Abstract
Post-traumatic stress disorder (PTSD) is a prevalent, disabling anxiety disorder that constitutes a major health care burden. Despite evidence supporting a genetic predisposition to PTSD, the precise genetic loci remain unclear. Herein we review the current state and limitations of genetic research on PTSD. Although recent years have seen an exponential increase in the number of studies examining the influence of candidate genes on PTSD diagnosis and symptomatology, most studies have been characterized by relatively low rates of PTSD, with apparent inconsistencies in gene associations linked to marked differences in methodology. We further discuss how current advances in the genetics field can be applied to studies of PTSD, emphasizing the need to adapt a genome-wide approach that facilitates discovery rather than hypothesis testing. Genome-wide association studies offer the best opportunity to identify novel “true” risk variants for the disorder that in turn has the potential to inform our understanding of PTSD etiology.
Keywords: Post-traumatic stress disorder, Trauma, Genetics, Genome-wide association, Gene–environment interaction
Introduction
Post-traumatic stress disorder (PTSD) occurs following exposure to a traumatic event and is defined by distinct symptom clusters of re-experiencing, avoidance and numbing, and arousal persisting for more than 1 month after trauma [1]. At least 1 in 9 American women and 1 in 20 American men will meet criteria for the diagnosis in their lifetime [2]. Individuals who develop PTSD have an increased risk of major depression, substance dependence, and other health conditions, as well as impaired role functioning and reduced life course opportunities [3, 4]. Among the 50% to 85% of Americans who are exposed to a traumatic event, the risk of PTSD ranges from 2% to 50%, depending on the type of trauma exposure [5, 6].
Why some individuals develop PTSD following trauma exposure while others are resilient remains a key question in trauma research. The importance of genetic influences on PTSD risk have been recognized for half a century [7]; however, little progress has been made in identifying true or causal risk genetic variants for PTSD. The genetic epidemiology of PTSD has been primarily limited to twin and candidate gene association studies, and there have been no linkage studies of PTSD. Twin studies have all shown monozygotic twins to have significantly higher concordance for PTSD than dizygotic twins, resulting in heritability estimates in the range of 30% to 40% [8, 9]. Despite evidence supporting a genetic predisposition to PTSD, an insufficient amount of research has focused on identifying the precise genetic loci that account for the moderate heritability estimate. This article reviews the current state and limitations of genetic research on PTSD. We then discuss how these limitations could be addressed through genome-wide association studies (GWAS), which, combined with well-powered replication samples, offer the best opportunity to identify novel “true” risk variants for the disorder.
Candidate Gene Association Studies
The candidate gene association design has been the most commonly used approach in the field of PTSD genetics to date. In this approach, allele or genotype frequencies are compared between a sample of PTSD patients and a sample of trauma-exposed, non-PTSD controls. The two most common types of genetic variations, referred to as polymorphisms, studied are single nucleotide polymorphisms (SNPs, in which one single nucleotide base differs) and variable number tandem repeats (VNTRs, in which the nucleotide sequence repeat pattern differs).
In the candidate gene study design, genetic regions are typically selected for study based on their hypothesized putative relationship with the neurobiological processes underlying the development and/or maintenance of PTSD. Table 1 presents a list of candidate genes for PTSD that have been the focus of at least one published study. Most of the extant molecular genetic studies of PTSD have focused on the dopaminergic and serotonergic systems. In fact, 18 of 30 genetically informed studies of PTSD have focused on genes in these systems. Markers of the hypothalamic-pituitary-adrenal axis (FKBP5, GCCR, CNR1), components of the locus coeruleus/noradrenergic systems (NPY, DBH), and neurotrophins (BDNF) also have been studied. Reviews detailing the neurobiological mechanisms whereby these genes are hypothesized to exert their effects are available elsewhere [10•, 11].
Table 1.
Genetic loci implicated in post-traumatic stress disorder, by neurobiological system
| Gene | Common name(s) | Chr | dbSNP | Description | Function | Allele frequency
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Cau | Y | JP | Ch | ||||||
| RD2 (D2R, D2DR) | Dopamine receptor D2 | 11q23 | rs1799732 | Ins/del,−141C | Promoter | 0.09 | ? | 0.22 | 0.09 |
| rs1801028 | Ser311Cys, C > G | Exon 7 | 0.02 | 0 | – | – | |||
| rs1079597 | “TaqIB,” G > A | Intron 1 | 0.16 | 0.17 | 0.39 | 0.45 | |||
| rs1800498 | “TaqID,” C > T | Intron 2 | 0.6 | 0.15 | 0.05 | 0.07 | |||
| rs6277 | 957C > T | Exon 7 | 0.53 | 0.03 | 0.05 | 0.06 | |||
| rs1800497 | “TaqIA,” Glu713Lys, C > T | Exon 8 | 0.22 | 0.45 | 0.42 | 0.48 | |||
| DRD4 (D4DR) | Dopamine receptor D4 | 11p15.5 | – | VNTR, 2–11 48-bp rpt | Exon 3 | – | – | – | – |
| 2 rpt | 0.09 | 0.03 | 0.12 | 0.19 | |||||
| 4 rpt | 0.66 | 0.83 | 0.81 | 0.77 | |||||
| 7 rpt | 0.19 | 0.11 | 0.01 | 0 | |||||
| SLC6A3 (DAT1) | Dopamine transporter | 5p15.3 | – | VNTR, 3–13 40-bp rpt | 3 UTR | – | – | – | – |
| 9 rpt | 0.25 | 0.13 | 0.02 | 0.05 | |||||
| 10 rpt | 0.75 | 0.75 | 0.95 | 0.9 | |||||
| SLC6A4 (HTT, 5HTT, SERT, 5-HTTLPR) | Serotonin transporter | 17q11 | rs4795541 | Ins/del, 44 or 43 bp, “5-HTTLPR” | Promoter | – | – | – | – |
| rs25531 | 3609A > G | Promoter | – | – | – | – | |||
| rs57098334 | VNTR | Intron 2 | – | – | – | – | |||
| 9 rpt | 0.01 | 0 | 0 | 0 | |||||
| 10 rpt | 0.32 | 0.2 | 0.25 | 0.1 | |||||
| 12 rpt | 0.65 | 0.74 | 0.76 | 0.86 | |||||
| HTR2 (5-HT2A) | 5-hydroxytryptamine (serotonin) receptor 2A | 13q14-q21 | rs6311 | −1438G > A | Promoter | 0.45 | 0.38 | 0.5 | 0.47 |
| FKBP5 | FK506 binding protein 5 | 6p21 | rs3800373 | T > G | 3 UTR | 0.72 | 0.5 | – | 0.29 |
| rs992105 | A > C | Intron 7 | 0.17 | 0.2 | 0.14 | 0.13 | |||
| rs9296158 | G > A | Intron | 0.24 | 0.39 | 0.32 | 0.22 | |||
| rs737054 | C > T | Intron 5 | 0.23 | 0.1 | 0.28 | 0.31 | |||
| rs1360780 | C > T | Intron 2 | 0.24 | 0.36 | 0.19 | 0.2 | |||
| rs1334894 | C > T | Intron 1 | 0.06 | 0 | 0.03 | 0.06 | |||
| rs9470080 | C > T | Intron 1 | 0.28 | 0.44 | 0.32 | 0.24 | |||
