Abstract
The Cys-loop ligand-gated ion channel superfamily is a major group of neurotransmitter–activated receptors in the central and peripheral nervous system. The superfamily includes inhibitory receptors stimulated by GABA and glycine and excitatory receptors stimulated by acetylcholine and serotonin. General anesthetics at clinical concentrations inhibit the excitatory receptors and enhance the inhibitory receptors.
The location of general anesthetic binding sites on these receptors is being defined by photoactivable analogs of general anesthetics. The muscle type nicotinic acetylcholine receptor (nAChR) is the most extensively studied, and progress is now being made with γ-aminobutyric acid type A receptors (GABAARs). There are three categories of sites, all in the transmembrane domain: (1) Within a single subunit's four-helix bundle (intrasubunit site; halothane and etomidate on the δ–subunit of AChRs); (2) Between five subunits in the transmembrane conduction pore (channel lumen sites; etomidate and alcohols on nAChR), and (3) between two subunits (subunit interface sites; etomidate between the β1 and β2/3 subunits of the GABAAR). These binding sites function allosterically. Certain conformations of a receptor bind the anesthetic with greater affinity than others. Time resolved photolabeling of some sites occurs within milliseconds of channel opening on the nAChR but not before. In GABAARs, electrophysiological data fits an allosteric model in which etomidate binds to and stabilizes the open state, increasing both the fraction of open channels and their lifetime. As predicted by the model, etomidate's channel–stabilizing action is so strong that at higher concentrations it opens the channel in the absence of agonist.
Introduction
The Cys-loop ligand-gated ion channels have remained a major focus of research on the molecular mechanisms of general anesthesia, as evidence continues to accumulate implicating these targets, particularly the gamma-aminobutyric acid-A (GABAA) receptors in major anesthetic actions, the glycine receptor in immobility, and the nAChRs in memory, autonomous action and neuromuscular relaxation (1-7). Furthermore, our understanding of how and where general anesthetics modulate these targets has progressed dramatically. This review first presents current evidence on the location of the anesthetic binding sites on these channels and the mechanism by which binding to these sites alters their function. The best-characterized mechanisms involve selective allosteric interactions of general anesthetics with the open state of these channels, so the second part of the review will address the basis for this selectivity. The third part will describe the predictive power of a quantitative allosteric model of etomidate's actions on GABAARs.
Allosterism and binding sites
An elegant exposition on allosterism first appeared 45 years ago (8-10). Today it is an accepted concept with ramifications that extend to medicine (11), signal transduction (12, 13) and anesthetic action (14-18). The requirement for an allosteric anesthetic binding site is that the affinity of the site in certain conformations should be higher than that in other conformations. In this way the anesthetic stabilizes certain conformations over others. Thus, consider a protein that contains a single anesthetic binding site and that can exist in three different native conformations each of which has a different affinity for anesthetics (figure 1). The fraction of the protein in a given conformation depends on its free energy. When an anesthetic binds to a site on only one of the conformations, its free energy is decreased by the binding free energy, so that the fraction of the protein in that conformation increases. If such a conformation was the open channel state of a ligand-gated ion channel, the fraction of channels open would be increased and their lifetime would likely be longer and more current would pass. This is the case with GABAARs (15). A special case occurs if the stabilizing anesthetic binding site happens to be in the channel lumen. In nAChRs many agents cause open channel inhibition. The anesthetic has higher affinity for the open state and binds to it as soon as the agonist opens the channel, but once bound it sterically obstructs the flow of ions through the channel.
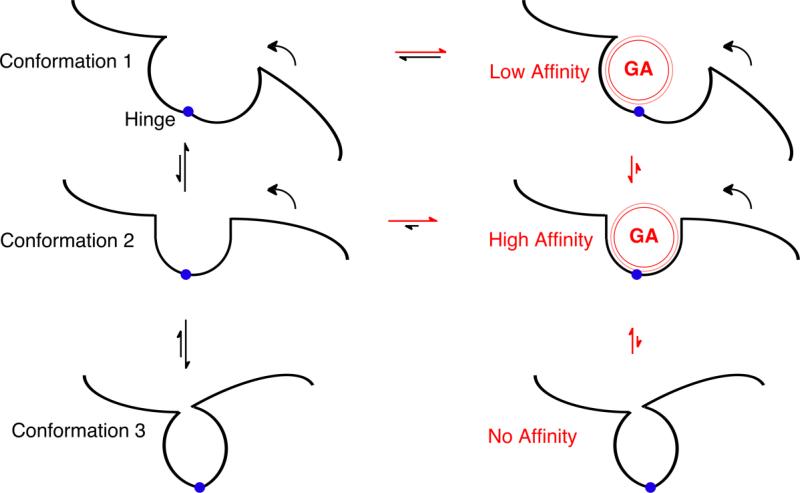
Figure 1. Principles of Allosterism.
The black lines depict part of a protein in a region where two domains are joined by a structural hinge (blue circle). In the left hand column, the protein can adopt three different conformations depending on movement of the right hand domain around the hinge (curved arrows). The relative size of the two black vertical straight arrows between each pair of conformations suggest the equilibrium distribution between the conformations; Conformations 1 and 3 are favored relative to Conformation 2. In the right hand column, general anesthetics (red sphere, GA) have the opportunity to bind to each of these conformations in the pocket between the domains. Because the intermolecular dispersion forces between the general anesthetic and the protein are very short range (depicted by the thin red outer line), strong, high affinity interactions only occur when the anesthetic fits snugly in the pocket (i.e. in Conformation 2). If the hinge closes too far (Conformation 3), steric hindrance prevents the anesthetic from binding. The horizontal red and black arrows depict how anesthetic binding perturbs the equilibrium between anesthetic free (left) and anesthetic bound (right) protein. In this example, Conformation 2 is sufficiently stabilized by the anesthetic's binding energy, that it is now the most stable relative to the Conformation 1 and 3.
Two main factors determine the affinity of an anesthetic for a binding pocket. First, the attractive energy falls off very rapidly with distance, so a snug fit is needed, which means that the pocket needs to be of comparable size to the anesthetic (conformation 2, figure 1). The maximum attraction occurs when two atoms are separated by only 12–20% (the distance increases with polarity) of the sum of their radii (for a review see (19)). Second, the pocket cannot be smaller than the anesthetic because the repulsive energy increases very rapidly as two atoms approach each other (they interact as hard spheres). Thus, an anesthetic can't squeeze down into a smaller pocket. Because of these constraints, a binding site that changes its geometry with the protein's conformation will only bind the anesthetic tightly in one conformation (figure 1).
The structure of Cys-loop ligand-gated ion channels
To resolve in atomic detail anesthetics bound to a protein requires a resolution of 2 Å. In addition, to understand allosterism, the same structure should be determined in several conformations. However, there are currently no structures of mammalian Cys-loop ligand-gated ion channels that achieve this resolution. The only structure of a vertebrate receptor, is the cryoelectron microscopy structure of the muscle subtype of the acetylcholine receptor from Torpedo electric tissue determined in the absence of agonist in the resting, or closed, state at 4 Å resolution (20). Such resolution is not sufficient to resolve the detailed structure of the amino acid side chains, let alone the presence of an anesthetic. The only other structures that include the transmembrane domain are those recently determined for two bacterial members of the same superfamily (21, 22). Although they lack the intracellular domain, they confirm the secondary structure of the transmembrane and extracellular domains seen in the cryoelectron microscopy structure above. One structure, ELIC, is thought to be in the closed state. The other protein, GLIC, is thought to be in the open state, but there is no independent confirmation of this, and no structure of a single receptor in more than one state has been determined. Nonetheless, the bacterial channels' structures are of sufficient resolution to allow bound anesthetics to be detected. Xenon has been imaged in the ELIC structure near the top of the pore. GLIC is sensitive to anesthetics (23) and bound anesthetic can be detected crystallographically (24).
