 week.
Add 1 ml borate buffer (pH 8.0) into dried micelle.
The reconstituted drug-loaded micelle without any excipient in aqueous solution was phase-separated and precipitated immediately. However, the dried DPHSM with PEG, P85, and F127 were redispersed well without phase-separation by hand shaking within 5 s. Recovery after filtration (0.8µm) was relatively high (85–95%), which suggested that the micelle structure prepared by above methods was maintained during freeze-drying and could be reconstituted using these excipients. The stability of dispersions of DPHSM with the excipients was examined by measuring the size of particles as a function of time (Fig. 1). The size of redispersed micelles with PEG was increased and the stability of dispersion was less than the fresh micellar dispersion prepared by dialysis. The micellar dispersion including PEG (both 2000 and 400 Da) after reconstitution was phase-separated at less than 1 h and 1 day, respectively.
Fig. 1.
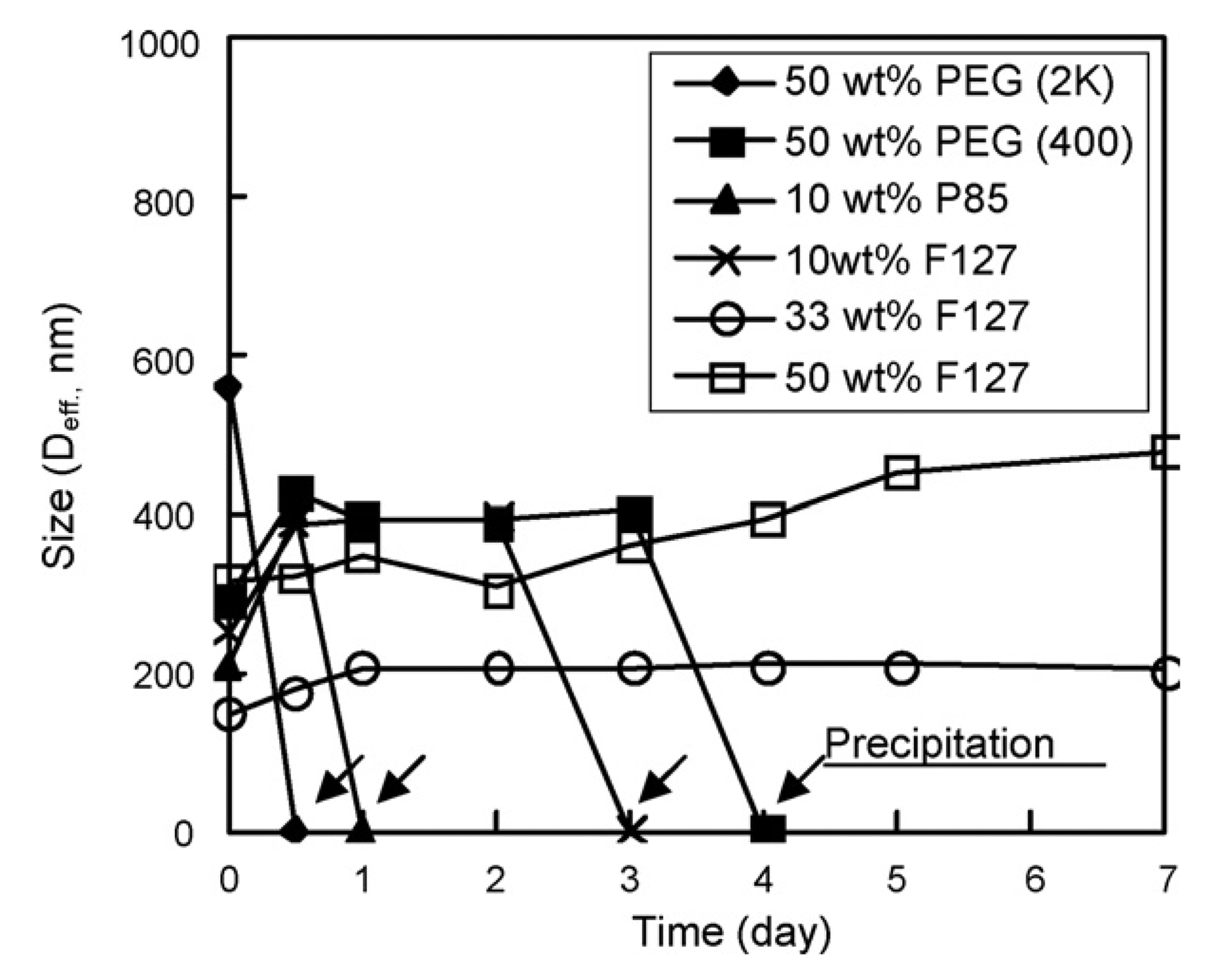
Particle size of pH-sensitive micelles with excipients: 50 wt% PEG (2k) (♦), 50 wt% PEG (400) (■), 10 wt% P85 (▲), 10 wt% F127 (×), 33 wt% F127 (○), 50 wt% F127 (□); as a function of time. The 0 nm of particle size (arrows) indicates the precipitation of particle observed visually.
In the case of P85 and F127, the micelles’ dispersion showed similar sizes of micelles after reconstitution when compared to the fresh micelles. However, moderately hydrophobic P85 and a small amount (10 wt%) of hydrophilic F127 led to aggregation of reconstituted micelles, resulting in increased particle size and phase-separation with time. Interestingly, the micelle formulation incorporating 33 wt% F127 (DPHSM-F127) was stable without precipitation for more than a month and the size of micelle remained unchanged for 5 days. In addition, the size of reconstituted micelle was smallest with the highest (approximately 95%) recovery yield among all tested groups. The results suggest that F127 in a proper weight ratio may prevent the aggregation of hydrophobic micelles and increase the stability of micelles by decreasing the interaction between micelles.