| rs4713916 | G > A | Promoter | 0.35 | – | – | 0.27 | |||
| BDNF | Brain-derived neurotrophic factor | 11p13 | rs6265 | Val66Met, G > A | Missense | 0.18 | 0 | 0.34 | 0.63 |
| – | 270C > T | Exon 5 | – | – | – | – | |||
| – | −712G > A | 5 UTR | – | – | – | – | |||
| NPY | Neuropeptide Y | 7p15.1 | rs16139 | Leu7Pro, T > C | Exon 2 | 0.7 | 0 | 0 | 0 |
| GCCR (NR3C1) | Glucocorticoid receptor | 5q31.3 | rs6189 | Glu22Glu, G > A | Exon 2 | – | – | – | – |
| rs6190 | Arg23Lys, G > A | Exon 2 | – | – | – | – | |||
| rs56149945 | Asn363Ser, A > G | Exon 2 | 0.2 | 0 | – | – | |||
| DBH | Dopamine β-hydroxylase | 9q34 | rs1611115 | −1021C > T | Promoter | 0.18 | 0.13 | 0.16 | 0.22 |
| CNR1 (CB1,CNR) | Cannabinoid receptor 1 (brain) | 6q14-q15 | rs806369 | C > T | Promoter | 0.32 | 0.15 | 0.44 | 0.43 |
| rs1049353 | Thr453Thr, A > G | Exon 1 | 0.26 | 0.01 | 0.09 | 0.08 | |||
| rs806377 | T > C | 5 UTR | 0.47 | 0.63 | 0.3 | 0.42 | |||
| rs6454674 | T > G | Intron | 0.29 | 0.24 | 0.23 | 0.39 | |||
| GABRA2 | GABAA | 4p12 | rs279836 | T > A | Intron 3 | 0.49 | 0.15 | 0.45 | 0.57 |
| rs279826 | G > A | Intron 3 | 0.49 | 0.5 | 0.55 | 0.43 | |||
| rs279858 | Lys132Lys, G > A | Exon 5 | 0.48 | 0.84 | 0.63 | 0.5 | |||
| rs279871 | A > G | Intron 3 | – | – | – | – | |||
| COMT | Catechol-O-methyltransferase | 22q11 | rs4680 | Val158Met, G > A | Exon 6 | 0.52 | 0.29 | 0.24 | 0.26 |
| APOE | Apolipoprotein E | 19q13 | rs429358 | Cys130Arg, T > C | Exon 4 | 0.21 | 0.02 | 0.01 | 0 |
| rs7412 | Arg176Cys, C > T | Exon 4 | 0.28 | – | – | 0.1 | |||
| ε1 = C + T | – | – | – | – | |||||
| ε2 = T + T | – | – | – | – | |||||
| ε3 = T + C | – | – | – | – | |||||
| ε4 = C + C | – | – | – | – | |||||
| RGS2 | Regulator of G-protein signaling 2 | 1q31 | rs4606 | C > G | 3 UTR | 0.29 | 0.34 | – | 0.6 |
Cau Caucasian, Ch Han Chinese, Chr chromosome, GABA γ-aminobutyric acid, JP Japanese, rpt repeat, UTR untranslated region, VNTR variable number tandem repeat, Y Yoruba
(Data from Szantai et al. [80], Rajeevan et al. [81], and http://www.ncbi.nlm.nih.gov/snp)
Table 2 summarizes the 30 genetic association studies of PTSD published to date. Five studies examined the association between SNPs of the dopamine receptor D2 (DRD2) region and chronic PTSD [12–16]. The first two studies [12, 13] found a positive association between risk and a SNP commonly known as TaqIA within the coding region of the ankyrin repeat and kinase domain containing 1 (ANKK1) gene, located downstream of DRD2. Young et al. [15] replicated these findings, but only in a subset of PTSD patients who engaged in harmful drinking. A fourth study found no association with this or any other DRD2 variant or haplotype [14]. Voisey et al. [16] also reported no significant effect of the TaqIA SNP on risk of PTSD but reported a significant association with another DRD2 variant (rs6277) that has yet to be replicated. All five studies included non-Hispanic white, combat-exposed patients, but only one included controls who were specifically selected for trauma exposure [13]. Two studies examined a VNTR in a dopamine transporter gene (DAT1), and both reported an increased risk of PTSD with 9 40-bp repeats compared with 10 repeats despite differences in traumatic exposure across studies [17, 18]. Finally, a VNTR in the gene encoding the dopamine receptor D4 (DRD4) was examined in relation to PTSD diagnosis and symptoms within 3 months of exposure to a flood [19]. Findings supported significantly higher levels of avoidance/numbing symptoms in carriers of the long (seven or eight repeats) allele, as well as higher levels of PTSD symptoms as measured by a questionnaire indexing the intensity of PTSD symptoms. However, genotype did not predict PTSD diagnosis, and although a trend was observed, it did not significantly predict PTSD symptoms on a measure of clinical symptoms. Although most studies of the DRD4 VNTR have compared long-allele carriers with short-allele carriers, fine-mapping and resequencing studies suggest potential functional differences among these subgroups that may in turn impact association studies using the traditional long/short classification [20].
Table 2.
Genetic association studies of PTSD organized by neurobiological system
| Reference (year) |
Trauma type |
Cases, n (% male) |
Mean age, y (SD) |
Case ascertainment | Controls, n (% male) |
Mean age, y (SD) | Control exposed |
Comorbid, current and history |
Nation/ ethnicity |
Gene and dbSNP | Finding | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||||||
| [12] (1991) | “Severe” combat | 35 (100) | NR | Setting: VA clinic; method: NR; time from E: NR | 314 (100) | NR | No | Yes | Yes | United States/NHW | DRD2; rs1800497 | Excess T in PTSD cases (P=0.007); P=0.0008 when controls screened for alcoholism |
| [13] (1996) | Combat | 37 (100) | ~44 | Setting: VA clinic; method: SI: DSM-III; time from E: NR | 19 (100) | ~44 | Yes | Yes | Yes | United States/NHW | DRD2; rs1800497 | Excess T in PTSD cases (P=0.00001); T positively associated with symptoms |
| [14] (1999) | Combat | 52 (100) | 45 (4) | Setting: VA clinic; method: SI: SCID, SADS-L; time from E: NR | 87 (100) | NR | No | Yes | No | United States/NHW | DRD2; rs1800497, rs1079597, rs1800498 | No significant association between SNPs/haplotypes and PTSD |
| [15] (2002) | Combat | 91 (100) | 52 (1) | Setting: inpatient unit; method: SI: DSM-IV; time from E: NR | 51 (35) | 39 (2) | No | Yes | NR | Australia/NHW | DRD2; rs1800497 | Excess T only in PTSD cases with harmful (≥60 g) drinking (P< 0.001); T associated with alcohol consumption among cases |
| [16] (2009) | Combat | 127 (100) | NR | Setting: hospital; method: SI: DSM-IV; time from E: “decades” | 228 (NR) | NR | NSF | No | NR | Australia/NHW | DRD2; rs1800497, rs6277, rs1799732 | Excess rs6277 C in PTSD (P=0.021) |
| [19] (2009) | Flood | 24 (~47) | ~36 | Setting: epidemiologic exposure; method: SI: PTSD-F, PTSD-C, DSM-IV symptoms; time from E: 3 mo | 83 (~47) | ~36 | Yes | NR | NR | Poland/NR | DRD4; VNTR (exon 3) | “Long” (7- and 8-repeat carriers) predicted more intense PTSD symptoms (P=0.048), more specifically those related to avoidance and numbing (P=0.035); no association with PTSD risk |
| [17] (2002) | Various | 102 (56) | 40 (12) | Setting: PTSD research studies/medical health clinic; method: SI: CAPS, SCID; time from E: “chronic” | 104 (47) | 34 (10) | Yes | No | No | Israel/Ashkenazi and non- Ashkenazi | DAT1; VNTR (3 UTR) | Excess 9-repeat in PTSD cases (P=0.012) |