The cryoelectron microscopy structure of the Torpedo nAChR is shown in figure 2A. It consists of three domains. First, an extracellular domain of some 200 residues that binds the agonist and that largely consists of a β-sheet structure. Second, a transmembrane domain containing four α-helices arranged as a four–helix bundle (M1 – M4; Figure 2B), in which M1 and M2 and M2 and M3 are separated by short loops. Third, there is a long intracellular loop, much of whose structure could not be defined, between M3 and M4. The secondary structure of the bacterial channels is similar, although the lengths of the transmembrane helices and loops differ, and the intercellular domain between M3 and M4 is short. Thus, it seems reasonable to assume that other members of the superfamily, particularly the GABAARs, have a similar secondary structure (see below). To facilitate the following discussion of photolabeling of the channel lumen, which is bounded by the M2 helices of five subunits, we use the prime numbering system for residues that aligns the M2 helix of all receptor subunits in such a way that the conserved leucine is always at M2–9'.
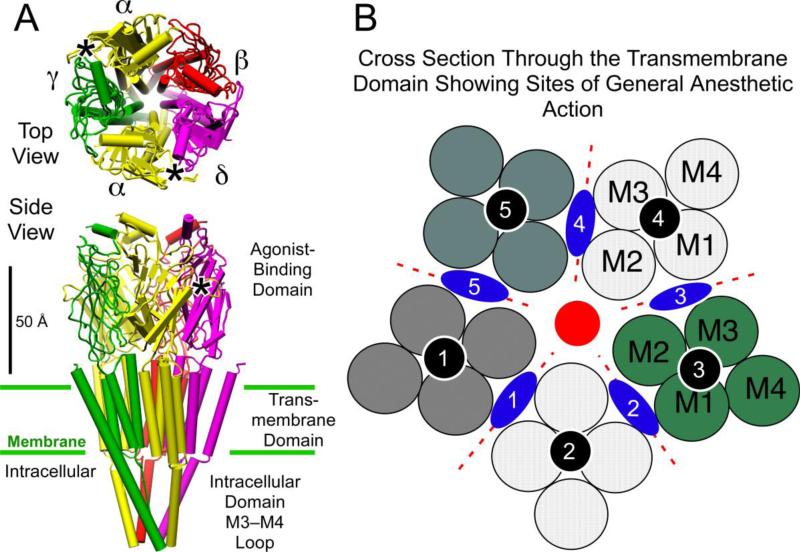
Figure 2.
General anesthetic binding sites on ligand-gated ion channels of the Cys-loop receptor superfamily. Panel A shows the structure of the nicotinic acetylcholine receptor (Unwin 2005), with its five subunits arranged centro-symmetrically around a central ion pore or channel, in both top view from the extracellular side and side view. The agonist site (*)in the extracellular agonist-binding domain is on the a-subunits in the interface with the g- and d-subunits. Panel B shows a schematic of a cross section through the transmembrane region. Each subunit, separated by dotted red lines, consists of four transmembrane helices shown as circles and numbered in the order they appear in the sequence. Three categories of anesthetic binding site (see text) are superimposed; intrasubunit sites (black circles); a channel lumen site (red circle), and subunit interface sites (blue lozenges). Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081) (74).
A high resolution structure of the GABAARs is not yet available. In such situations, structural biologists build a homology model. This is achieved by using a known published structure from the same superfamily, aligning its sequence with the new receptor, and substituting the residues of the new receptor onto the known structure. Finally, energy minimization is performed to sort out steric clashes and optimize positive interactions, such as H-bonds or charge-charge interactions. In a sense, the homology model is just a three-dimensional alignment algorithm. It should be regarded as providing no more than a hypothesis or cartoon for designing experiments. Indeed, the homology models from different research groups often differ in detail (25, 26). Generally, the secondary structure is conserved within the superfamily and the variation between models arises mainly from uncertainties in how to align the sequences. For example, the length of the M2 – M3 loop differs in nAChR and GABAARs introducing considerable uncertainty in the alignment of M3 (27). Cross-linking of introduced cysteines can reduce the uncertainties somewhat (28, 29).
Within the framework of these structures, photolabeling has defined three categories of general anesthetic sites in the transmembrane domain of ligand-gated ion channels. We will outline them here before considering the detailed results. The first is an intrasubunit site (Fig. 2B, black circles) located within the four-helix bundle of individual subunits; halothane and etomidate can bind here on the nAChR. The second is a channel lumen site (Fig. 2B, red circle) located between all five subunits, alcohols and etomidate can bind here on the nAChR. The third is a subunit interface site (Fig. 2B, blue lozenges) located between the subunits in the subunit–subunit interfaces; etomidate binds here on the GABAAR. Note the importance of the subunit composition. A homomeric receptor, such as the glycine or serotonin type 3A receptor might have five copies of one subunit interface site, whereas a receptor consisting of three different subunits, such as certain GABAARs, has more possibilities.
The art of photolabeling
Currently, the only unambiguous information on the location of general anesthetic sites on different conformations of a given mammalian receptor comes from photolabeling studies. The method involves developing and characterizing new general anesthetics that have the additional property of becoming chemically reactive when exposed to light of a certain wavelength. If this process occurs while the agent is bound to its target ion channel, it becomes possible to identify residues in contact with the agent in its binding pocket. The main limitations of this approach follow from the fact that the amino acid sequencing required is very difficult because of the hydrophobicity of the transmembrane domain where the anesthetic sites are located. To overcome this, large amounts of protein are required, and the most detailed information is available for the Torpedo acetylcholine receptor because it is readily available in adequate quantity. However, recent progress in the heterologous expression of human neuronal GABAARs largely resolves this problem (30).
Here we will review the location of general anesthetic sites established by photolabeling with halothane, two etomidate derivatives and 3-azioctanol (figure 3). Each of these has advantages and disadvantages that we will point out in the appropriate sections. Furthermore, to avoid discussing nonspecific binding, the emphasis is on sites where the degree of photoincorporation is modulated by changes in conformation between the equilibrium resting and desensitized states of the receptor. In a subsequent section we will consider how these sites change during gating.
Figure 3.
Formulae of photolabels referred to herein.
Location of general anesthetic sites on nicotinic acetylcholine receptors
Halothane was the earliest photoactivatible anesthetic (31, 32). It reacts covalently with tyrosines and tryptophans and can tell us about binding pockets that contain these residues. Unfortunately, some regions of interest, the channel lumen, for example, lack such residues. However, halothane does not react with all the tyrosines and tryptophans in the nAChR, so those it does react with likely represent true binding sites. In the transmembrane domain, halothane photoincorporated into δY228 at higher levels in the desensitized state than in the resting state. It also photolabeled the equivalent residue on the α–subunits, αY213, but at a level too low to assess state–dependence (see figure 4A). These residues are at the extracellular end of M1, two helical turns above the conserved proline. On the nAChR structure, δY228 faces into an intrasubunit site within the four-helix bundle. In the α-subunit, there is one more residue between the tyrosine and the conserved proline, so that the two copies of αY213 face into the α – β and α – γ intersubunit interfaces, which may explain why they are labeled 10-fold less than δY228.
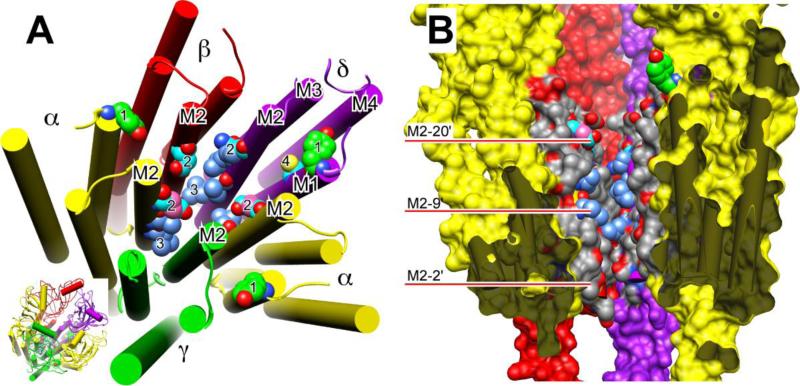
Figure 4.