3.3. Characterization of the reconstituted micelle formulation
From the above results, the formulation containing 33 wt% F127 was selected as an optimized formulation for further studies. To compare the reconstituted formulation with freshly made micelles, pH-sensitive destabilization of the formulation containing 33 wt% F127 and cytotoxicity against an MDR cancer cell line were evaluated. The pH sensitivity was evaluated by particle size measurement using the mixed micelles containing F127 without the DOX. The size of reconstituted mixed micelle containing F127 when redispersed in PBS (pH 8.0), was approximately 165 nm, which was slightly larger than the freshly made formulation of DPHSM-F127. The size of reconstituted DOX unloading pH-sensitive micelles with F127 increased as pH was lowered, especially below pH 7.4, which may be due to micelle destabilization (Lee et al., 2003a,b). Below pH 7.0, the sizes and polydispersity indices of the mixed micelle with F127 were abruptly increased, subsequently; the nanoparticle size distribution became bimodal below pH 6.8 (Fig. 2). These results may be attributed to the dissociation of PLLA-PEG and PHis-PEG from the mixed micelles with F127 due to the ionization of His component at below pKb of the PHis-PEG diblock copolymer (pH 7.0) (Lee et al., 2003b). The contents of F127 in the mixed micelle can theoretically be estimated from the value of critical micelle concentration (CMC) of F127. The CMC of F127 at 25 °C is about 1% (w/v) (10mg/ml) (Alexandridis et al., 1994) Therefore, the F127 proportion in the systems could be changed depending on the drug concentration. For example, in case of 1mg/ml DOX (10%, w/v content in micelle) which is a target concentration for further pre/clinical study, all F127 (0.5%, w/v) might exist as unimers. If the DOX in DPHSM containing 33 wt% F127 increased to 5mg/ml, F127 (2.5%, w/v) may theoretically exist with a component of about 40% unimers and 60% of mixed micelles containing F127 or F127 micelles. Interestingly, in this condition, the DPHSM formulation still had pH sensitivity. The change of particle size at different pHs was similar to the above results (data not shown). This suggests that a certain portion of F127 micelles may not affect the pH sensitivity of PHSM. Furthermore, F-127 resulted in a more stable dispersion of DPHSM after reconstitution may be by preventing secondary aggregation during lyophilization (Oh et al., 2004). The advantage of this formulation was further supported by MDR cell viability study. The cytotoxicity of DPHSM-F127 was similar to that of DPHSM without F127 against MDR cancer cells as shown in Fig. 3. Since the pH-sensitivity of DPHSM-F127 was unaltered, the reversal of MDR by DPHSM-F127 was equivalent to the previous results (Lee et al., 2003a, 2005; Mohajer et al., 2007). In summary, DPHSM-F127 may be used as an optimized formulation to treat MDR cancers.
Fig. 2.
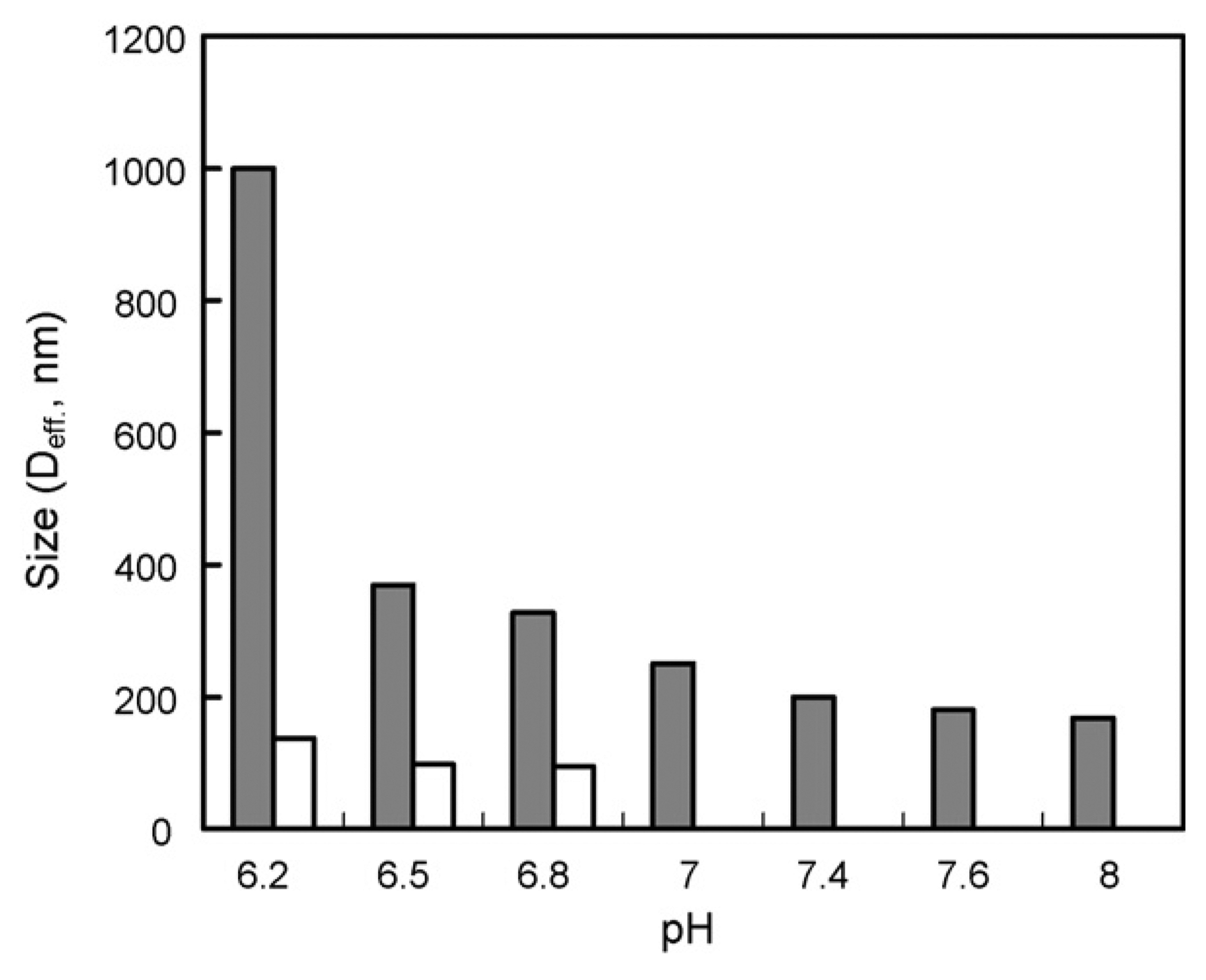
Particle size of pH-sensitive micelles with F127 at various pHs. From pH 8.0 to 7.0 unimodal particle distribution and below pH 7.0, bimodal particle distributions were observed, respectively.
