Abstract
Abstract
Background
DNA methylation of cytosine residues in CpG dinucleotide controls gene expression and dramatically changes during development. Its pattern is disrupted in cloned animals suggesting incomplete reprogramming during somatic cell nuclear transfer (the first reprogramming). However, the second reprogramming occurs in the germ cells and epigenetic errors in somatic cells of cloned animals should be erased. To analyze the DNA methylation changes on the spermatogenesis of bulls, we measured DNA methylation levels of three repetitive elements in blastocysts, blood and sperm.
Methods
DNA from PBLs (peripheral blood leukocytes), sperm and individual IVF (in vitro fertilized) and parthenogenetic blastocysts was isolated and bisulfite converted. Three repetitive elements; Satellite I, Satellite II and art2 sequences were amplified by PCR with specific pairs of primers. The PCR product was then cut by restriction enzymes and analyzed by agarose gel electrophoresis for determining the DNA methylation levels.
Results
Both Satellite I and Satellite II sequences were highly methylated in PBLs, whereas hypo-methylated in sperm and blastocysts. The art2 sequence was half methylated both in PBLs and sperm but less methylated in blastocysts. There was no difference in DNA methylation levels between IVF and parthenogenetic blastocysts.
Conclusions
These results suggest that there is a dynamic change of DNA methylation during embryonic development and spermatogenesis in cattle. Satellite I and Satellite II regions are methylated during embryogenesis and then de-methylated during spermatogenesis. However, art2 sequences are not de-methylated during spermatogenesis, suggesting that this region is not reprogrammed during germ cell development. These results show dynamic changes of DNA methylation levels during bovine embryogenesis, especially genome-wide reprogramming in germ cells.
Background
DNA methylation is a major physiological modification in mammalian genome. Cytosine residues in CpG dinucleotide pairs are selectively methylated by DNA methyltransferases and these methylation patterns are maintained throughout cell division. DNA methylation alters gene expression patterns in cells and is crucial for normal mammalian development [1,2]. Usually, repetitive elements such as centromeric repeats and transposon sequences are highly methylated and transcriptionally silenced, called a heterochromatic state. The methylation patterns are dramatically changed during embryonic development, from a fertilized egg to a lot of types of differentiated cells. As DNA methylation controls gene expression, some sets of genes are activated/inactivated in particular types of cells during differentiation. Genomic imprinting, which causes parent-of-origin specific gene expression in mammals and X chromosome inactivation, which compensates X chromosome genes dosage between females (XX sex chromosomes) and males (XY sex chromosomes), are controlled by DNA methylation and are crucial for normal mammalian development [3]. Aberrant DNA methylation patterns were observed in many kinds of tumour cells [4].
In mouse germ cells development, it was previously shown that imprinted genes and repetitive elements were de-methylated in primordial germ cells [5] and then re-methylated during spermatogenesis or oogenesis [6]. De novo DNA methyltransferases, Dnmt3a and Dnmt3b and Dnmt3-like protein Dnmt3L are responsible for establishing sex-specific DNA methylation patterns both in males and females [7-10]. After fertilization, there is a passive DNA demethylation in the preimplantation embryos depending on DNA replication, however, methylation of imprinted gene escapes this genome-wide demethylation event. De novo methylation begins after implantation by Dnmt3a and Dnmt3b, and these methylation patterns are maintained throughout development by the maintenance DNA methyltransferase Dnmt1 [11,12]. However, little is known about the changes of DNA methylation during embryogenesis in cattle. Here we report the dynamic changes of DNA methylation patterns at three repetitive sequences in bovine blastocysts, somatic cells and sperm.
Methods
DNA preparation
Blood and frozen sperm samples from six Japanese Black bulls (Wagyu), two Japanese Brown bulls (Aka-ushi) and one Holstein bull were obtained and genomic DNA was extracted by using DNeasy Blood & Tissue Kit (QIAGEN). Sperm DNA was extracted using the lysis buffer with 200mM dithiothreitol (Sigma-Aldrich). DNA from individual blastocysts was isolated as described previously [12]. IVF and PA embryos were produced by a standard method [13].