Residues photolabeled on nicotinic receptors by three classes of general anesthetics. Panel A shows a slightly tilted top view of just the transmembrane domain of the nAChR of the nicotinic acetylcholine receptor (nAChR) from Torpedo using the cryoelectron microscopy structure (20). The agonist-binding domain has been omitted for clarity, but is shown in the inset at bottom right, where the orientation is the same as in the larger diagram. The subunits are color-coded and labeled as in Fig. 2. The helices are shown as rods and the atoms of photolabeled residues, including the backbone atoms, are shown in space-filled mode. All other residues are omitted for clarity. Oxygen (red) and nitrogen (blue) atoms are colored conventionally. The carbon atoms are color coded to denote which anesthetics photolabeled the residue: green, halothane; cyan, azietomidate; cornflower blue, TDBzl-etomidate; salmon, azioctanol. In some cases, more than one agent photolabels the same residue and individual carbons are given different colors accordingly. Number code: 1. αY213 & δY228 photolabeled on M1 by halothane; 2. αE262, βD268 & δQ276 (the M2-20' residues) photolabeled by azietomidate and azioctanol; 3. αL251, δL265 (the M2-9' residues) photolabeled by TDBzl-etomidate; 4. δC236 on M1 Photolabeled by azietomidate. Panel B shows a cross-section through the transmembrane domain of the same nAChR structure. The γ-subunit has been removed to facilitate a view of the ion pore. The viewer is situated at the γ-subunit and the two α-subunits are closest to the viewer. The subunits have the same color code as before but are presented surfaced. The dark grey regions denote where the surface of the α-subunits have been cut through and serve to emphasize the free space that exists within the 4-helix bundle of subunits. The central ion channel is open to view. The M2 helices are colored conventionally with grey for carbon, red for oxygen and blue for nitrogen, except that in photolabeled residues some of the carbons have the same color code as in panel A. The red bars point to residues on M2 using the prime numbering system, where 1' is the residue following the last charged residue before the M2 helix and M2-9' is always the conserved leucine.
Halothane's photochemistry is not ideal because it is activated by wavelengths of light that also activate bonds in the protein. Diazirines, like azioctanol (33), azietomidate (34) and aziisoflurane (35) (figure 3), do not have this disadvantage. In the transmembrane domain 3-azioctanol, an open-state inhibitor of the ion channel (33), photolabels the α-subunit at αE262 M2–20'. It is near the top of M2 and points into the channel lumen (36) (figure 4A & B). It is located above a largely hydrophobic region of the channel lumen that stretches down to the conserved ™L251 (M2–9'), so it is tempting to assume that the hydrophobic tail of the alcohol projects in the intracellular direction down the channel. However, this hypothesis remains untestable because hydrophobic residues rarely react with aliphatic diazirines.
In anesthetics that have more complex chemical structures, it is possible to insert either an aliphatic or an aromatic diazirine at a particular position. Because each type of diazirine reacts with a different spectrum of residues, such a pair of general anesthetics would get around the issue of each photolabel exhibiting certain “blind spots”. Such a pair of derivatives has been added to the etomidate structure (Fig. 3) to provide a more complete picture of sites on the nAChR. The first of these was azietomidate, an aliphatic diazirine. Like 3-azioctanol, it photolabels αE262 on M2–20', but it also photolabels the equivalent M2–20' residues (βD268 & δQ276) (the γ-subunit was not examined), confirming that the upper end of the channel lies within an anesthetic binding site. On the β– and δ–subunits, there are reactive residues at M2–24' one helical turn above their respective photolabeled M2–20' residues. These residues, βE272 & δE280, define the limits of the binding site because they are not photolabeled, suggesting that the site extends in an intracellular direction. This hypothesis is confirmed by a second etomidate derivative bearing an aromatic diazirine in place of the aliphatic diazirine. TDBzl-etomidate, was designed to react with the hydrophobic residues more intracellular to the M2–20' site photolabeled by azietomidate and azioctanol. Indeed, it photolabeled the channel lumen-facing residues in the α– and δ–subunits on M2 as far below M2–20' as M2–9', a distance along the channel of some 16.5 Å.
A number of other sites on the nAChR have been photolabeled by these agents, illustrating that a large receptor can have multiple anesthetic binding sites, although not all produce functional effects. The first of these sites is the agonist-binding pocket on the α-subunit, nearly 50 Å from the transmembrane domain (36-38). It is photolabeled in the absence but not in the presence of agonist. This competitive behavior is consistent with the observation that at very high concentrations anesthetics decrease [3H]acetylcholine binding (39), even though at low concentrations they enhance [3H]acetylcholine binding by stabilizing the desensitized over the resting state.
Three further regions have been consistently photolabeled, usually at much lower levels of photoincorporation than the residues in the transmembrane domain, but no function has been ascribed to them and they will not be discussed in detail here. The first is just before the intracellular end of M4 (40), close to the intracellular fenestrations described by Unwin (20). The second is in the lipid-protein interface. This is commonly considered to be nonspecific photolabeling because of the high concentration of photolabel that must be present in the lipid bilayer. The third is the hint of a subunit interface site between αM2–10' and the δ–subunit (41).
Location of general anesthetic sites on GABAARs
The specific activity of GABAARs in brain is some 10,000-fold lower than that of nAChR in Torpedo electric tissue (25). To improve the chances of success it was necessary to develop a highly selective and potent general anesthetic photolabel. Steroids, propofol and etomidate fall into this category. While photolabels are being developed for all three, etomidate was an attractive initial candidate because of it is less hydrophobic than steroids, while possessing stereoselectivity and a structure amenable to chemical modification at multiple positions (figure 3). The synthesis of azietomidate proved the value of this strategy. In spite of the aliphatic diazirine group, azietomidate retains the pharmacological properties of etomidate. Both are equipotent as general anesthetics and as modulators of the GABAAR, at which they enhance GABA-induced currents and activate the channels in the absence of agonist (34). The R-enantiomer of both is more potent than the S-enantiomer (the clinical drug is R-etomidate). Furthermore, in knock–in mice rendered less sensitive to etomidate by a β3–N265M point mutation in their GABAARs, both R-etomidate and R-azietomidate are about 10-fold less potent than in wild type mice (42).
In a heterogeneous mixture of GABAARs purified from cow brain, R-azietomidate was found to photolabel two residues in a GABA-sensitive allosteric manner (25). The first, lay within the βM3 transmembrane helix at βMet-286 (the exact β–subtype could not be determined). This region was a known anesthetic determinant (43-45). The second residue was novel. There was no hint of its importance from any previous site–directed mutagenesis studies. Azietomidate photolabeled α1M236 (and/or the homologous methionines in α2, 3 & 5), which is located on the αM1 transmembrane helix, three residues after the conserved proline. The ability to find a novel site illustrates the power of photolabeling; it provides binding site information at the level of the primary structure, independent of any preconceived hypothesis.
Using the homology model discussed earlier in this review (25), the two methionine residues (αM236 & βM286) on separate subunits photolabeled by azietomidate are predicted to face each other across the interface between the α- and β-subunits in the transmembrane domain. Support for this arrangement comes from subsequent cysteine cross-linking studies (29). Thus, the etomidate site is not within a single subunit's intrahelical bundle, but instead is in the interface between two subunits (figure 5). The αM1 M236 is predicted to be more intracellular than βM3 M286, and their α-carbons are separated by 13 Å. This suggests that the etomidate site is between them, which is consistent with the lack of photolabeling at βM3 M283, which is located one helical turn more extracellular to the photolabeled M286. The arrangement of the subunits in α1β3γ2L GABAARs is, reading clockwise, α1β3α1β3γ2L, so there are two α – β interfaces and consequently two etomidate binding sites. This is consistent with the prediction of the allosteric model discussed below (15).