| [18] (2009) | New Orleans Hurricane Katrina/2005 | Total: 88 (59) | 3–6 | Setting: epidemiologic exposure; method: SSI: preschool age psychiatric assessment, DSM-IV; time from E: <3 y | Total: 88 (59) | 3–6 | Yes | No | No | United States/AA; United States/NHW; United States/other | DAT1; VNTR (3 UTR) | Significant difference in PTSD risk by genotype classification (P<0.05, 9/9 highest risk); 9 carriers exhibited greater total symptoms compared with 10/10 genotype (driven by criterion D: arousal) |
| [21] (2005) | Various | 100 (43) | 35 (10) | Setting: medical health clinic; method: SI: DSM-IV; time from E: NR | 197 (39) | 35 (11) | NSF | No | No | Korea/Korean | SLC6A4; rs4795541 | Excess s allele in PTSD cases (P=0.04) |
| [22] (2010) | Rwandan civilian war (36 war/nonwar events) | 331 (~53) | ~35 | Setting: epidemiologic exposure; method: SI: PDS, DSM-IV; time from E: 13 y postwar | 77 (~53) | ~35 | Yes | No | No | Rwanda/NR | SLC6A4; rs4795541, rs25531 | s/s associated with increased risk of lifetime PTSD (P=0.008); no gene– environment (number of TE types, or time since trauma) interaction; significant dose–response between number of event types and lifetime PTSD among s/l and l/l, but not among s/s (interaction not significant); no association with current PTSD or remission from lifetime PTSD |
| [23••] (2007) | Hurricane 2005 (Florida) | 19 (32) | Adults | Setting: epidemiologic exposure; method: telephone interview, National Women’s Study PTSD module; time from E: 6 mo (“current”) | 570 (37) | Adults | Yes | Yes | Yes | United States/NHW; United States/AA, HW, As | SLC6A4; rs4795541, rs25531 | Significant association between s/s genotype and PTSD in adults with high hurricane exposure and low social support prior to hurricane (P<0.03 for interaction); similar effect pattern observed for MD |
| [24••] (2009) | Hurricane 2004 (Florida); various | 19 (32) | Adults | Setting: epidemiologic exposure; method: telephone interview, National Women’s Study PTSD module; time from E: 6 mo (“current”) | 571 (36) | Adults | Yes | Yes | Yes | United States/NHW; United States/AA, HW, As | SLC6A4; rs4795541, rs25531 | Significant interaction between genotype and crime rate (P=0.03) or unemployment (P=0.007) for risk of PTSD; s allele was associated with decreased risk of PTSD in low-risk environments (low crime/unemployment) and increased risk of PTSD in high-risk environments |
| [25] (2007) | Various | 107 (42) | 34 (10) | Setting: medical center; method: SI: DSM-IV, SCID-Kor; time from E: NR (“chronic”) | 161 (32) | 32 (10) | NSF | No | No | Korea/Korean | 5-HTR2A; rs6311 | Excess GG in female PTSD case (P=0.04) |
| [26•] (2009) | Motor vehicle accidents, other trauma | 24 (~46) | ~30 | Setting: prospective study of emergency department trauma patients (convenience sample); method: SI: development and persistence and SI, PDI, PDEQ, CAPS, DSM-IV, MINI; time from E: 1-mo and 12-mo follow-up for remission (acute) or persistence (chronic) | 17 (~46) | ~30 | Yes | NR | NR | United States/NHW; United States/other | SLC6A4; rs4795541 | At 12 mo, excess l/l in chronic PTSD vs non-PTSD and acute cases (P=0.052) |
| [27] (2009) | Various | 229 (42) | 39 (10) | Setting: hospital/medical health clinic; method: SSADDA, includes PTSD interview; time from E: “lifetime” | 1023 (54) | 39 (11) | Yes | Yes | Yes | United States/NHW; United States/AA | SLC6A4; rs4795541, rs25531 | No main effect of gene/variant on PTSD risk; gene–adult trauma interaction: NHW, P=0.03, AA, P=0.04; gene– child adversity interaction: NHW, P=0.02, AA, P=0.16; highest risk group: “ss” and event; gene–(adult trauma and child adversity) interaction: NHW, P=0.002, AA, P=0.04 |
| [28] (2009) | Various | 67 (36) | 58 (17) | Setting: study of health in Pomerania; method: SCID; time from E: “lifetime” | 1596 (51) exposed; 1382 (46) not exposed | 58(16); 50 (13) | NSF | Yes | Yes | German/NHW | SLC6A4; rs4795541, rs25531 | La increased risk of PTSD (P=0.009); in individuals with more than 3 traumatic life events, an additive interaction was found with the La allele conferring risk (P<0.05 for interaction) |
| [29] (2009) | Various | 55 (24) | 40 (16) | Setting: university clinics; method: CAPS for PTSD, SCID for MD and other psychotic disorders, life event checklist; time from E: NR (“lifetime”) | 63 (45) | 40 (17) | Yes | Yes | Yes | United States/AA | SLC6A4; rs4795541, rs25531; 5-HT2A; rs6311 | No significant association between SLC6A4 SNPs and PTSD; excess rs6311 G allele in PTSD cases (P=0.008) |
| [30] (2010) | Physical trauma, stroke | 29 (38) | NR | Setting: emergency department; method: telephone interview, CAPS; time from E: assessed 6 mo after trauma (“lifetime”) | 48 (75) | NR | Yes | Yes | Yes | Turkey/NHW | SLC6A4; rs4795541, rs57098334 | No association with lifetime PTSD; L carriers associated with milder hyperarousal symptoms (P=0.05), and carriers of “12” associated with more severe avoidance symptoms (P<0.05); S carriers related to more severe PTSD (P=0.05) |
| [38] (2008) | Various | 762 (~43) | ~41 (14) | Setting: hospital/medical health clinic; method: PSS, CAPS; time from E: NR (“lifetime”) | – | – | – | Yes | – | United States/AA; United States/other | FKBP5; rs3800373, rs992105, rs9296158, rs737054, rs1360780, rs1334894, rs9470080, rs4713916 | rs3800373 (risk C), rs9296158 (A), rs1360780 (T), and rs9470080 (T) each significantly interacted with severity of child abuse in prediction of adult PTSD symptoms (P< 0.0004) |
| [39••] (2010) | Various | 343 (~54) | ~39 (11) | Setting: hospital/medical health clinic; method: SSADDA, includes PTSD, interview; time from E: “lifetime” | 2084 (~54) | ~39 (11) | NSF | Yes | Yes | United States/NHW; United States/AA | FKBP5; rs3800373, rs9296158, rs1360780, rs9470080 | rs3800373, rs9296158, and rs9470080 associated with PTSD in AA only (P<0.05); AA with rs9470080 T/T had the lowest risk of PTSD compared with other genotypes in the absence of childhood adversity exposure but had the highest risk when exposed (P=0.008 for interaction); NHW rs3800373 (risk A), rs9296158 (G), rs1360780 (C), and rs9470080 (C) carriers had higher risk of PTSD if they were also alcohol dependent compared with those with other genotypes (P for interaction, NR) |
| [33] (2005) | Combat | 118 (100) | 56 (4) | Setting: PTSD clinic; method: SI: CAPS, DSM-IV; time from E: NR | 42 (100) | 61 (7) | Yes | No | No | Australia/NHW | GCCR; rs6189, rs6190, rs56149945 | No significant association between GCCR SNPs and PTSD |
| [40] (2008) | Not specified | Child: 6 (67); parents: 25 (24) 17 (29) |
NR | Setting: ADHD genetic study; method: SSI: KSADS-PL (child), SADS-LAR (adult); time from E: NR (“lifetime”) Setting: cohort; method: SADS-LAR?; time from E: NR (“lifetime”) |
Child: 181 (70); parent: 291 (52) 292 (67) |