DNA methylation analysis
Genomic DNA was bisulfite converted by EpiTect Bisulfite Kits (QIAGEN) according to the manufacturer’s instructions. Three repetitive elements were amplified with specific pairs of primers previously described [14]. The amplified PCR products were then cut by restriction enzymes (Satellite I by AciI, Satellite II by AccII and art2 by TaqI). After digestion, each size of the digested PCR fragments was isolated by 2% agarose gel electrophoresis.
Results
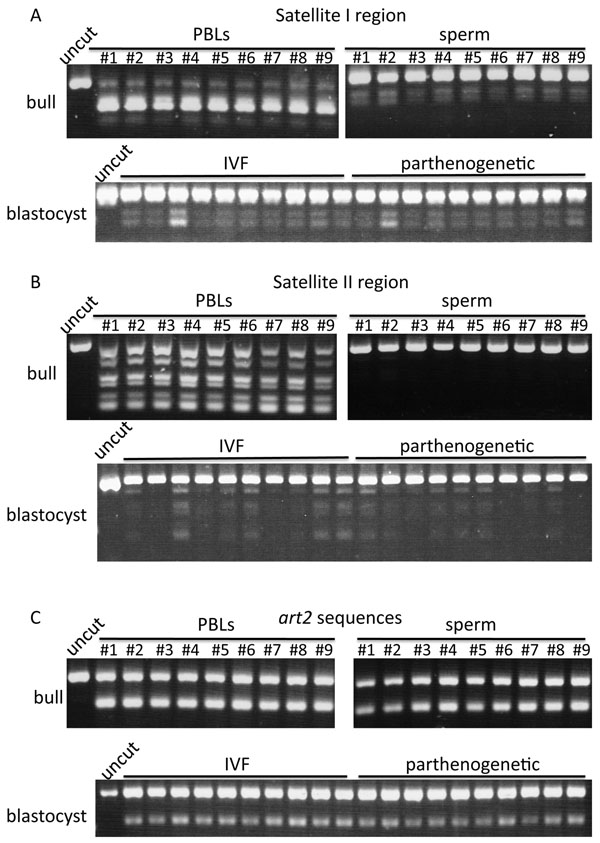
We analyzed genomic DNA methylation patterns to monitor the changes of epigenetic patterns during bovine embryogenesis and spermatogenesis. We chosen three repetitive regions; Satellite I, Satellite II and art2 sequences. Satellite sequences are repetitive sequences at the peri-/centromeric regions of the chromosomes, whereas art2 sequences are Alu-like short interspersed nuclear elements (SINEs). First, we analyzed genomic DNA of bull blood (peripheral blood leukocytes, PBLs) and sperm for methylation status of the same regions. We found a large difference in methylation status using restriction enzyme analysis (Figure 1A-C). Satellite I sequences were highly methylated in PBLs (almost PCR fragments were cut by AciI), whereas hypo-methylated in sperm (almost PCR fragments were not cut by AciI) (Figure 1A). Satellite II sequences were also hyper-methylated in PBLs but hypo-methylated in sperm (Figure 1B). However, there were no differences in art2 sequence methylation levels between PBLs and sperm (Figure 1C). These results clearly indicated that both Satellite I and Satellite II sequences, which are located on the centromeric heterochromatic regions, were de-methylated during spermatogenesis, whereas art2 sequences, which are located on euchromatic regions, were not methylated/de-methylated during spermatogenesis. Of nine bulls analyzed, there were no differences in DNA methylation patterns of three repetitive elements among individuals and breeds (bulls #1 and #6-9 are Japanese Black, bulls #3-5 are Japanese Brown and Bull #2 is Holstein).
Figure 1.