Fig. 3.
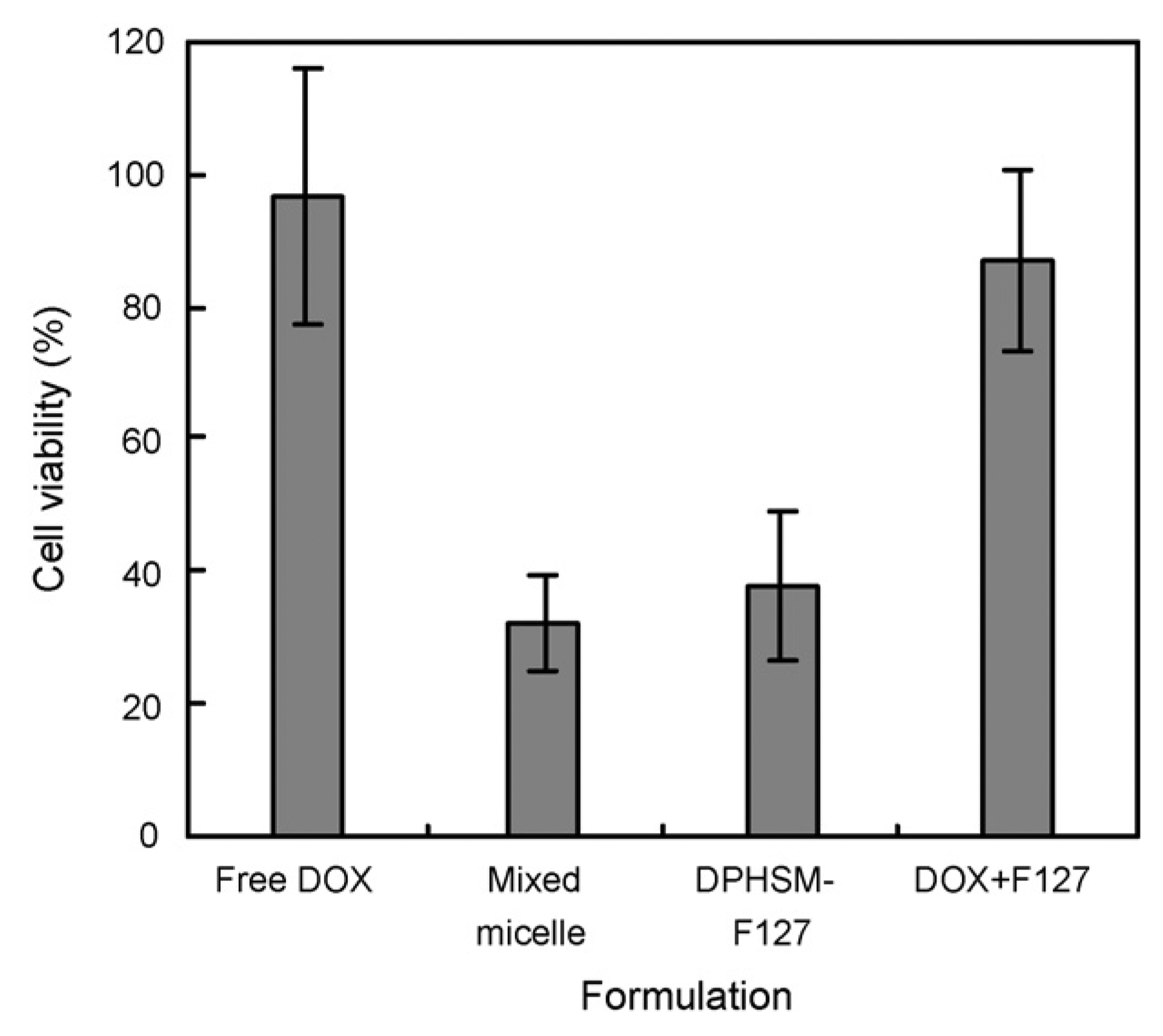
Cell viability of free DOX, fresh DPHSM, DPHSM-F127, and mixture of free DOX/F127 against A2780/DOX cells at pH 7.4. The equivalent DOX in each formulation is 10 µg/ml. (Assessment by MTT assay; mean±S.D., n = 5).