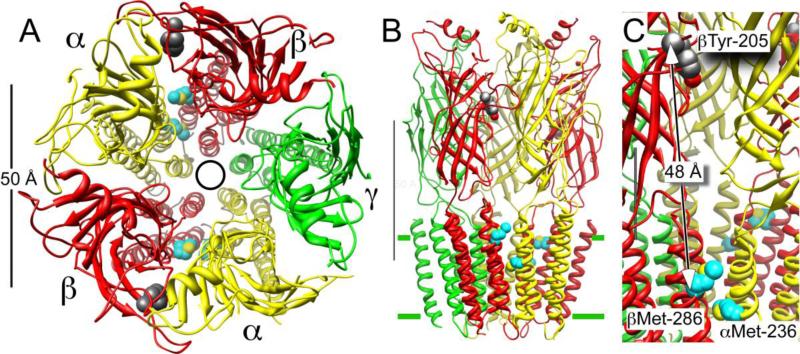
Figure 5.
Azietomidate photolabels the GABAAR in the transmembrane domain between the a- and b-subunits. Panel A shows a top view from the extracellular side of the α1β2γ2L GABAAR homology model of Li et al (25). The five subunits, which are consistently color coded in all panels, are arranged centrosymmetrically around the pore, denoted by a circle. Panel B shows the same receptor in side view; the M3–M4 intracellular loop is omitted in this model. The scale bar is 50 Å in both pannels. Panel C shows a detail of Panel B. The partly obscured scale bar is 20 Å. Only the secondary structure is shown except for two sets of residues. The residues with carbons shown in cyan are those photolabeled by azietomidate, αMet-236 and βMet-286, and that with dark grey carbons is βTyr-205, which is a residue in the GABA–binding pocket, nearly 50 Å away.
Thus, azietomidate photolabeling has provided the first evidence that placed an anesthetic binding site in a subunit interface on a ligand-gated ion channel. While a novel concept for anesthetic mechanisms, agonist sites have long been thought to lie partly in such interfaces (46). The agonist site in the extracellular domain, like the etomidate site in the transmembrane domain, lies in the α–β interface. The two sites are separated by ~50 Å (figure 5C). If the agonist induces gating by perturbing the α–β interface in the transmembrane domain, it is evident that an etomidate binding site in the same interface and adjacent to the ion pore, might allosterically modulate agonist gating. We will return to this theme in a later section of this review.
Other classes of general anesthetic have variable effects on photoincorporation of [3H]azietomidate into GABAARs. These agents may have different binding sites (47), so that further photolabeling work with other anesthetic analogs is eagerly awaited.
Evidence for conformationally sensitive general anesthetic binding
How can we establish that the affinity of general anesthetic modulatory sites vary with the protein's conformational state (figure 1)? General anesthetics have such low affinities that, with rare exceptions (14, 48), nonspecific binding prevents binding to receptors from being detected. Furthermore, kinetic measurements suggest the highest affinity is often for the open state of a receptor, a transient state that is present for far too short a time for a binding measurement to be accomplished. Faced with this issue, time resolved photolabeling has been adopted as a strategy for seeking sites on transient states of receptors (49). To date this demanding strategy has only been applied to the abundant Torpedo nAChR. When rapidly exposed to an agonist, the resting state of this nAChR is converted to the open state in less than a millisecond (50, 51). The population of open receptors is all converted to the fast desensitized state a second later and to the slow desensitized state within minutes. Thus, by using time resolved photolabeling in the absence of agonist, or 1 – 50 ms, 1 s and minutes after rapid mixing with agonist, it is possible to determine the level of photoincorporation of an anesthetic as a function of the receptor's conformation (resting, open, fast desensitized and slow desensitized states, respectively) (52).
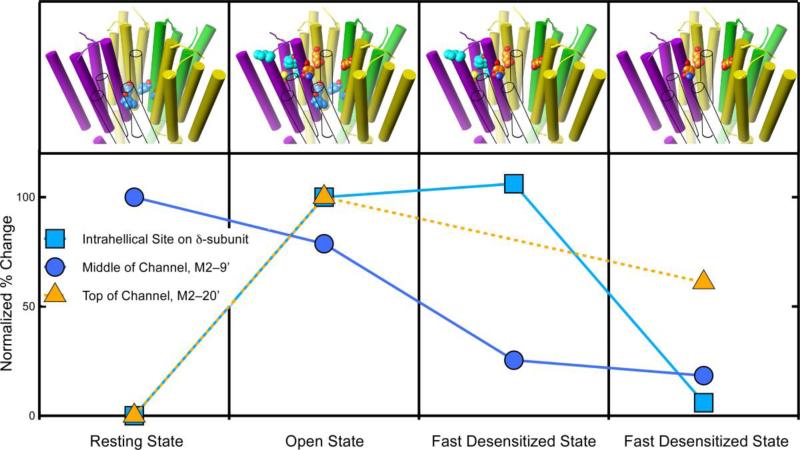
The time–dependence of photoincorporation into three different anesthetic sites on the nAChR has been characterized to date (figure 6). All three exhibit different behaviors. Of the general anesthetics, the best characterized is azietomidate (40), which photolabels the M2–20' residues. These channel–lumen residues are not photolabeled in the resting state, but as soon as the channel opens photoincorporation into these residues is robust (Fig. 6, triangles). No data is available for the fast-desensitized state, but photoincorporation falls during slow desensitization. The most complete set of data is for the hydrophobic aromatic diazirine, 125I-TID (3-(trifluoromethyl)-3-(m-iodophenyl) diazirine (figure 3) (52, 53). It photolabels the nAChR in the resting state in the center of the channel at M2–9', the second of the three sites. During channel opening, photoincorporation at M2–9' falls but by surprisingly little. It is not until the receptor enters the fast desensitized state that photoincorporation into the channel dramatically declines (figure 6, circles).
Figure 6.
The degree of photolabeling of three sites on the nAChR varies with the receptor's conformation, supporting allosteric action. The graph shows the relative level of photoincorporation for three different sites on the nicotinic acetylcholine receptor. The photoincorporation level is normalized to that first observed (either the resting or the open state). The upper panel depicts the transmembrane domain of the nAChR and the photolabeled residues in each state. The subunit colors are yellow for α, green for γ, and purple for δ. The β-subunit is shown in outline only to allow the channel residues to be seen. The carbon atoms are colored with the same color as the symbols on the graph. At two sites photolabeling is negligible in the resting state but increases dramatically when the channel opens. One of these sites (orange triangles) is in the upper part of the channel (M2–20') and the other (cyan squares) is in the intrahelical bundle of the β-subunit. The behavior of these sites diverges during desensitization. The channel site changes modestly, whereas the intrahelical site remains unchanged during fast desensitization and then decreases dramatically upon slow desensitization. The third site (blue circles) is also in the channel but at the conserved M2–9' leucines. It behaves differently from the site in the upper part of the channel. It is photolabeled in the resting state and in the open state, but photolabeling decreases dramatically during fast desensitization and remains unchanged during slow desensitization. Data for azietomidate (triangles) is from (40) and for TID (circles and squares) is from (52, 53).
The third site is the transmembrane domain intrahelical site in the four–helix bundle on the δ–subunit. It is photolabeled by halothane and by azietomidate, but the best time resolved photolabeling is with TID. This site is not photolabeled in the resting state, but within 1 ms of adding acetylcholine it is photolabeled (figure 6, squares). Furthermore, photoincorporation remains unchanged when the nAChR enters the fast-desensitized state, but falls dramatically upon slow desensitization. It is interesting to note that halothane photolabels this site in the resting state, suggesting that this much smaller molecule can fit into the pocket in a conformation that cannot accommodate the larger probes, azietomidate and TID.