NR | NSF – |
Yes – |
Yes – |
United States/NHW Finland/NHW |
CNR1; rs806369, rs1049353, rs806377, rs6454674 rs806369, rs1049353, rs806377, rs6454674 |
rs1049353 A associated with PTSD in parents (P=0.011); haplotype (rs806369, rs1049353): excess C–A and C–G variants in parents of youth with ADHD who report PTSD No association with PTSD |
| [34] (2002) | Combat | 77 (100) | NR | Setting: VA clinic; method: SCID; time from E: NR | 202 (100) | NR | NSF | Yes | Yes | United States/NHW | NPY; rs16139 | No association between NPY SNP and PTSD |
| [37] (2007) | Combat | 133 (100) | 40 (7) | Setting: military unit; method: SCID, DSM-IV; time from E: “current” and “chronic” | 34 (100) | 38 (4) | Yes | No | No | Croatia/Caucasian | DBH; rs1611115 | No association between SNP and PTSD; SNP associated with DBH levels (P=0.0001) |
| [41] (2009) | Various | 46 (NR) | NR | Setting: adult twin study; method: telephone interview, DSM-IV; time from E: NR | 213 (NR) | NR | NSF | Yes | Yes | NR | GABRA2; rs279836, rs279826, rs279858, rs279871 | No main effect of gene/variant on PTSD risk; significant interaction (P<0.05) between composite lifetime history of trauma exposure and 3 of 4 risk alleles for adult PTSD |
| [42] (2010) | 1994 Rwandan genocide/war; TE: 36 war- and non–war- related types | 340 (~53) | ~35 | Setting: refugee during war; method: SI: PDS; time from E: 12–13 y (“lifetime” and “current”) | 84 (~53) | ~35 | Yes | No | No | Rwanda | COMT; rs4680 | No main effect of gene/variant on PTSD risk; significant interaction (P=0.04) between genotype and number of traumatic event types. Met/Met were at high risk for lifetime PTSD independent of number of traumatic events types, whereas Val/Val showed typical dose–response of traumatic event types and risk for PTSD |
| [36] (2006) | Various | 107 (42) | 34 (10) | Setting: hospital; method: SCID-Kor, DSM-IV; time from E: NR | 161 (32) | 32 (10) | NSF | Yes | Yes | Korea/Korean | BDNF; rs6265 | No association between BDNF SNP and PTSD |
| [35] (2006) | Various | 96 (76) | 44 (7) | Setting: VA clinic; method: SCID, SADS-L, DSM-III; time from E: NR | 250 (41) | 38 (20) | NSF | NR | No | United States/NHW | BDNF, G-712A, C270T; rs6265 | No association between BDNF SNPs and PTSD |
| [43] (2005) | Combat | 54 (100) | 53 (6) | Setting: PTSD treatment program; method: CAPS-2, SCID, DSM-IV; time from E: NR | – | – | – | Yes | – | United States/NHW | APOE | APOE ε2 allele associated with higher CAPS-2 re-experiencing scores (P=0.001) |
| [44] (2009) | Hurricane 2004 (Florida); various | 607 (35) | Adults | Setting: epidemiologic exposure; method: telephone interview, National Women’s Study PTSD module; time from E: 6 mo (“current” and “lifetime”) | – | – | – | Yes | – | United States/NHW; United States/AA, HW, As | RGS2; rs4606 | Significant 3-way interaction for posthurricane PTSD symptoms (P=0.03) and lifetime PTSD symptoms (P<0.001); “C” allele increased risk under high environmental stress conditions (high hurricane exposure/PTE and low social support) |
AA African American, ADHD attention-deficit/hyperactivity disorder, As Asian, CAPS Clinician Administered Posttraumatic Stress Disorder Scale, dbSNP Single Nucleotide Polymorphism Database, E trauma exposure, HW Hispanic white, KSADS-PL Kids’ Schedule for Affective Disorders and Schizophrenia-Present and Lifetime, MD major depression, MINI Mini International Neuropsychiatric Interview, NHW non-Hispanic white, NR not reported, NSF not selected for, PDEQ Peritraumatic Dissociative Experiences Questionnaire, PDI Peritraumatic Distress Inventory, PDS Posttraumatic Diagnostic Scale, PSS Posttraumatic Stress Disorder Symptom Scale, PTE post-traumatic event, PTSD post-traumatic stress disorder, PTSD-C Post-Traumatic Stress Disorder Clinical Questionnaire, PTSD-F Post-Traumatic Stress Disorder Factor Questionnaire, SADS-L Schedule for Affective Disorders and Schizophrenia-Lifetime, SADS-LAR Schedule for Affective Disorders and Schizophrenia, SCID Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, SCID-Kor SCID, Korean version, SI structured interview, SNP single nucleotide polymorphism, SSADDA Semi-Structured Assessment for Drug Dependence and Alcoholism, SSI semistructured interview, TE traumatic event, UTR untranslated region, VA Veterans Affairs, VNTR variable number of tandem repeats
Among 10 studies investigating the serotonergic system [21, 22, 23••, 24••, 25, 26•, 27–30], all but one [25] examined an insertion/deletion polymorphism in the promoter region of the serotonin transporter (SLC6A4, locus 5-HTTLPR) commonly annotated as “long (l)” and “short (s)” alleles with inferred high and low expression, respectively [31]. The first reported an excess of s/s genotypes in Korean PTSD patients compared with controls who were not selected for exposure [21]. Mellman et al. [29] and Sayin et al. [30] reported no effect of the 5-HTTLPR polymorphism on risk of lifetime PTSD following various post-traumatic exposures. Sayin et al. [30], however, observed a positive association between the s allele and severity of PTSD and hyperarousal symptoms [30]. In a prospective study of emergency department physical trauma patients (n=41), Thakur et al. [26•] found that 5-HTTLPR was not significantly associated with initial risk for PTSD diagnosis. To examine the variant’s association with PTSD chronicity, the authors compared participants continuing to evidence PTSD at 12 months with those who no longer met criteria for PTSD at 12 months (including participants who did not meet initial diagnosis and participants who evidenced remission of early PTSD diagnosis). Findings supported excess l/l genotypes in chronic PTSD patients compared with a group of acute PTSD patients and exposed nonpatients (P= 0.052). Although this study was significantly limited by a small sample size and by the grouping of participants who met initial diagnostic criteria along with participants who did not meet initial diagnosis, the results suggest that predictors of onset may differ from predictors of chronicity. Additionally, the 5-HTTLPR polymorphism has been found to be triallelic in that a third functional allele LG, has been identified [32]; LG is characterized by an A > G substitution at nucleotide 6 of the first of two extra 22-bp repeats in the l allele, resulting in transcriptional capacity comparable with that of the s allele. Following, it has become common practice to classify the 5-HTTLPR triallelically. Accordingly, investigations that have examined only the insertion/deletion may have included less transcriptionally efficient variants in their “l” allele groups.