3.4. Effect and toxicity of polymers
Concentration-dependent toxic effects of the diblock copolymers in MDR cells were evaluated by determining the cytotoxicity of DOX. Fig. 4 presents a typical DOX resistance of MDR cell line, reversal of MDR by verapamil (a reversal agent for MDR), and no effects of each polymer at concentrations below CMC. Kabanov’s group reported the effect of unimers of hydrophobic Pluronics (e.g. P85) on MDR reversal (Batrakova et al., 1999, 2001, 2003). They have suggested that Pluronic might inhibit Pgp through ATP depletion, and inhibition of Pgp ATPase activity. However, PHis-PEG or PLLA-PEG did not influence the activity of DOX (Fig. 4). This suggests that DPHSM can reverse the MDR by high local concentration of DOX triggered by endosomal pH after folate receptor mediated endocytosis.
Fig. 4.
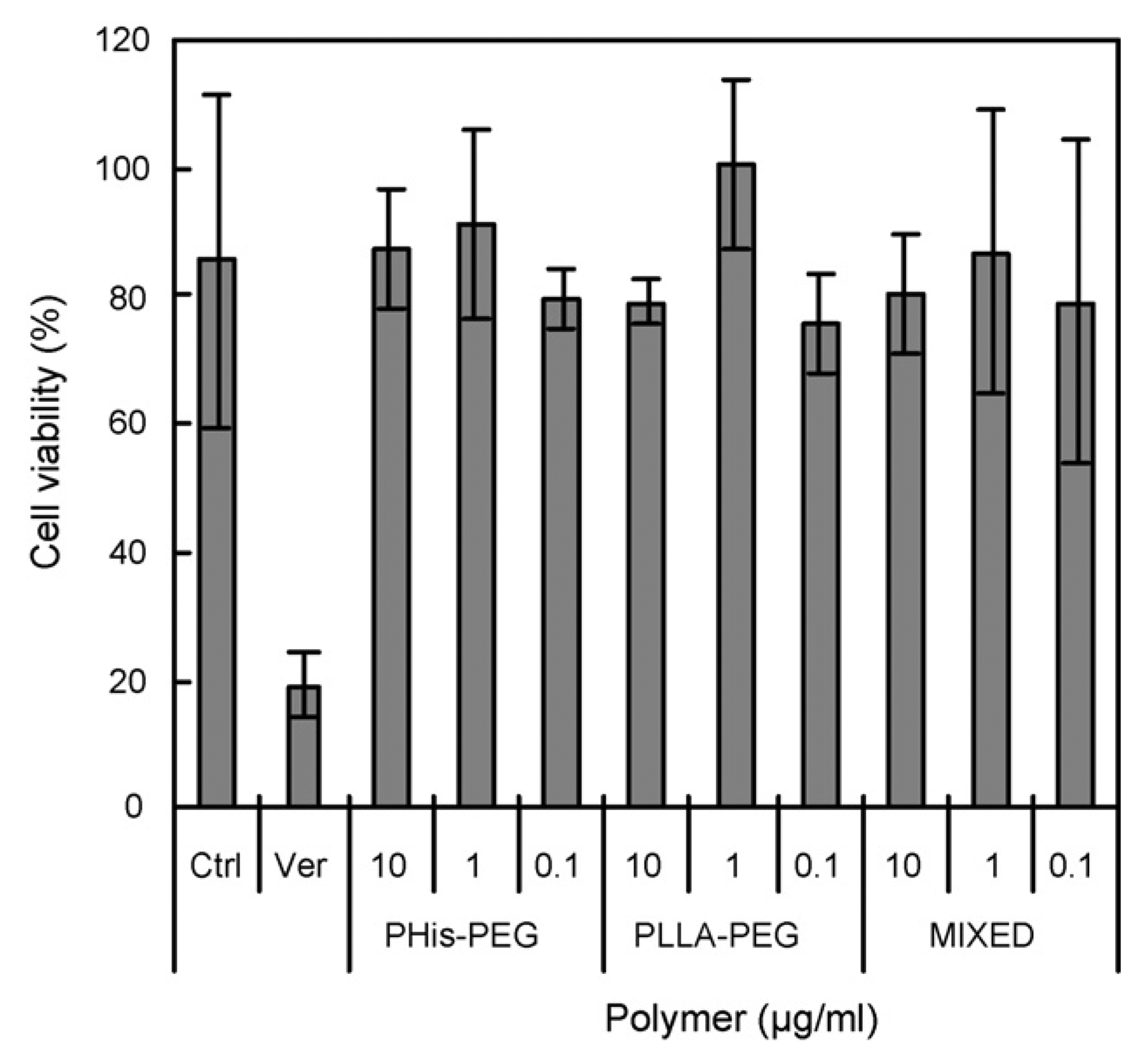
Effect of polymers with concentrations below CMC on cell viability of MDR cell line (A2789/DOX) incubated with 10 µg/ml free DOX after 24 h (n = 5). Ctrl and Ver indicate control and 0.3 mM verapamil without polymers, respectively.