Do current concepts of gating and desensitization help us understand why photoincorporation into each of the three sites above has a different dependence on the nAChR conformation (figure 6)? The two-gate hypothesis of Auerbach and Akk (54), which postulates distinct activation and desensitization gating structures in the nAChR channel (54), offers an explanation of why sites at two different levels within the channel lumen, at M2–9' and at M2–20', behave differently as the channel's conformation changes (figure 6). The hypothesis states that in the resting state, the desensitization gate is open and the activation gate is closed. Agonist opens the activation gate and conduction occurs. During fast desensitization, the desensitization gate closes while the activation gate remains open. Thus, the structural changes associated with the activation gate correlate with photoincorporation at M2–20' residues, whereas those associated with the desensitization gate correlate with photoincorporation at M2–9'. It is as though the channel's long sausage–shaped cavity is squeezed at different points during the conformation changes associated with opening and fast desensitization (and presumably during slow desensitization when both gates might be closed, although the model did not address this issue).
The uncoupled model of desensitization postulates that each subunit has only one desensitized structure, and that the difference between fast and slow desensitized states is in the number of subunits in their desensitized conformation (55). Fast desensitization is associated with the γ–α subunit pair desensitizing, whereas slow desensitization is associated the δ–α subunit pair. The M2–9' site will be affected by the motion in any subunit, so that desensitization of the γ–α subunit pair and then the δ–α subunit pair would be sufficient to lower photoincorporation during both fast and slow desensitization, as is observed (Fig. 6). The uncoupled model is also consistent with the behavior of the anesthetic site in the intrahelical bundle of the δ–subunit, because it remains unchanged during fast desensitization and only changes during slow desensitization. The structural basis for the δ–subunit being the only one to exhibit intrahelical binding may be that the δ–subunit is the only one that has a proline near the middle of M4. This causes the upper part of the helix to bend out into the lipid-protein interface, creating more space within the intrasubunit four-helix bundle.
Thus, a structural basis is beginning to emerge for the way in which general anesthetics interact with their binding sites as ligand-gated ion channels pass through their various functional states. That the allosteric principle applies means that it is now possible to conceive of designing agents that are not just selective for a given receptor but also for a given conformation of that receptor. Such specific design requires more detailed structural insights than are currently available, but these are likely to come. Meanwhile, functional studies are also often consistent with allosteric action. The combination of functional and structural studies will be necessary to obtain a complete description of general anesthetic action.
Allosteric co-agonism: A formal mechanism for general anesthetic actions in GABAA receptors
Formal functional paradigms for general anesthetic modulation of GABAA receptors have not been widely accepted or applied in molecular studies. The best-established paradigm is that for etomidate effects at α1β2γ2L GABAA receptors (15), essentially a two-state Monod-Wyman-Changeux (MWC) allosteric co-agonist model (figure 7). The underlying assumptions of MWC allosteric models, outlined in 1965, include the existence of interchangeable conformations both in the presence and absence of ligands, and maintenance of symmetry during structural transitions (8). The simplest equilibrium MWC models have two interchangeable states: resting and active. Agonists in this formalism are any ligands (orthosteric or allosteric) that bind with higher affinity to active states than to inactive states. Thus addition of agonist preferentially stabilizes open-channel conductive (active) states of ion channels. The concept of inverse agonism is also implicit in these mechanisms; agonist site ligands that bind better to inactive rather than active states are inverse agonists, and act as competitive antagonists in the presence of full agonists.
Figure 7.
The scheme depicts equilibrium two-state allosteric co-agonism for GABA and etomidate actions on GABAA receptors, as described by Rüsch et al (15). There are two equivalent GABA sites and two equivalent etomidate sites. Only doubly-bound states are shown, both for simplicity and because they are the most highly populated states when ligands are present. GABA binding transitions are blue, etomidate binding transitions are red, and gating (opening and closing) transitions are black. The L0 parameter describes the basal equilibrium between closed (R) and open (O) states. KG is the dissociation constant for GABA interactions with R-state receptors and KG* is the dissociation constant for GABA interactions with O-state receptors. The GABA efficacy factor, c, is defined as KG*/KG. KE is the dissociation constant for etomidate interactions with R-state receptors and KE* is the dissociation constant for etomidate interactions with O-state receptors. The anesthetic efficacy factor, d, is defined as KE*/KE.
The co-agonist model for GABA and etomidate emerged in part from research on GABAA receptor mutations and other anesthetics. A similar model has been previously considered for barbiturate actions on GABAA receptors (56). In 1999, Chang & Weiss demonstrated that a two-state equilibrium MWC allosteric model describes GABA-dependent activation of GABAA receptors harboring mutations at the highly conserved M2 - 9’ leucines (57). When L9’ residues are mutated to serines or threonines, enhancement of both basal receptor-channel gating activity and apparent GABA sensitivity is produced, effects that are similar to those observed in the presence of general anesthetics. In addition, studies showed that potentiation of GABAA receptor responses to partial agonists by propofol (58) and barbiturates (59) is due to increased agonist efficacy rather than enhanced agonist binding at the orthosteric (GABA) binding site. For etomidate, other critical observations supported a MWC co-agonist mechanism. Firstly, etomidate displays significant stereospecific actions as an anesthetic, paralleling its stereospecificity at GABAA receptors, and providing convincing evidence for a protein site or sites of action (34, 60, 61). Like a number of other general anesthetics, etomidate potentiates GABA-dependent receptor activation, resulting in enhanced apparent GABA sensitivity. Furthermore, high concentrations of etomidate directly activate GABAA receptors in the absence of orthosteric agonists, an action termed direct activation or GABA-mimetic activity (61-63). Notably, the ten-fold to twenty-fold R(+)/S(-) stereoselectivity observed for GABA potentiation of GABAA receptors was the same magnitude as that seen for direct activation by etomidate (34, 60). In addition, subunit substitutions and mutations that reduced GABAA receptor sensitivity to GABA potentiation also reduced direct activation by etomidate (63, 64). These observations suggest that a single class of allosteric etomidate sites may underlie both potentiation of GABA responses and direct receptor activation.
An alternative hypothesis is that the GABA-potentiating and direct activating effects of general anesthetics are respectively mediated by distinct high- and low-affinity sites on GABAA receptors (65). Support for the existence of high-affinity anesthetic sites depends on the methods used to quantify GABA potentiation. In particular, the presence of high affinity sites frequently has been inferred from measurements of anesthetic potentiation at a single GABA concentration, usually EC5 or EC10. The flaw in this approach is that the half-effect anesthetic enhancing concentration varies depending on the amount of GABA activation being enhanced. Thus if one assumes that saturating GABA activates nearly all receptors (i.e. intrinsic efficacy is about 1.0) then an anesthetic concentration that enhances activation ten-fold will produce a maximum effect in the presence of GABA at EC10. In other words, the observable amount of enhancement has a “ceiling” around ten-fold. Under these conditions, the half-effect anesthetic concentration will produce a 4.5-fold enhancement (halfway between 1-fold and 10-fold). However, in a similar experiment performed using GABA at EC25, the anesthetic concentration that quadruples activation will produce maximal activation and the half effect anesthetic concentration will enhance activation only 1.5-fold. In contrast, assessing allosteric enhancement from shifts in agonist response curves eliminates the “ceiling effect.” When etomidate-induced GABA potentiation was assessed using leftward shifts of concentration-responses curves, no evidence for high affinity sites was found (15), suggesting both direct activation and agonist enhancement might be mediated by anesthetic binding to a single class of low affinity sites.
The MWC co-agonist model (figure 7) explains anesthetic actions by postulating a single class of allosteric anesthetic sites that act as co-agonist sites linked to the receptor gating equilibrium. MWC analysis of wild-type α1β2γ2L GABAA receptor function quantitatively accounts for direct activation, enhancement of currents elicited with GABA EC5, and etomidate-dependent shifts in GABA EC50. It also quantitatively accounts for effects of combining etomidate with a partial agonist (15). Moreover, models postulating two equivalent etomidate sites fit the data significantly better than models assuming different numbers of etomidate sites, a conclusion that accurately predicted subsequent azi-etomidate photolabeling results (25).