The remaining four studies considered potential gene–environment (G × E) interactions, and all these studies classified the 5-HTTLPR triallelically [23••, 24••, 27, 28]. Kilpatrick et al. [23••] found the inferred low expression “s” variant of the 5-HTTLPR increased risk of post-hurricane PTSD only under conditions of high environmental stress exposure (high hurricane exposure and low social support). Using the same study population of hurricane-exposed adults, Koenen et al. [24••] reported a similar G × E interaction when a high-risk environment was defined by a high county-level crime rate and county-level unemployment rate. Notably, this is the first demonstration of a gene by social environment interaction. Moreover, and relevant to the pattern of inconsistencies reported for this genetic variation was the observation of a protective effect of the “s” variant under conditions of low risk [24••]. Grabe et al. [28] reported an increased risk of lifetime PTSD associated with the high expression variant as well as an additive interaction with number of traumatic events in a population-based sample of German adults (20–79 years of age). In contrast to the Kilpatrick et al. [23••] and Koenen et al. [24••] investigations, both a strength and a limitation of this investigation is the heterogeneity of the timing and type of trauma(s) experienced by participants. Xie et al. [27] observed a significant interaction between variation in 5-HTTLPR and adult and/or child trauma for risk of lifetime PTSD. More specifically, increased risk of PTSD was evidenced in “s” allele carriers who experienced childhood and adulthood trauma.
Yet another serotonergic polymorphism, a G →A substitution (rs6311) in 5-hydroxytryptamine (serotonin) receptor 2A (5-HT2A), was examined in a sample of Koreans by Lee et al. [25] and in a sample of Americans by Mellman et al. [29]. Both reported an increased risk of PTSD associated with the G allele, although Lee et al. [25] observed this effect only among women.
The remaining studies explored genetic polymorphisms across alternative neurobiological pathways, with mixed success. These included markers of the hypothalamic-pituitary-adrenal axis (FKBP5, GCCR, CNR1) and components of the locus coeruleus/noradrenergic systems (NPY, DBH, COMT, GABRA2). Loci-encoding neurotrophins (BDNF), lipoproteins (APOE), and regulators of G-protein signaling (RGS2) also have been investigated. No significant associations were reported between chronic PTSD and variation in genes encoding glucocorticoid receptor (GCCR) [33], neuropeptide Y (NPY) [34], or brain-derived neurotrophic factor (BDNF) [35, 36]. Two variants in DBH encoding dopamine β-hydroxylase were also not associated with current or chronic PTSD following exposure to combat [37]. Among a population of predominantly African Americans, Binder et al. [38] reported significant interactions between four highly linked variants in FKBP5 (FK506 binding protein 5) and severity of child abuse in prediction of adult PTSD symptoms. The same four variants were recently examined by Xie et al. [39••] in a population of non-Hispanic whites and African Americans. Three of the variants were associated with risk of PTSD only among African Americans. Moreover, Xie et al. [39••] observed a significant interaction between one of these four FKBP5 variants and childhood adversity that was specific to the African American subgroup, which was consistent with results reported by Binder et al. [38]. Lu et al. [40] reported a significant association between lifetime PTSD and one of four SNPs in CNR1 (cannabinoid receptor 1) among parents and a haplotype of two CNR1 SNPs among parents of youth with attention-deficit/hyperactivity disorder. The same study, however, reported no relationship between any CNR1 polymorphism and PTSD among an independent population of similar ancestry [40]. Significant G × E interactions for risk of PTSD were recently reported in studies of GABRA2 (γ-aminobutyric acid A receptor, α2) [41] and COMT (catechol-O-methyltransferase) [42]. Several variants of GABRA2 interacted with composite lifetime history of trauma exposure [41], while a well-characterized amino acid substitution (Val158Met) in COMT interacted with the number of traumatic event types [42]. A single study examined the association between the commonly investigated APOE variation and PTSD symptoms among PTSD veterans [43]. The APOE ε2 allele was associated with higher re-experiencing scores [43]. Additionally, a variant in the regulator of G-protein signaling 2 (RGS2) was found to be associated with increased risk of PTSD (current and lifetime) symptoms under conditions of high stress [44].
Our review of genetic association studies as presented in Table 2 leads to four conclusions. First, relatively few genetic association studies of PTSD—when compared with mental disorders of similar heritability such as depression—have been conducted. Second, a very limited number of candidate genes selected from a few relevant neurobiological pathways have been studied. Third, sample sizes have been small, and range of exposure type and duration limited. Fourth, existing studies have produced conflicting results. For example, in six studies [21, 22, 23••, 24••, 27, 29], the low expression “s” allele of the serotonin transporter polymorphism increased risk of PTSD, and in two studies, the high expression “l” allele increased risk [26•, 28]. These inconsistencies are likely a result of differences in study design and underscore the need to attend to these differences for not only interpretative purposes but also as a means to move forward efficiently and successfully in the field of PTSD genetics.
Genome-Wide Association Studies of Post-Traumatic Stress Disorder
Advances in cost-effective, high-throughput genotyping platforms have led to the new era of GWAS. Such studies take an agnostic approach to risk loci discovery by comparing frequencies of hundreds of thousands of SNPs across the entire genome of cases with those of controls. GWAS are especially powerful when genetic variations with appreciable frequency in the population at large but relatively low penetrance are the major contributors to genetic susceptibility to common diseases; this is often described as the “common disease, common variant” hypothesis [45]. Thus far, GWAS have been successful in uncovering more than 600 new loci for more than 130 complex diseases and traits, including psychiatric conditions such as schizophrenia, bipolar disorder, and attention-deficit/hyperactivity disorder [46]. A notable absence, however, is any GWAS of PTSD.
The candidate gene association approach used by all genetic epidemiologic studies of PTSD to date relies on biological hypotheses to guide the choice of candidate genes. Given the relative paucity of information regarding the biological underpinnings of PTSD, this approach has been limited to a few biological pathways. Moreover, only a few SNPs from each candidate gene have been examined. Therefore, a null finding does not necessarily rule out the role of the gene in PTSD etiology, even under ideal study conditions. We believe GWAS is the next necessary step in genetic research of PTSD. Large, well-designed GWAS with well-powered replication samples offer the best opportunity to identify the true causal variants that underlie the disorder. In the remainder of this article, we discuss important design considerations specific to GWAS of PTSD. Design considerations for GWAS in psychiatric disorders have been well-described elsewhere [47•, 48••] and thus are not a focus of this article.
Trauma-Exposed Controls
Appropriate control selection remains a major challenge to PTSD genetic studies. Because PTSD is conditional on trauma exposure, a substantial proportion of the population that is not trauma exposed may carry an unexpressed genetic vulnerability for PTSD. Selecting controls independently of their trauma exposure would impede detection of PTSD risk loci, especially if these loci have modest effect sizes [8, 49]. In addition, a significant genetic association with PTSD may not be distinguishable from a gene–trauma correlation. To reduce both type 1 and type 2 errors, controls should be selected from the same underlying population as cases, with both groups evidencing comparable levels of trauma exposure (including severity and duration).
Fourteen of the published PTSD genetic studies have used the standard epidemiologic study design in which a random sample is drawn from an underlying population and assessed for trauma exposure and PTSD to ensure appropriate control selection. Prospective population-based designs or prospective exposed cohort designs, in which individuals are enrolled in a study upon exposure to a traumatic event and observed over time to see who develops PTSD (cases) and who does not (controls), are preferable options but also require additional effort and resources.