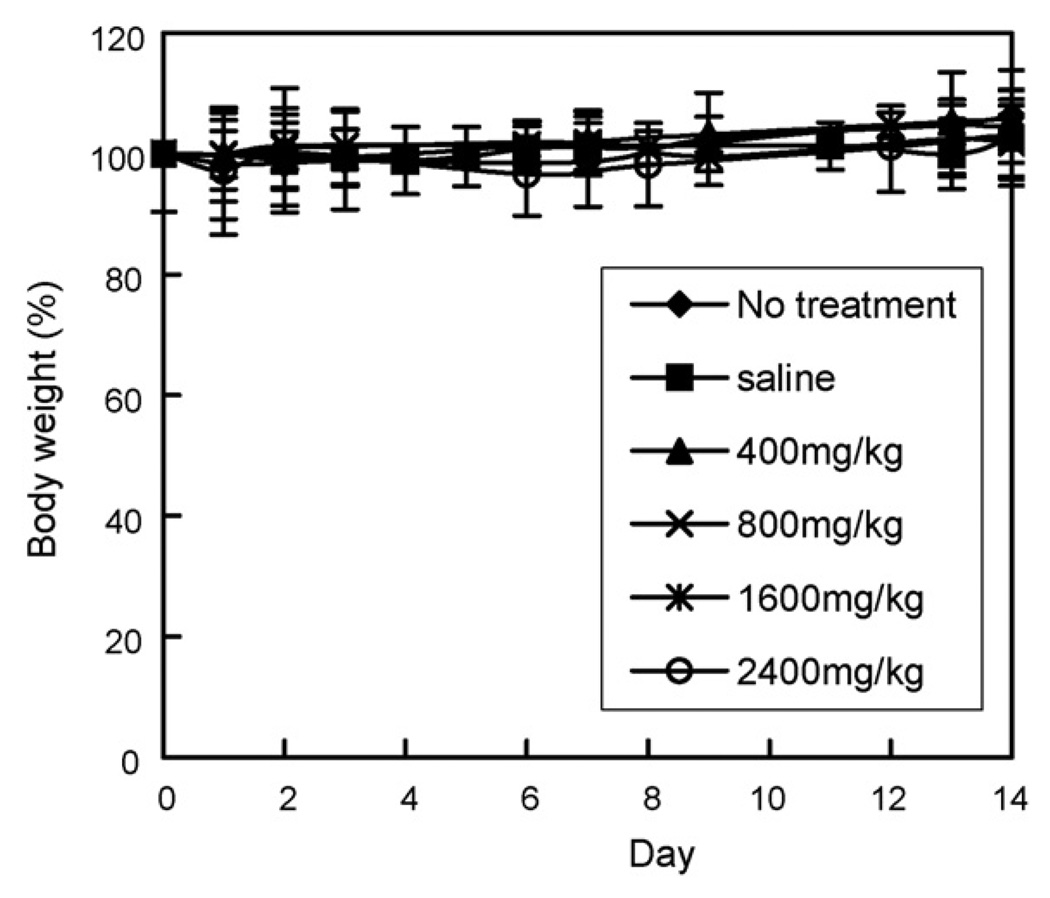
We reported no cytotoxicity of blank micelles (above CMC) by in vitro cell viability study (Lee et al., 2003a). In this study, the systemic toxicity of PHis-PEG was briefly tested in vivo with Balb/C mice. The body weight of mice injected with the polymer did not decrease and their vital behavior appeared equivalent to the control groups (no treatment receiving saline only). The polymer showed no apparent toxicity at concentrations up to 2400 mg/kg, i.v. for 2 weeks (Fig. 5). This dose was achieved by injecting 800mg/kg, which is a maximum polymer concentration for a single injection, daily for 3 days.
Fig. 5.
Effect of PHis-PEG on body weight of BALB/c mice (n = 5). Mice were injected i.v. with saline (■), 400mg/kg (▲), 800mg/kg (×), 1600 mg/kg (✴), and 2400 mg/kg (◊) compared with mice with no treatment (♦).
3.5. MTD determination of DPHSM-F127
Dose-finding studies were conducted to establish the MTD for DPHSM given intravenously in non-tumor-bearing female Balb/c mice. MTD is the highest dose of a chemical that can be administered without altering the animals’ life span and without causing any severe detrimental effects to the animals’ health. This data is used to correlate the plasma concentration–time curve (AUC), providing the basis for subsequent dose escalation in phase I clinical trials (Collins et al., 1986). Once the MTD is established following the single dose study, one tenth of the MTD can be selected as an appropriate starting dose in clinical phase I studies.
To investigate the tolerance of DPHSM-F127 in mice, five mice per group were i.v. injected at a dose range of 50–100 mg/kg. After dosing, the body weight and toxic death were monitored (Fig. 6). For each group, the median body weight was obtained over time. DPHSM-F127at the doses of50–75 mg/kg of equivalent DOX to mice weight caused a median body weight loss of 3.5 ± 6% during the first week, and the mice regained the weight at the end of the second week, but not to the control levels. However, a transient fall in body weight was observed in 85 mg/kg groups. All mice showed a sharp decrease in the body weight on Day 1 and resulted in the death of three mice within 2 days and other two mice were sacrificed on Day 4 due to the loss of body weight more than 20%. In case of 100mg/kg dose, natural mortality was seen in all mice within 2 days. The sudden death by the injection was not observed in all mice. This demonstrated that the formulation caused all mice to die within 1 week in over 85 mg/kg because of toxicity of DOX. From these results a MTD of 75 mg/kg for DPHSM-F127 was approximated.
Fig. 6.