DNA methylation analysis in PBLs, sperm and blastocysts. Bulls #1-#9 shows each number of analyzed bulls. Uncut band indicates the PCR product not cut by restriction enzymes. (A) Satellite I regions were cut by AciI. (B) Satellite II regions were cut by AccII. (C) art2 sequences were cut by TaqI.
We also analyzed DNA methylation levels in individual blastocysts; 10 in vitro fertilized (IVF) and 10 parthenogenetically activated (PA) embryos. Both Satellite I and Satellite II regions were hypo-methylated, whereas art2 sequences were moderately methylated in IVF and PA blastocysts. There were no differences in DNA methylation patterns between IVF and PA embryos, however, some blastocysts showed more methylated patterns (less cut by restriction enzymes) compared to others (Figure 1A and 1B).
Discussion
This study shows the DNA methylation changes of repetitive elements during bovine development. The methylation pattern differences of imprinted genes IGF2 and SNRPN in bovine oocytes and sperm have been reported [15,16] and the methylation levels of repetitive elements in bovine blastocysts, especially embryos produced by somatic cell nuclear transfer (SCNT) technology showing high levels of DNA methylation, have also been described [14,17]. However, the DNA methylation status of repetitive elements in sperm DNA has not been well understood. We found that two satellite sequences on the centromeric regions of chromosomes, Satellite I and Satellite II sequences, were highly methylated in PBLs but hypo-methylated in sperm. As these regions were not methylated at blastocyst stages, it is suggested that they are methylated after implantation, and then de-methylated during spermatogenesis. In contrast, art2 sequences were moderately methylated in blastocysts but more methylated both in PBLs and sperm, suggesting this region is methylated after implantation but not de-methylated during spermatogenesis. There were no differences between IVF and PA blastocysts, suggesting that these repetitive elements are not methylated by a parent-of-origin specific manner. We analyzed total nine bulls with three different breeds; six Japanese Black, two Japanese Brown and one Holstein, however, there were no difference in DNA methylation patterns among individuals. These results suggest that in adults DNA methylation patterns are firmly maintained during embryogenesis and uniformly reprogrammed during spermatogenesis. In contrast, we observed differences of DNA methylation patterns among the individual IVF and PA blastocysts. This could be explained that the variation itself confers the developmental competence of embryos because SCNT embryos with high DNA methylation levels mostly die in utero and even IVF embryos, only half of them transferred to the uterus develop to term.
In SCNT embryos, Satellite I, Satellite II regions and art2 sequences are hyper-methylated compared to IVF embryos [14]. As donor cells also have high DNA methylation levels, it is suggested that the first reprogramming step (transfer donor nucleus to the enucleated oocyte) is not sufficient to fully reprogram the donor genome. SCNT technology has been developed to rewind the differentiation mechanism, however, the efficiency of this artificial reprogramming is quite low so that still now, more than ten years has past since the first cloned sheep Dolly was born, the success rate of SCNT is still less than 5-10% in cattle and other species. This incomplete reprogramming in SCNT and the resulting alternation of DNA methylation and gene expression were described [18]. However, it is hypothesized that the epigenetic errors that were not corrected during the first reprogramming step are erased and then properly reprogrammed (the second reprogramming step) during germ cell development [19]. Therefore, offspring from cloned animals do not show any abnormalities observed in cloned animals themselves. In fact, the obese phenotype frequently observed in cloned mice does not transmitted to the next generation [20]. In cattle, there is no remarkable difference in health status and food products among non-cloned, cloned cattle developed to adulthood and their offspring [21-23]. By applying this study for cloned cattle, it will be possible to prove proper epigenetic reprogramming during cloned cattle gametogenesis and thus contribute to the normality of cloned cattle offspring.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MK carried out DNA extraction, DNA methylation analysis and analyzed the data. SA made IVF and PA embryos. SW provided blood and sperm samples from bulls. TN participated in the design of the study and contributed to discussion of the results and revision of the paper. All authors read and approved the final manuscript.