Allosteric co-agonism as a framework for structure-function analysis
The MWC allosteric co-agonist model has proven to be a powerful tool for analysis of structure-function studies of etomidate interactions with GABAA receptors. An idea that emerges directly from MWC allosteric principles is that receptors (including certain wild-type GABAA subunit combinations, and also mutants) can spontaneously activate in the absence of agonists. Furthermore, as Chang & Weiss observed, more spontaneous activity is associated with a higher apparent affinity for agonists. Thus, the co-agonist mechanism correctly predicts that spontaneously active GABAA receptors with an L9'S or L9'T gating mutation are exquisitely sensitive to direct activation by etomidate (15). Indeed, assessment of spontaneous activity in GABAA receptor mutants is a critical factor in MWC allosteric models. Wild-type α1β2γ2L receptors are estimated to have a very low spontaneous activity (P0 < 0.0001), and experimentally it is difficult to measure activity this low. However, when the impact of a mutation on spontaneous activity is small, it can be estimated using mutant-cycle analysis, by adding a second mutant subunit containing an L9'S or L9'T (66).
To date, the MWC co-agonist framework has been used to analyze the effects of GABAA receptor mutations at three amino acids that are hypothesized to interact with etomidate (66, 67). Two of these residues, αM236 and βM286, were identified by azi-etomidate photolabeling of purified detergent-solubilized bovine brain receptors (25) (see Location of general anesthetic sites on GABAA receptors, above). Stewart et al (67) hypothesized that bulky hydrophobic tryptophan sidechains at these residues would occupy the space where etomidate binds. The β2M286W mutation in a α1β2γ2 background increases receptor sensitivity to GABA and produces spontaneous gating in the absence of GABA, which is detectable using picrotoxin (Table 1). These functional characteristics of the mutant channel mimic the impact of etomidate binding (67). In addition, in the presence of etomidate β2M286W channels display neither GABA modulation nor direct activation. In the absence of etomidate-dependent effects, model fitting to these data fails to indicate whether etomidate binding or efficacy is reduced by the mutation (Table 2). Similar to β2M286W, the α1M236W mutation produces receptors with increased GABA sensitivity relative to wild-type and spontaneous activation, mimicking the effects of etomidate binding to wild-type receptors. Notably, receptors harboring α1M236W mutations are potently and efficaciously activated by etomidate, while modulation of GABA responses by etomidate is much weaker than that observed in wild-type receptors. MWC modeling proves valuable in making sense of this pattern of results. The small leftward shift of GABA responses results in a low etomidate efficacy factor in the fitted model, but this low efficacy is sufficient to fully activate channels that, based on their spontaneous activity, have a strong propensity to open.
Table 1.
Functional Characteristics of Wild-Type and Mutant GABAA Receptors Activated with GABA +/- Etomidate
| Receptor | Spontaneous Activation* | GABA EC50 (μM) | GABA Efficacy† | Etomidate EC50 (μM) | Etomidate Efficacy‡ | Left-Shift Ratio (CNTL/ETO) |
|---|---|---|---|---|---|---|
| α1β2γ2L | <0.001 | 26 | 0.9 | 36 | 0.4 | 20 |
| α1M236Wβ2γ2L | 0.16 | 2.0 | 0.99 | 12 | 0.97 | 1.7 |
| α1β2M286Wγ2L | 0.04 | 6.6 | 1.0 | NA | <0.001 | 1.1 |
| α1β2N265Sγ2L | <0.001 | 27 | 0.93 | 78 | 0.03 | 2.3 |
| α1β2N265Mγ2L | <0.001 | 32 | 0.84 | NA | <0.001 | 0.95 |
Spontaneous activation is estimated using picrotoxin to block constitutively active receptors in the absence of agonists. The picrotoxin-sensitive current was normalized to the maximum GABA current.
GABA efficacy is estimated using positive allosteric modulators (etomidate or alphaxalone) to enhance the maximum current elicited by high GABA concentrations. We assume that the combination of high GABA plus allosteric enhancer activates all receptors.
Etomidate efficacy is the maximum current elicited by etomidate, normalized to the maximum current elicited with GABA.
Table 2.
MWC Coagonist Model Parameters for Wild-Type and Mutant GABAA Receptors Activated with GABA +/- Etomidate
| Receptor | L0 | KG(μM) | c | KE (μM) | d |
|---|---|---|---|---|---|
| α1β2γ2L | 25,000 | 70 | 0.0019 | 40 | 0.0077 |
| α1M236Wβ2γ2L | 6.2 | 51 | 0.021 | 24 | 0.18 |
| α1β2M286Wγ2L | 31 | 32 | 0.029 | NA | NA |
| α1β2N265Sγ2L | 25,000 | 68 | 0.0018 | 88 | 0.038 |
| α1β2N265Mγ2L | 50,000 | 59 | 0.0019 | NA | NA |
Another GABAA receptor residue of great interest is β2N265 (M2 - 15′), which faces away from the transmembrane pore in homology models. Mutations at this site on the pore-forming M2 helix were first reported to dramatically reduce etomidate and loreclezole sensitivity (63). Furthermore, mice with knock-in mutations β2N265S or β3N265M have normal baseline phenotypes, but markedly alter behavioral sensitivity to various etomidate, propofol, and barbiturate actions (1, 68). Compared with wild-type receptors in oocytes, α1β2N265Sγ2L receptors display no change in basal gating, GABA EC50 or GABA efficacy (66). The β2N265M mutation slightly increases GABA EC50 and reduces GABA efficacy. Combining this mutation with the α1L264T mutation that produces spontaneous activation reveals that the β2N265M mutation reduces spontaneous gating about two-fold, which accounts for both its altered GABA EC50 and efficacy. Both α1N265 mutations also confer etomidate insensitivity. β2N265S reduces both GABA modulation and direct activation by etomidate, while β2N265M eliminates these etomidate effects. MWC co-agonist model fitting to the data set for β2N265S suggests that etomidate efficacy is reduced far more than etomidate affinity to resting receptors. Nonetheless, efficacy in the context of MWC models represents the relative affinity for active versus inactive receptors. Thus, βN265 mutations appear to have no effect on etomidate binding to resting (closed) receptors, but βN265 may contact etomidate in open receptors, where M and S mutations weaken binding.
The framework of MWC allosteric co-agonism has also proven valuable in designing protection studies based on cysteine substitutions and modification with small sulfhydryl-modifying probes. Prior work, notably in the labs of Akabas (43, 69) and Czajkowski (70), have shown that the rate of sulfhydryl modification is often dependent on whether receptors are closed versus active. Therefore, to properly interpret protection studies when it is hypothesized that ligands sterically interfere with sulfhydryl modification, one must identify control and protection conditions where the distribution of resting versus activated receptors are similar. Furthermore, protection can best be demonstrated when ligand occupancy is high, and when that ligand has agonist activity, as with anesthetics at GABAA receptors, the problem is further complicated by the protective ligand altering the distribution of resting and activated receptors. The approach used in our lab has been to “phenotype” cysteine substituted mutant receptors using the MWC co-agonist model, including measurement of spontaneous activation, GABA binding and efficacy, and anesthetic binding and efficacy to provide a framework for designing optimal protection experiments. The results (not yet published) provide a more solid basis for inferences about which GABAA receptor residues interact with general anesthetics.
Can allosteric co-agonism at GABAA receptors be generalized to other drugs and experiments?
So far, simple equilibrium MWC co-agonist models appear to work well for the analysis of oocyte electrophysiology data for GABAA receptors, where the relatively slow rate of drug concentration changes may result in mixing of receptors in activated and desensitized states. Further experiments will be required to determine if similar or related models will be useful for interpreting rapid kinetic patch-clamp electrophysiological data, single-channel kinetic data, or other types of dynamic signals, such as those from fluorescent labels within GABAA receptors. The concept of allosteric agonism will likely be a useful framework for interpreting the actions of other general anesthetic drugs. Structural studies locating the high-affinity benzodiazepine binding site at a position homologous to the orthosteric agonist (GABA) binding sites suggested that these drugs might be allosteric agonists (71). This was shown to be the case in two ways. First, benzodiazepine agonists were shown to directly gate spontaneously active mutant α1L264Tβ2γ2L GABAA receptors, approximately tripling open probability. Thus, benzodiazepine agonists directly enhance the gating of these receptors in the absence of orthosteric agonists. Secondly, when wild-type α1β2γ2L GABAA receptors are maximally activated with the partial agonist P4S, addition of benzodiazepine agonists increases the maximal current, indicating that these drugs also increase gating efficacy under conditions where orthosteric sites are fully occupied by agonist.