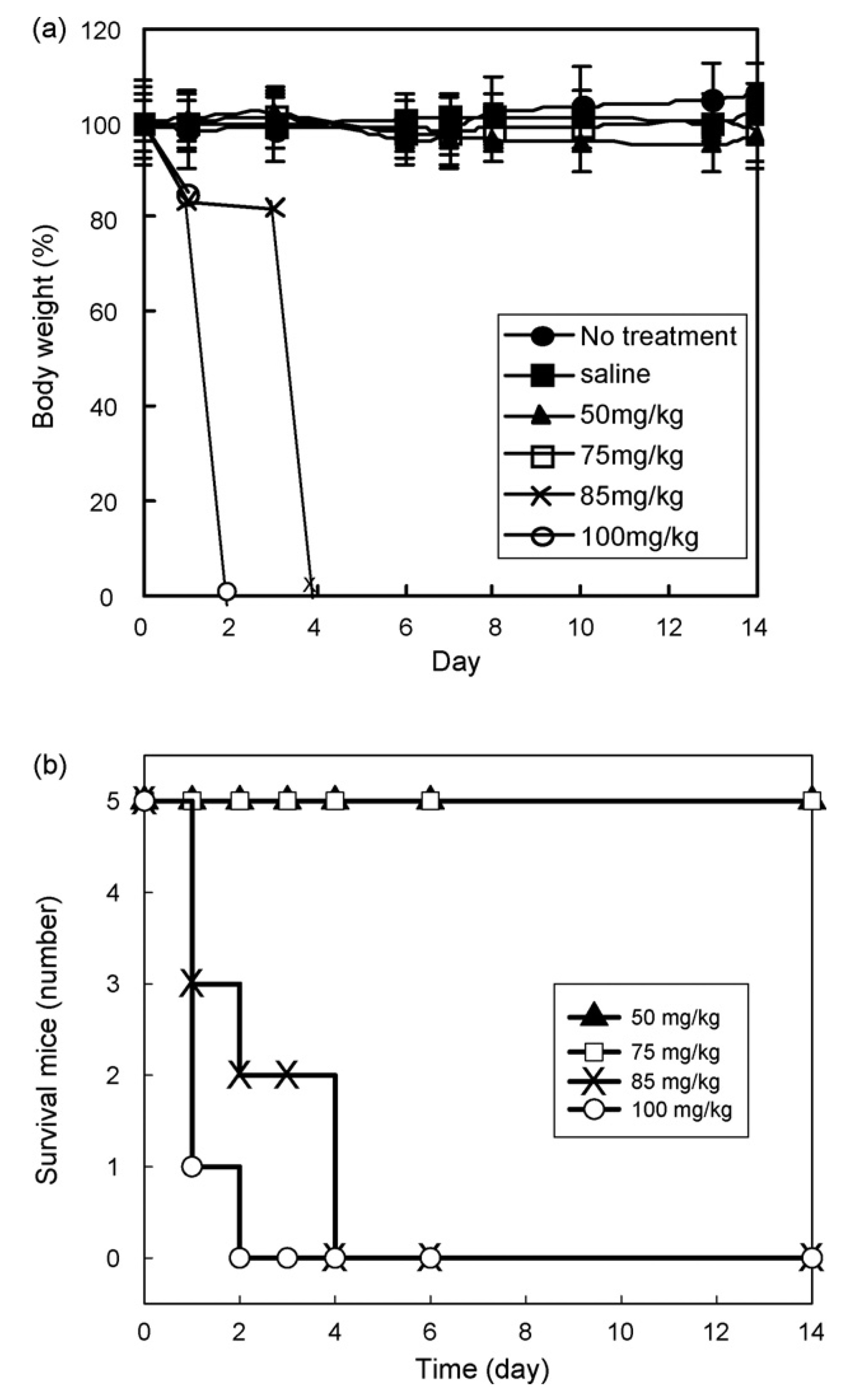
Effect of DPHSM-F127 on (a) body weight and (b) survival of mice (n = 5): no treatment (●), saline (■), 50 mg/kg (▲), 75 mg/kg (□), 85 mg/kg (×), and 100mg/kg (○). The body weight in case of 85 and 100 mg/kg indicates only average weight of survival mice and 0% represents the death of all mice.
The MTD of free DOX was previously determined to be 8–12mg/kg from several independent studies (Boven et al., 1990, 1992; Yokoyama et al., 1990, 1991; Breistol et al., 1998; Colbern et al., 1999; Bae et al., 2005). The MTD of DOX formulation with carriers may deviate from those of free DOX in mice, depending on the carrier effects on pharmacokinetics, biodistribution, and the drug release rate (Allen and Cullis, 2004). For example, the MTD of pegylated liposomal DOX (DOXIL) was lower but the potency was improved (Colbern et al., 1999; Gabizon, 2001; Gabizon et al., 2003). When the drug is inactive by polymer conjugation or given in a prodrug form, a large increase in the MTD has been reported, as in the case of N-(2-hydroxypropyl)methyacrylamide (HPMA) copolymer-DOX (4.5-fold) (Duncan et al., 1998), PEO-bpoly(l-aspartate) conjugated of DOX (NK911) (20-fold) (Yokoyama et al., 1990, 1991), N-l-leucyl-DOX (threefold) (Bennis et al., 1993; Breistol et al., 1998, 1999), and glucuronide prodrug DOX (25-fold) (Houba et al., 2001). In addition, the changes in PK profile of the drug associated with a carrier can increase the MTD as shown by PEO-b-poly(β-benzyl-l-aspartate) (PBLA) micelle (2.3-fold) (Kwon et al., 1997; Kataoka et al., 2000) and SP1049 (1.4-fold) (Danson et al., 2004).