Gene–Trauma Correlations
Twin studies have highlighted potential G × E correlations, whereby selection of environment and, subsequently, potential for exposure to trauma is partially determined by genetic factors [9, 50, 51]. Data from civilian and non-civilian twin studies suggest that heritability for traumatic events ranges widely, from negligible for disasters and accidents and to more than 50% for being awarded a combat metal in Vietnam (which correlates highly with self-reported combat exposure) [9, 51, 52]. Individual personality and behavioral characteristics are moderately heritable and may explain in part reported gene–trauma correlations [52, 53]. These correlations may impact the ability to detect susceptibility loci specific to PTSD. Indeed, some of the candidates listed in Table 1 have been associated with particular personality or behavioral characteristics that may be tied to likelihood of experiencing a traumatic event [54, 55]. Consequently, PTSD researchers need to consider issues related to gene–trauma correlation carefully in their study design and statistical analysis. Modeling PTSD development following exposure to natural (eg, hurricanes) or human-made disasters (eg, large-scale terrorist attacks) is one viable strategy to attempt to control for gene–trauma correlations, as the occurrence of these events is largely independent of the individual victim’s behavior or personality. However, it should be noted that although the exposure to these forms of events may be largely random, the effects of these events are not distributed at random. For example, individuals who are at low socioeconomic status may be affected by a disaster to a higher degree than individuals who are socioeconomically privileged (eg, they may be less likely to be able to evacuate or afford rapid repairs to their home or belongings).
Case Definition
Published studies are characterized by marked heterogeneity in definition and assessment of “caseness.” Whereas some studies rely on self-report questionnaire assessment of trauma exposure and PTSD, others have conducted formal clinical interviews in person or by telephone. Additionally, studies vary markedly in the degree to which they report the influence of genotype on diagnostic status versus symptom severity or subsets of symptoms. Most published investigations made limited to no efforts to address duration of time since trauma; time lapsed since trauma exposure is an important consideration because it may be associated with remission or change in PTSD symptoms. Individuals selected into the case group therefore may be a mix of acute and chronic PTSD cases, and those selected into the control group a mix of individuals with no history of PTSD and individuals in remission at the time of PTSD assessment. Factors that influence the onset of the disorder may differ from those that influence the course, chronicity, or recovery from the disorder once it develops [56, 57]; thus, attention should be paid to distinguishing these phenotypes. Genetic influences may differ for acute versus chronic PTSD. Interestingly, all six studies that included incident (acute) cases within 6 months of trauma reported significant genetic effects. The probability of remission (or persistence) also may vary by trauma type [42]. Even studies designed to implement follow-ups at specific time points after trauma (as may be conducted following natural disaster) are challenged by the potential that some participants may have experienced trauma before the index trauma and may experience new traumas after the index trauma. Lifetime PTSD may be a better case definition under conditions in which information on type as well as duration of time between event and assessment is limited.
PTSD (acute or chronic) is a heterogeneous phenotype, with clusters of symptoms likely representing a defined reaction to trauma, modified by a unique set of genetic variants [58, 59]. Some argue that “endophenotypes,” measurable intermediate phenotypes that are generally closer to the action of the gene, may function as a better index of genetic liability for disease that overcomes the limitations in PTSD diagnosis [59]. A variety of endophenotypes have been proposed to index PTSD, ranging from behavioral symptoms to more biological measures, such as those obtained via neuroimaging [59–61]. Although a quantitative measure of PTSD may improve the power to detect genetic loci, the overall feasibility of the approach and generalizability of results remain unclear.
Post-Traumatic Stress Disorder Comorbidity
PTSD is highly comorbid with other psychiatric disorders, which may be explained by a common genetic diathesis [5]. A positive family history of psychiatric disorders is a consistent risk factor for development of PTSD [4, 62, 63]. Preexisting psychiatric disorders, particularly conduct disorder, major depression, and nicotine dependence, also increase PTSD risk [4, 64, 65]. At the same time, PTSD increases risk of first-onset major depression; alcohol, drug, and nicotine dependence; and smoking [66, 67]. The incidence of other psychiatric disorders is not higher in individuals who experience trauma but do not develop PTSD, suggesting that PTSD represents a generalized vulnerability to psychopathology following trauma [4]. Twin studies have demonstrated that genetic influences common to major depression, generalized anxiety disorder, panic disorder, or substance dependence account for up to 60% of the genetic variance in PTSD [64, 68, 69]. Variants implicated in PTSD also have been associated with other psychiatric conditions [70–72]. These findings raise the question of how to address other disorders in GWAS of PTSD.
An unscreened sample would be preferable to a screened sample because it would ensure that noncases and cases are identical for all characteristics other than affection status. Screening controls for other psychiatric conditions would reduce the genetic variance shared by cases and controls but at the expense of PTSD specificity. Screening both cases and controls would generate a very refined PTSD phenotype and limit the generalizability of results. Any of these approaches may be taken, but each will potentially inform different aspects of PTSD development. Indeed, the Psychiatric GWAS Consortium (PGC) recognizes that the comorbid nature of psychiatric conditions presents an opportunity rather than solely a challenge. Consequently, the PGC aims to coordinate and facilitate large-scale collaborative analyses using not only the traditional disorder categories but also nontraditional analyses that cut across diagnostic categories [48••]. Regardless of the approach taken to address PTSD comorbidities, investigators will need to carefully consider their approach when interpreting results, comparing across studies, and designing a suitable follow-up study for replication [11].
Gene–Environment Interactions
Trauma timing, type, and severity seem to modify genetic risk in PTSD. Individuals whose first trauma occurs in childhood as opposed to adolescence or adulthood are at particularly high risk of developing the disorder [62, 63, 73, 74]. Childhood abuse prospectively predicts trauma exposure in adolescence and adulthood; victims of childhood sexual abuse in particular are at increased risk of being raped later in life [73]. The conditional risk of developing PTSD is higher for interpersonal violence events such as rape than for other types of traumatic events (eg, sudden unexpected death) [75, 76]. A dose–response relation between severity of exposure and conditional risk of developing PTSD also has been well-documented [5].
A G × E interaction occurs when the effect of genotype on risk of a disorder differs by the presence or absence of an environmental pathogen, or vice versa. For example, degree of exposure to childhood abuse, but not adult trauma, modifies the association between polymorphisms in FKBP5 and PTSD symptoms in adults [38]. The presence of G × E interactions may mask our ability to detect susceptibility loci and might explain in part the inconsistent results observed across genetic association studies conducted to date. Thus far, 10 PTSD genetic association studies have specifically accounted for G × E interactions. Five have examined association with genetic variation in 5-HTTLPR [22, 23••, 24••, 27, 28].
Factors impacting power to detect main genetic effects will also apply to tests for G × E interactions. The prevalence and effect of the environmental pathogen, as well as the type and size of interaction effect will also determine study power. A rule of thumb is that a fourfold increment in sample size is required to test for a multiplicative interaction of two main effects [77]. Clearly, most of the existing studies in Table 2 were underpowered to detect G × E.