There is related data supporting the idea that propofol and barbiturates, and perhaps neurosteroids act as allosteric agonists at GABAA receptors. We have already noted that propofol and barbiturates enhance the efficacy of partial agonists, indicating that they affect gating rather than agonist binding. At supra-clinical concentrations, barbiturates, propofol, and volatiles also directly activate synaptic GABAA receptors. The single-channel conductance elicited with barbiturates matches that elicited using GABA, supporting the assumption that similar, if not identical conformational changes are triggered by these two classes of agonist (59, 72). Similarly, cysteine accessibility and cross-linking studies in the channel-lining M2 helices of GABAA receptors indicate that channel structure is similar when opened by both GABA and propofol (73).
Can propofol actions at GABAA receptors be described by an allosteric co-agonist mechanism similar to that for etomidate? We have addressed this question by comparing the linkages between direct activation and GABA modulation for both propofol and etomidate. Using oocyte electrophysiology, we identified a propofol concentration that is equi-potent with 10 μM etomidate for direct activation of α1β2γ2L GABAA receptors. Preliminary studies demonstrate that the shift in GABA concentration-responses produced by this propofol concentration matches that produced by 10 μM etomidate. Thus, the linkage between direct agonism and GABA modulation inherent in co-agonist models is maintained for propofol. Additional experiments are underway to establish the number of equivalent propofol sites that best fits the MWC model.
Emerging hypotheses regarding anesthetic modulation sites on cys-loop ion channels
Research during the last two decades has tremendously improved our understanding of the mechanisms underlying general anesthetics. In the case of etomidate, transgenic animal studies (1, 68) have confirmed that the major molecular targets are GABAA receptors, the major functional effects of drug binding to targets are explained by allosteric co-agonism (15), and photolabeling has identified the binding sites amidst transmembrane helices (25). Additional potential binding sites of this type exist both within the four TM helices of single cys-loop ion channel subunits, but also within the transmembrane pore and at subunit interfaces. Indeed, recent data suggests that the sites where neurosteroids and propofol bind to GABAA receptors differ from those for etomidate (47). The formal functional paradigm presented for etomidate may apply to these other potent general anesthetic drugs. Combining photolabeling with structure-function mutational studies in the context of allosteric mechanisms should lead us to a more detailed understanding of how and where these important drugs act.
Acknowledgments
Funded by a grant from the National Institute of General Medical Sciences (GM 58448) and by the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital.
Footnotes
The authors have no conflicts of interest.
Implication Statement
We review the location of general anesthetic binding sites on GABAA and ACh receptors, and the allosteric mechanism by which they act to change the receptor's function. This work provides the necessary background for rational development of improved general anesthetics.
Citations
- 1.Jurd R, Arras M, Lambert S, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB Journal. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 2.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 3.Hemmings HC, Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 5.Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–79. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- 6.Zeller A, Jurd R, Lambert S, et al. Inhibitory ligand-gated ion channels as substrates for general anesthetic actions. Handb Exp Pharmacol. 2008:31–51. doi: 10.1007/978-3-540-74806-9_2. [DOI] [PubMed] [Google Scholar]
- 7.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 8.Monod J, Wyman J, Changeux J. On the nature of allosteric transitions: A plausible model. Journal of Molecular Biology. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 9.Perutz MF. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989;22:139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- 10.Cui Q, Karplus M. Allostery and cooperativity revisited. Protein Sci. 2008;17:1295–307. doi: 10.1110/ps.03259908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Changeux JP, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. doi: 10.1016/j.molmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–50. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 13.Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–8. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 14.Dodson BA, Braswell LM, Miller KW. Barbiturates bind to an allosteric regulatory site on nicotinic acetylcholine receptor-rich membranes. Mol Pharmacol. 1987;32:119–26. [PubMed] [Google Scholar]
- 15.Rüsch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA-A receptors accounts for both direct activation and agonist modulation. Journal of Biological Chemistry. 2004;279:20982–92. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 16.Arias HR, Kem WR, Trudell JR, Blanton MP. Unique general anesthetic binding sites within distinct conformational states of the nicotinic acetylcholine receptor. Int Rev Neurobiol. 2003;54:1–50. doi: 10.1016/s0074-7742(03)54002-8. [DOI] [PubMed] [Google Scholar]
- 17.Baenziger JE, Corringer PJ. 3D structure and allosteric modulation of the transmembrane domain of pentameric ligand-gated ion channels. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Olsen RW, Chang CS, Li G, Hanchar HJ, Wallner M. Fishing for allosteric sites on GABA(A) receptors. Biochem Pharmacol. 2004;68:1675–84. doi: 10.1016/j.bcp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Miller KW. The nature of sites of general anaesthetic action. Br J Anaesth. 2002;89:17–31. doi: 10.1093/bja/aef167. [DOI] [PubMed] [Google Scholar]
- 20.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–89. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Bocquet N, Nury H, Baaden M, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–4. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 22.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–8. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 23.Weng Y, Yang L, Corringer PJ, Sonner JM. Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth Analg. 2010;110:59–63. doi: 10.1213/ANE.0b013e3181c4bc69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nury H, Van Renterghem C, Weng Y, et al. X-Ray Structures Of General Anaesthetics Bound To A Pentameric Ligand-Gated Ion Channel. Nature. 2010 doi: 10.1038/nature09647. in press. [DOI] [PubMed] [Google Scholar]
- 25.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. Journal of Neuroscience. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campagna-Slater V, Weaver DF. Molecular modelling of the GABAA ion channel protein. J Mol Graph Model. 2007;25:721–30. doi: 10.1016/j.jmgm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: important insights in pharmacology and function. Mol Pharmacol. 2005;68:1291–300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- 28.Jansen M, Akabas MH. State-dependent cross-linking of the M2 and M3 segments: functional basis for the alignment of GABAA and acetylcholine receptor M3 segments. J Neurosci. 2006;26:4492–9. doi: 10.1523/JNEUROSCI.0224-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bali M, Jansen M, Akabas MH. GABA-induced intersubunit conformational movement in the GABAA receptor alpha 1M1-beta 2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site. J Neurosci. 2009;29:3083–92. doi: 10.1523/JNEUROSCI.6090-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dostalova Z, Liu A, Zhou X, et al. High-level expression and purification of Cys-loop ligand-gated ion channels in a tetracycline-inducible stable mammalian cell line: GABA(A) and serotonin receptors. Protein Sci. 2010 doi: 10.1002/pro.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosterling B, Trevor A, Trudell JR. Binding of halothane-free radicals to fatty acids following UV irradiation. Anesthesiology. 1982;56:380–4. doi: 10.1097/00000542-198205000-00010. [DOI] [PubMed] [Google Scholar]
- 32.el-Maghrabi EA, Eckenhoff RG, Shuman H. Saturable binding of halothane to rat brain synaptosomes. Proc Natl Acad Sci U S A. 1992;89:4329–32. doi: 10.1073/pnas.89.10.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Husain SS, Forman SA, Kloczewiak MA, et al. Synthesis and properties of 3-(2-hydroxyethyl)-3-n-pentyldiazirine, a photoactivable general anesthetic. J Med Chem. 1999;42:3300–7. doi: 10.1021/jm9806300. [DOI] [PubMed] [Google Scholar]