In this study, the MTD of DPHSM-F127was 7.5-fold higher when compared to the free DOX. Interestingly, when compared with other amphiphilic block copolymer micellar formulations, the toxicity by DOX in the formulation significantly decreased. This may be linked to the feature of controlled release of DOX from DPHSM-F127 as a function of pH.
4. Conclusion
In this study, we developed a lyophilized pH-sensitive micelle formulation for DOX which is constituted of PHis-PEG and PLLA-PEG-folate with an excipient, F127 and is readily injectable upon adding a buffer solution and mild agitation. The reconstituted solution showed high stability and high concentrations of DOX for further pre/clinical study and was able to provide the same properties of pH sensitivity and cytotoxicity in MDR tumor cell similar to the freshly made micelle formulation. The brief toxicity study with MTD determination presented minimal systemic toxicity of the formulation. The MTD approximated in this study was 75mg/kg. These physicochemical and safety properties support the study of the formulation on pre/clinical evaluation.
Acknowledgements
The authors thank Dr. Haiqing Yin (University of Utah) and Dr. Ajay Taluja (University of Utah) for a valuable discussion related to physicochemical characterization and reading the manuscript carefully, respectively. This work was supported by NIH CA101850.
References
- Abdelwahed W, Degobert G, Fessi H. Freeze-drying of nanocapsules:impact of annealing on the drying process. Int. J. Pharm. 2006;324:74–82. doi: 10.1016/j.ijpharm.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Advani R, Lum BL, Fisher GA, Halsey J, Chin DL, Jacobs CD, Sikic BI. A phase I trial of liposomal doxorubicin, paclitaxel and valspodar (PSC-833), an inhibitor of multidrug resistance. Ann. Oncol. 2005;16:1968–1973. doi: 10.1093/annonc/mdi396. [DOI] [PubMed] [Google Scholar]
- Alakhov V, Klinski E, Li S, Pietrzynski G, Venne A, Batrakova E, Bronitch T, Kabanov AV. Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surf. B: Biointerf. 1999;16:113–134. [Google Scholar]
- Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: thermodynamics of copolymer association. Macromolecules. 1994;27:2414–2425. [Google Scholar]
- Allen TM, Cheng WW, Hare JI, Laginha KM. Pharmacokinetics and pharmacodynamics of lipidic nano-particles in cancer. Anticancer Agents Med. Chem. 2006;6:513–523. doi: 10.2174/187152006778699121. [DOI] [PubMed] [Google Scholar]
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm. Res. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm. Res. 2003;20:1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: selective energy depletion. Br. J. Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis S, Garcia C, Robert J. Aspects of the cellular pharmacology of N-l-leucyldoxorubicin in human tumor cell lines. Biochem. Pharmacol. 1993;45:1929–1931. doi: 10.1016/0006-2952(93)90453-4. [DOI] [PubMed] [Google Scholar]
- Boven E, Hendriks HR, Erkelens CA, Pinedo HM. The anti-tumour effects of the prodrugs N-l-leucyl-doxorubicin and vinblastine-isoleucinate in human ovarian cancer xenografts. Br. J. Cancer. 1992;66:1044–1047. doi: 10.1038/bjc.1992.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven E, Schluper HM, Erkelens CA, Pinedo HM. Doxorubicin compared with related compounds in a nude mouse model for human ovarian cancer. Eur. J. Cancer. 1990;26:983–986. doi: 10.1016/0277-5379(90)90626-5. [DOI] [PubMed] [Google Scholar]
- Breistol K, Hendriks HR, Berger DP, Langdon SP, Fiebig HH, Fodstad O. The antitumour activity of the prodrug N-l-leucyl-doxorubicin and its parent compound doxorubicin in human tumour xenografts. Eur. J. Cancer. 1998;34:1602–1606. doi: 10.1016/s0959-8049(98)00152-x. [DOI] [PubMed] [Google Scholar]
- Breistol K, Hendriks HR, Fodstad O. Superior therapeutic efficacy of N-l-leucyl-doxorubicin versus doxorubicin in human melanoma xenografts correlates with higher tumour concentrations of free drug. Eur. J. Cancer. 1999;35:1143–1149. doi: 10.1016/s0959-8049(99)00074-x. [DOI] [PubMed] [Google Scholar]
- Chavanpatil MD, Patil Y, Panyam J. Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoprotein-mediated drug efflux. Int. J. Pharm. 2006;320:150–156. doi: 10.1016/j.ijpharm.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Colbern GT, Hiller AJ, Musterer RS, Working PK, Henderson IC. Antitumor activity of Herceptin in combination with STEALTH liposomal cisplatin or nonliposomal cisplatin in a HER2 positive human breast cancer model. J. Inorg. Biochem. 1999;77:117–120. doi: 10.1016/s0162-0134(99)00138-5. [DOI] [PubMed] [Google Scholar]
- Collins JM, Zaharko DS, Dedrick RL, Chabner BA. Potential roles for preclinical pharmacology in phase I clinical trials. Cancer Treat. Rep. 1986;70:73–80. [PubMed] [Google Scholar]
- Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, Brampton M, Halbert G, Ranson M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer. 2004;90:2085–2091. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donini C, Robinson DN, Colombo P, Giordano F, Peppas NA. Preparation of poly(methacrylic acid-g-poly(ethylene glycol)) nanospheres from methacrylic monomers for pharmaceutical applications. Int. J. Pharm. 2002;245:83–91. doi: 10.1016/s0378-5173(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Duncan R, Coatsworth JK, Burtles S. Preclinical toxicology of a novel polymeric antitumour agent: HPMA copolymer-doxorubicin (PK1) Hum. Exp. Toxicol. 1998;17:93–104. doi: 10.1177/096032719801700204. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- Gabizon A. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin. Cancer Res. 2000;6:1949–1957. [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Han SK, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation. Colloids Surf. A: Physicochem. Eng. Aspects. 2003;214:49–59. [Google Scholar]