While aforementioned issues pertaining to study design impact any PTSD genetic study, attending to each is particularly important in the context of GWAS. For example, sample heterogeneity in combination with multiple testing penalties will severely reduce the power of a single GWAS. Given the small sample sizes of existing PTSD studies, pooling or meta-analyzing data may be the only means by which to attain the necessary sample sizes for a successful GWAS. However, the power of this collective approach will need to be weighted against the need to account for a second dimension of heterogeneity—that which occurs between studies. Interest is also growing in extending GWAS to discovery of gene–gene and G × E interactions. Statistical approaches to detect interactions, however, presently are less standardized relative to statistical tests for main genetic effects. When applying traditional tests for interactions, sample size requirements clearly exceed those for main effects analysis. Nevertheless, new methods for G × E interaction testing have been and will continue to be developed to boost statistical power for detection while maintaining low type 1 error [78, 79].
Conclusions
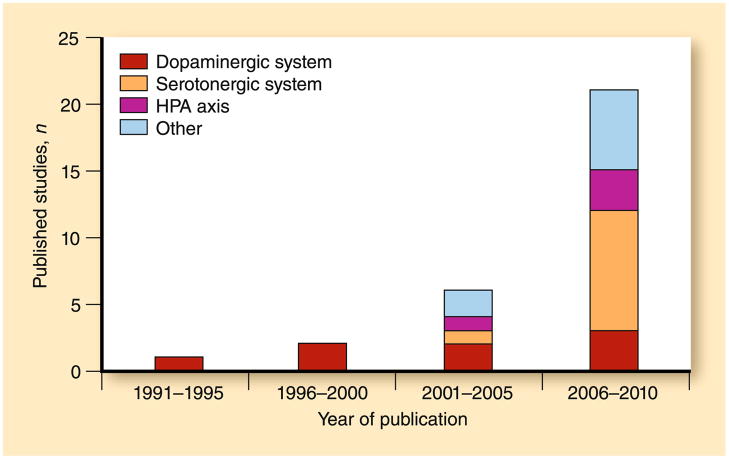
PTSD is a prevalent, disabling anxiety disorder that constitutes a major health care burden. Despite intensive research efforts during the past few decades, PTSD remains poorly understood in terms of etiology and shows modest response to current treatment interventions. Identifying the specific genes associated with PTSD risk should provide critical insight into the cause of this disorder that may lead to the development of novel diagnostic and therapeutic strategies. Although recent years have seen an exponential increase in the number of studies examining the influence of candidate genes on PTSD diagnosis and symptomatology (Fig. 1), most studies have been characterized by relatively low rates of PTSD, with apparent inconsistencies in gene associations linked to marked differences in methodology. Extant studies evidence many of the challenges common to trauma research, including control group trauma exposure, comorbidity in both case and control groups, influences on likelihood of exposure to trauma, time since index trauma, and number/type/timing of trauma(s) experienced. The combination of important methodologic differences and relatively few studies examining most of the variants make interpretation of findings across studies difficult; observed findings may indicate specificity of real genetic effects or may simply reflect design limitations.
Fig. 1.
Number of post-traumatic stress disorder candidate gene association studies published by year and neurobiological system. HPA hypothalamic-pituitary-adrenal
Progress in the development of powerful new techniques for locating and identifying human susceptibility genes and genetic variations contributing to common diseases has created new opportunities to advance our understanding of the etiology of mental disorders. These opportunities, however, have not been sufficiently recognized in the field of PTSD genetics. A completely untapped avenue for future research in measured genes and PTSD is GWAS. PTSD is uniquely fitting for this innovative approach, but its application will require a dramatic shift from our current hypothesis-driven science to a data-driven science. Large-scale collaborations will be crucial for success of the GWAS approach by increasing sample sizes, enabling replication of findings from individual studies, and optimizing methods for analysis. PTSD investigators therefore must recognize the need to cooperate and share data to maximize the knowledge obtained from GWAS. Upon effective implementation, GWAS will be a first step toward harnessing the accruing advancements in genetic research that will undoubtedly enhance our understanding of PTSD etiology and identify opportunities for treatment and prevention.
Acknowledgments
Dr. Cornelis is a recipient of a Canadian Institutes of Health Research Fellowship.
Dr. Nugent is supported by National Institute of Mental Health grant K01 MH087240.
Dr. Amstadter is supported by National Institutes of Health grants K12HD055885 and KL2RR029880.
Dr. Koenen is supported by National Institutes of Health grants K08MH070627-06 and R01MH078928-03 and a Junior Faculty Sabbatical from the Harvard University School of Public Health.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Marilyn C. Cornelis, Department of Nutrition, Harvard School of Public Health, Boston, MA, USA
Nicole R. Nugent, Bradley/Hasbro Children’s Research Center of Rhode Island Hospital, Department of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, RI, USA
Ananda B. Amstadter, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, USA
Karestan C. Koenen, Email: kkoenen@hsph.harvard.edu, Departments of Society, Human Development and Health and Epidemiology, Harvard School of Public Health, 677 Huntington Avenue, Kresge 613, Boston, MA 02115, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- 4.Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder, and other psychiatric disorders. Can J Psychiatry. 2002;47:923–929. doi: 10.1177/070674370204701003. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AL, Gilman SE, Breslau J, et al. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment seeking for post-traumatic stress disorder in the United States. Psychol Med. 2010;29:1–13. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slater E, Slater P. A heuristic theory of neurosis. In: Sheilds J, Gottesman I, editors. Man, Mind & Heredity: Selected Papers of Eliot Slater on Psychiatry and Genetics. Baltimore, MD: Johns Hopkins Press; 1944. pp. 216–227. [Google Scholar]
- 8.True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 9.Stein MB, Jang KL, Taylor S, et al. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 10•.Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: fear conditioning as a model for future research. Psychiatr Ann. 2009;39:358–367. doi: 10.3928/00485713-20090526-01. This article provides a comprehensive review of biological pathways and genes implicated in PTSD development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. J Trauma Stress. 2007;20:737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- 12.Comings DE, Comings BG, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- 13.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 14.Gelernter J, Southwick S, Goodson S, et al. No association between D2 dopamine receptor (DRD2) “A” system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biol Psychiatry. 1999;45:620–625. doi: 10.1016/s0006-3223(98)00087-0. [DOI] [PubMed] [Google Scholar]
- 15.Young RM, Lawford BR, Noble EP, et al. Harmful drinking in military veterans with post-traumatic stress disorder: association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002;37:451–456. doi: 10.1093/alcalc/37.5.451. [DOI] [PubMed] [Google Scholar]
- 16.Voisey J, Swagell CD, Hughes IP, et al. The DRD2 gene 957C > T polymorphism is associated with posttraumatic stress disorder in war veterans. Depress Anxiety. 2009;26:28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- 17.Segman RH, Cooper-Kazaz R, Macciardi F, et al. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- 18.Drury SS, Theall KP, Keats BJ, Scheeringa M. The role of the dopamine transporter (DAT) in the development of PTSD in preschool children. J Trauma Stress. 2009;22:534–539. doi: 10.1002/jts.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dragan WL, Oniszczenko W. The association between dopamine D4 receptor exon III polymorphism and intensity of PTSD symptoms among flood survivors. Anxiety Stress Coping. 2009;22:483–495. doi: 10.1080/10615800802419407. [DOI] [PubMed] [Google Scholar]
- 20.Wang E, Ding YC, Flodman P, et al. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. Am J Hum Genet. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 22.Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010 Apr 6; doi: 10.4088/JCP.08m04787blu. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 23••.Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. This was the first study reporting a G × E interaction for risk of PTSD. The inferred low expression ‘s’ variant of the 5-HTTLPR increased risk of post-hurricane PTSD only under conditions of high environmental stress exposure (high hurricane exposure and low social support) [DOI] [PubMed] [Google Scholar]
- 24••.Koenen KC, Aiello AE, Bakshis E, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–711. doi: 10.1093/aje/kwn397. Using the same study population of Kilpatrick et al. [23••], this more recent study reported a similar G × E interaction when a high-risk environment was defined by high county-level crime rate and county-level unemployment rate, re-emphasizing the potential modifying effect of physical or social stress on genetic susceptibility to PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Kwak S, Paik J, et al. Association between serotonin 2A receptor gene polymorphism and posttraumatic stress disorder. Psychiatry Investig. 2007;4:104–108. [Google Scholar]