- 34.Husain SS, Ziebell MR, Ruesch D, et al. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: A derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–65. doi: 10.1021/jm020465v. [DOI] [PubMed] [Google Scholar]
- 35.Eckenhoff RG, Xi J, Shimaoka M, Bhattacharji A, Covarrubias M, Dailey WP. Azi-isoflurane, a Photolabel Analog of the Commonly Used Inhaled General Anesthetic Isoflurane. ACS Chem Neurosci. 2010;1:139–45. doi: 10.1021/cn900014m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratt MB, Husain SS, Miller KW, Cohen JB. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem. 2000;275:29441–51. doi: 10.1074/jbc.M004710200. [DOI] [PubMed] [Google Scholar]
- 37.Chiara DC, Dangott LJ, Eckenhoff RG, Cohen JB. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42:13457–67. doi: 10.1021/bi0351561. [DOI] [PubMed] [Google Scholar]
- 38.Ziebell MR, Nirthanan S, Husain SS, Miller KW, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anesthetic. J Biol Chem. 2004;279:17640–9. doi: 10.1074/jbc.M313886200. [DOI] [PubMed] [Google Scholar]
- 39.Firestone LL, Sauter JF, Braswell LM, Miller KW. Actions of general anesthetics on acetylcholine receptor-rich membranes from Torpedo californica. Anesthesiology. 1986;64:694–702. doi: 10.1097/00000542-198606000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Chiara DC, Hong FH, Arevalo E, et al. Time-resolved photolabeling of the nicotinic acetylcholine receptor by [3H]azietomidate, an open-state inhibitor. Mol Pharmacol. 2009;75:1084–95. doi: 10.1124/mol.108.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nirthanan S, Garcia G, 3rd, Chiara DC, Husain SS, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for TDBzl-etomidate, a photoreactive positive allosteric effector. J Biol Chem. 2008;283:22051–62. doi: 10.1074/jbc.M801332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao M, Sonner JM, Husain SS, et al. R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the gamma-aminobutyric acid receptor beta3 subunit. Anesth Analg. 2005;101:131–5. doi: 10.1213/01.ANE.0000153011.64764.6F. table of contents. [DOI] [PubMed] [Google Scholar]
- 43.Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Molecular Pharmacology. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- 44.Krasowski MD, Koltchine VV, Rick CE, Ye Q, Finn SE, Harrison NL. Propofol and other intravenous anesthetics have sites of action on the gamma-aminobutyric acid type A receptor distinct from that for isoflurane. Mol Pharmacol. 1998;53:530–8. doi: 10.1124/mol.53.3.530. [DOI] [PubMed] [Google Scholar]
- 45.Mihic SJ, Ye Q, Wick MJ, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 46.Grutter T, Changeux JP. Nicotinic receptors in wonderland. Trends Biochem Sci. 2001;26:459–63. doi: 10.1016/s0968-0004(01)01921-1. [DOI] [PubMed] [Google Scholar]
- 47.Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285:8615–20. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth SH, Forman SA, Braswell LM, Miller KW. Actions of pentobarbital enantiomers on nicotinic cholinergic receptors. Mol Pharmacol. 1989;36:874–80. [PubMed] [Google Scholar]
- 49.Miller KW, Addona GH, Kloczewiak MA. Approaches to proving there are general anesthetic sites on ligand gated ion channels. Toxicol Lett. 1998;100-101:139–47. doi: 10.1016/s0378-4274(98)00178-7. [DOI] [PubMed] [Google Scholar]
- 50.Forman SA, Miller KW. High acetylcholine concentrations cause rapid inactivation before fast desensitization in nicotinic acetylcholine receptors from Torpedo. Biophysical Journal. 1988;54:149–58. doi: 10.1016/S0006-3495(88)82939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maconochie DJ, Steinbach JH. The channel opening rate of adult- and fetal-type mouse muscle nicotinic receptors activated by acetylcholine. J Physiol. 1998;506(Pt 1):53–72. doi: 10.1111/j.1469-7793.1998.053bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamodo IH, Chiara DC, Cohen JB, Miller KW. Conformational changes in the nicotinic acetylcholine receptor during gating and desensitization. Biochemistry. 2010;49:156–65. doi: 10.1021/bi901550p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arevalo E, Chiara DC, Forman SA, Cohen JB, Miller KW. Gating-enhanced accessibility of hydrophobic sites within the transmembrane region of the nicotinic acetylcholine receptor's {delta}-subunit. A time-resolved photolabeling study. J Biol Chem. 2005;280:13631–40. doi: 10.1074/jbc.M413911200. [DOI] [PubMed] [Google Scholar]
- 54.Auerbach A, Akk G. Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J Gen Physiol. 1998;112:181–97. doi: 10.1085/jgp.112.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andreeva IE, Pedersen SE. Conformational asymmetry of nicotinic acetylcholine receptor (AChR) desensitization. Biophysics Journal. 2005;89:3052–Pos. [Google Scholar]
- 56.Cash DJ, Subbarao K. Different effects of pentobarbital on two gamma-aminobutyrate receptors from rat brain: channel opening, desensitization, and an additional conformational change. Biochemistry. 1988;27:4580–90. doi: 10.1021/bi00412a053. [DOI] [PubMed] [Google Scholar]
- 57.Chang Y, Weiss DS. Allosteric activation mechanism of the alpha1beta2gamma2 gamma-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophysical Journal. 1999;77:2542–51. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Shea SM, Wong LC, Harrison NL. Propofol increases agonist efficacy at the GABA(A) receptor. Brain Res. 2000;852:344–8. doi: 10.1016/s0006-8993(99)02151-4. [DOI] [PubMed] [Google Scholar]
- 59.Steinbach JH, Akk G. Modulation of GABA(A) receptor channel gating by pentobarbital. Journal of Physiology. 2001;537:715–33. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belelli D, Muntoni AL, Merrywest SD, et al. The in vitro and in vivo enantioselectivity of etomidate implicates the GABAA receptor in general anaesthesia. Neuropharmacology. 2003;45:57–71. doi: 10.1016/s0028-3908(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 61.Tomlin SL, Jenkins A, Lieb WR, Franks NP. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–17. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Uchida I. Mechanisms of etomidate potentiation of GABAA receptor-gated currents in cultured postnatal hippocampal neurons. Neuroscience. 1996;73:69–78. doi: 10.1016/0306-4522(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 63.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11031–6. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. British Journal of Pharmacology. 1997;120:749–56. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 66.Desai R, Ruesch D, Forman SA. Gamma-Amino Butyric Acid Type A Receptor Mutations at beta2N265 Alter Etomidate Efficacy While Preserving Basal and Agonist-dependent Activity. Anesthesiology. 2009;111:774–84. doi: 10.1097/ALN.0b013e3181b55fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart DS, Desai R, Cheng Q, Liu A, Forman SA. Tryptophan mutations at azietomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol Pharmacol. 2008;74:1687–95. doi: 10.1124/mol.108.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKernan RM, Rosahl TW, Reynolds DS, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nature Neuroscience. 2000;3:587–92. doi: 10.1038/75761. [comment] [DOI] [PubMed] [Google Scholar]
- 69.Williams DB, Akabas MH. Gamma-aminobutyric acid increases the water accessibility of M3 Membrane-spanning segment residues in GABA-A receptors. Biophysical Journal. 1999;77:2563–74. doi: 10.1016/s0006-3495(99)77091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mercado J, Czajkowski C. Gamma-aminobutyric acid (GABA) and pentobarbital induce different conformational rearrangements in the GABA A receptor alpha1 and beta2 pre-M1 regions. J Biol Chem. 2008;283:15250–7. doi: 10.1074/jbc.M708638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rüsch D, Forman SA. Classic benzodiazepines modulate the open-close equilibrium in alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology. 2005;102:783–92. doi: 10.1097/00000542-200504000-00014. [DOI] [PubMed] [Google Scholar]
- 72.Akk G, Steinbach JH. Activation and block of recombinant GABA(A) receptors by pentobarbitone: a single-channel study. British Journal of Pharmacology. 2000;130:249–58. doi: 10.1038/sj.bjp.0703335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosen A, Bali M, Horenstein J, Akabas MH. Channel opening by anesthetics and GABA induces similar changes in the GABAA receptor M2 segment. Biophysical Journal. 2007;92:3130–9. doi: 10.1529/biophysj.106.094490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]