- Houba PH, Boven E, van der Meulen-Muileman IH, Leenders RG, Scheeren JW, Pinedo HM, Haisma HJ. Pronounced antitumor efficacy of doxorubicin when given as the prodrug DOX-GA3 in combination with a monoclonal antibody beta-glucuronidase conjugate. Int. J. Cancer. 2001;91:550–554. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1075>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-l-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J. Control. Release. 2000;64:143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- Kim GM, Bae YH, Jo WH. pH-induced micelle formation of poly(histidine-co-phenylalanine)-block-poly(ethylene glycol) in aqueous media. Macromol. Biosci. 2005;5:1118–1124. doi: 10.1002/mabi.200500121. [DOI] [PubMed] [Google Scholar]
- Kwon G, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Block copolymer micelles for drug delivery: loading and release of doxorubicin. J. Control. Release. 1997;48:195–201. [Google Scholar]
- Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(l-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002;54:169–190. doi: 10.1016/s0169-409x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003a;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flowerlike micelles of poly(l-lactic acid)-b-poly(ethylene glycol)-b-poly(l-histidine) J. Control. Release. 2007;123:19–26. doi: 10.1016/j.jconrel.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Shin HJ, Na K, Bae YH. Poly(l-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003b;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- Li F, Wang T, He HB, Tang X. The properties of bufadienolides-loaded nano-emulsion and submicro-emulsion during lyophilization. Int. J. Pharm. 2008;349:291–299. doi: 10.1016/j.ijpharm.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Li X, Ruan GR, Lu WL, Hong HY, Liang GW, Zhang YT, Liu Y, Long C, Ma X, Yuan L, Wang JC, Zhang X, Zhang Q. A novel stealth liposomal topotecan with amlodipine: apoptotic effect is associated with deletion of intracellular Ca2+ by amlodipine thus leading to an enhanced antitumor activity in leukemia. J. Control. Release. 2006;112:186–198. doi: 10.1016/j.jconrel.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Links M, Brown R. Clinical relevance of the molecular mechanisms of resistance to anti-cancer drugs. Expert Rev. Mol. Med. 1999;1999:1–21. doi: 10.1017/S1462399499001099X. [DOI] [PubMed] [Google Scholar]
- Meyuhas D, Lichtenberg D. Effect of water-soluble polymers on the state of aggregation, vesicle size, and phase transformations in mixtures of phosphatidylcholine and sodium cholate. Biophys. J. 1996;71:2613–2622. doi: 10.1016/S0006-3495(96)79453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K, Kakizawa Y, Nishiyama N, Yamasaki Y, Watanabe T, Kohara M, Kataoka K. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. J. Control. Release. 2005;109:15–23. doi: 10.1016/j.jconrel.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Mohajer G, Lee ES, Bae YH. Enhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells. Pharm. Res. 2007;24:1618–1627. doi: 10.1007/s11095-007-9277-5. [DOI] [PubMed] [Google Scholar]
- Oh KT, Bronich TK, Kabanov AV. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic block copolymers. J. Control. Release. 2004;94:411–422. doi: 10.1016/j.jconrel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Oh KT, Yin H, Lee ES, Bae YH. Polymeric nanovehicles for anticancer drugs with triggering release mechanisms. J. Mater. Chem. 2007;17:3987–4001. [Google Scholar]
- Pakunlu RI, Wang Y, Saad M, Khandare JJ, Starovoytov V, Minko T. In vitro and in vivo intracellular liposomal delivery of antisense oligonucleotides and anticancer drug. J. Control. Release. 2006;114:153–162. doi: 10.1016/j.jconrel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int. J. Pharm. 2004;277:155–162. doi: 10.1016/j.ijpharm.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Seo M-H, Choi I-J. US 6,616,941 B1. Polymeric composition for solubilixaing poorly water soluble drugs and process for the preparation thereof. 2003 September 9, 2003.
- Simon SM, Schindler M. Cell biological mechanisms of multidrug resistance in tumors. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3497–3504. doi: 10.1073/pnas.91.9.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Talsma H, Cherng J, Lehrmann H, Kursa M, Ogris M, Hennink WE, Cotten M, Wagner E. Stabilization of gene delivery systems by freeze-drying. Int. J. Pharm. 1997;157:233–238. doi: 10.1016/s0378-5173(97)00244-5. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Batrakova E, Li S, Kabanov A. Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells. Bioconjug. Chem. 1999;10:851–860. doi: 10.1021/bc990037c. [DOI] [PubMed] [Google Scholar]
- Wang JC, Liu XY, Lu WL, Chang A, Zhang Q, Goh BC, Lee HS. Pharmacokinetics of intravenously administered stealth liposomal doxorubicin modulated with verapamil in rats. Eur. J. Pharm. Biopharm. 2006;62:44–51. doi: 10.1016/j.ejpb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu L, Han L, Sha X, Fang X. Difunctional Pluronic copolymer micelles for paclitaxel delivery: synergistic effect of folate-mediated targeting and Pluronic-mediated overcoming multidrug resistance in tumor cell lines. Int. J. Pharm. 2007;337:63–73. doi: 10.1016/j.ijpharm.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, Inoue S. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res. 1990;50:1693–1700. [PubMed] [Google Scholar]
- Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–3236. [PubMed] [Google Scholar]