- 26•.Thakur GA, Joober R, Brunet A. Development and persistence of posttraumatic stress disorder and the 5-HTTLPR polymorphism. J Trauma Stress. 2009;22:240–243. doi: 10.1002/jts.20405. Despite its small sample size, this study supports the notion that (genetic) factors influencing the onset of PTSD may differ from those that influence chronicity or recovery from the disorder once it develops. [DOI] [PubMed] [Google Scholar]
- 27.Xie P, Kranzler HR, Poling J, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabe HJ, Spitzer C, Schwahn C, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- 29.Mellman TA, Alim T, Brown DD, et al. Serotonin polymorphisms and posttraumatic stress disorder in a trauma exposed African American population. Depress Anxiety. 2009;26:993–997. doi: 10.1002/da.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayin A, Kucukiyildirim S, Akar T, et al. A prospective study of serotonin transporter gene promoter (5-HTT gene linked polymorphic region) and intron 2 (variable number of tandem repeats) polymorphisms as predictors of trauma response to mild physical injury. DNA Cell Biol. 2010;29:71–77. doi: 10.1089/dna.2009.0936. [DOI] [PubMed] [Google Scholar]
- 31.Heils A, Teufel A, Petri S, et al. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm Gen Sect. 1995;102:247–254. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann AW, Sedgley TL, Jackson RV, et al. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology. 2005;30:297–306. doi: 10.1016/j.psyneuen.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Lappalainen J, Kranzler HR, Malison R, et al. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Ozbay F, Lappalainen J, et al. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Kang R, Lim S, et al. No association between the brain-derived neurotrophic factor gene Val66Met polymorphism and post-traumatic stress disorder. Stress Health. 2006;22:115–119. [Google Scholar]
- 37.Mustapic M, Pivac N, Kozaric-Kovacic D, et al. Dopamine beta-hydroxylase (DBH) activity and −1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1087–1089. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- 38.Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Xie P, Kranzler HR, Poling J, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010 Apr 14; doi: 10.1038/npp.2010.37. (Epub ahead of print). Xie et al. and Binder et al. [38] present complementary studies supporting the role childhood trauma experiences plays in modifying genetic predisposition to PTSD in adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu AT, Ogdie MN, Jarvelin MR, et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1488–1494. doi: 10.1002/ajmg.b.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson EC, Agrawal A, Pergadia ML, et al. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol Psychiatry. 2009;14:234–235. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolassa IT, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Freeman T, Roca V, Guggenheim F, et al. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2005;17:541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- 44.Amstadter AB, Koenen KC, Ruggiero KJ, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schork NJ, Murray SS, Frazer KA, Topol EJ. Common vs. rare allele hypotheses for complex diseases. Curr Opin Genet Dev. 2009;19:212–219. doi: 10.1016/j.gde.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hindorff LA, Junkins HA, Mehta JP, Manolio T. [Accessed January 2010];A catalogue of published genome-wide association studies. Available at http://www.genome.gov/gwastudies.
- 47•.Psychiatric GWAS Consortium Coordinating Committee. Cichon S, Craddock N, et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry. 2009;166:540–556. doi: 10.1176/appi.ajp.2008.08091354. This is suggested reading for a more comprehensive review of GWAS in the context of psychiatric disorders. The PGC Coordinating Committee discusses the rationale for GWAS of psychiatric disorders, results to date, limitations, and plans for GWAS meta-analyses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Cross-Disorder Phenotype Group of the Psychiatric GWAS Consortium. Craddock N, Kendler K, et al. Dissecting the phenotype in genome-wide association studies of psychiatric illness. Br J Psychiatry. 2009;195:97–99. doi: 10.1192/bjp.bp.108.063156. The PGC presents innovative strategies for identifying novel loci for psychiatric conditions that build on the well-established comorbid nature of psychiatric disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radant A, Tsuang D, Peskind ER, et al. Biological markers and diagnostic accuracy in the genetics of posttraumatic stress disorder. Psychiatry Res. 2001;102:203–215. doi: 10.1016/s0165-1781(01)00252-9. [DOI] [PubMed] [Google Scholar]
- 50.Kendler KS, Eaves LJ. Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- 51.Lyons MJ, Goldberg J, Eisen SA, et al. Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet. 1993;48:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 52.Jang KL, Stein MB, Taylor S, et al. Exposure to traumatic events and experiences: aetiological relationships with personality function. Psychiatry Res. 2003;120:61–69. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 53.Van Os J, Jones PB. Early risk factors and adult person–environment relationships in affective disorder. Psychol Med. 1999;29:1055–1067. doi: 10.1017/s0033291799001026. [DOI] [PubMed] [Google Scholar]
- 54.Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol Psychiatry. 2008;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 55.van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J. Dopamine system genes associated with parenting in the context of daily hassles. Genes Brain Behav. 2008;7:403–410. doi: 10.1111/j.1601-183X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 56.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 57.Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149:671–675. doi: 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- 58.Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 60.Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 61.Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 63.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 64.Koenen KC, Hitsman B, Lyons MJ, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- 65.Koenen KC, Fu QJ, Lyons MJ, et al. Juvenile conduct disorder as a risk factor for trauma exposure and posttraumatic stress disorder. J Trauma Stress. 2005;18:23–32. doi: 10.1002/jts.20010. [DOI] [PubMed] [Google Scholar]
- 66.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 67.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 68.Chantarujikapong SI, Scherrer JF, Xian H, et al. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 69.Xian H, Chantarujikapong SI, Scherrer JF, et al. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 70.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 71.Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 72.Lawford BR, Young R, Noble EP, et al. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- 74.Breslau N, Davis GC, Andreski P, et al. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 75.Breslau N, Chilcoat HD, Kessler RC, et al. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med. 1999;29:813–821. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- 76.Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 77.Smith PG, Day NE. The design of case-control studies: the influence of confounding and interaction effects. Int J Epidemiol. 1984;13:356–365. doi: 10.1093/ije/13.3.356. [DOI] [PubMed] [Google Scholar]
- 78.Chatterjee N, Carroll RJ. Semiparametric maximum likelihood estimation exploiting gene-environment independence in case-control studies. Biometrika. 2005;92:399–418. [Google Scholar]
- 79.Kraft P, Yen YC, Stram DO, et al. Exploiting gene-environment interaction to detect genetic associations. Hum Hered. 2007;63:111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 80.Szantai E, Szmola R, Sasvari-Szekely M, et al. The polymorphic nature of the human dopamine D4 receptor gene: a comparative analysis of known variants and a novel 27 bp deletion in the promoter region. BMC Genet. 2005;6:39. doi: 10.1186/1471-2156-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajeevan H, Osier MV, Cheung KH, et al. ALFRED: the ALlele FREquency Database. Update Nucleic Acids Res. 2003;31:270–271. doi: 10.1093/nar/gkg043. [DOI] [PMC free article] [PubMed] [Google Scholar]