Contributor Information
Masahiro Kaneda, Email: mkaneda@affrc.go.jp.
Satoshi Akagi, Email: akagi@affrc.go.jp.
Shinya Watanabe, Email: shw@affrc.go.jp.
Takashi Nagai, Email: taku@affrc.go.jp.
Acknowledgements
We thank Kumamoto Prefectural Agriculture Research Center and Ibaraki Prefectural Livestock Research Center for providing blood and sperm samples from Japanese Brown and Japanese Black bulls.
This article has been published as part of BMC Proceedings Volume 5 Supplement 4, 2011: Proceedings of the International Symposium on Animal Genomics for Animal Health (AGAH 2010). The full contents of the supplement are available online at http://www.biomedcentral.com/1753-6561/5?issue=S4.
References
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nature Reviews Genetics. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Barton SC, Kaneda M, Hajkova P, Surani MA. The continuing quest to comprehend genomic imprinting. Cytogenet Genome Res. 2006;113:6–11. doi: 10.1159/000090808. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mechanisms of development. 2002;117:15–23. doi: 10.1016/S0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- Bourc'his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- Bourc'his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M, Sasaki H. Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet. 2007;16:2272. doi: 10.1093/hmg/ddm179. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–258. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008;22:1607–1616. doi: 10.1101/gad.1667008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi S, Hosoe M, Matsukawa K, Ichikawa A, Tanikawa T, Takahashi S. Culture of Bovine Embryos on a Polydimethylsiloxane (PDMS) Microwell Plate. J Reprod Dev. 2010;56:475–459. doi: 10.1262/jrd.09-213H. [DOI] [PubMed] [Google Scholar]
- Kang YK, Koo DB, Park JS, Choi YH, Chung AS, Lee KK, Han YM. Aberrant methylation of donor genome in cloned bovine embryos. Nature genetics. 2001;28:173–177. doi: 10.1038/88903. [DOI] [PubMed] [Google Scholar]
- Gebert C, Wrenzycki C, Herrmann D, Gröger D, Reinhardt R, Hajkova P, Lucas-Hahn A, Carnwath J, Lehrach H, Niemann H. The bovine IGF2 gene is differentially methylated in oocyte and sperm DNA. Genomics. 2006;88:222–229. doi: 10.1016/j.ygeno.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Suzuki J, Bordignon V, Martel J, Vigneault C, Therrien J, Filion F, Smith LC, Trasler JM. Bovine SNRPN methylation imprint in oocytes and day 17 in vitro-produced and somatic cell nuclear transfer embryos. Biol Reprod. 2006;75:531–538. doi: 10.1095/biolreprod.106.051722. [DOI] [PubMed] [Google Scholar]
- Kang YK, Lee HJ, Shim JJ, Yeo S, Kim SH, Koo DB, Lee KK, Beyhan Z, First NL, Han YM. Varied patterns of DNA methylation change between different satellite regions in bovine preimplantation development. Mol Reprod Dev. 2005;71:29–35. doi: 10.1002/mrd.20249. [DOI] [PubMed] [Google Scholar]
- Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci U S A. 2001;98:13734. doi: 10.1073/pnas.241522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulka J, Miyashita N, Nagai T, Ogura A. Do cloned mammals skip a reprogramming step? Nat Biotechnol. 2004;22:25–26. doi: 10.1038/nbt0104-25. [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Wakayama T, Akutsu H, Yamazaki Y, Lachey JL, Wortman MD, Seeley RJ, D'Alessio DA, Woods SC, Yanagimachi R. Cloned mice have an obese phenotype not transmitted to their offspring. Nature Medicine. 2002;8:262–267. doi: 10.1038/nm0302-262. [DOI] [PubMed] [Google Scholar]
- Shiga K, Umeki H, Shimura H, Fujita T, Watanabe S, Nagai T. Growth and fertility of bulls cloned from the somatic cells of an aged and infertile bull. Theriogenology. 2005;64:334–343. doi: 10.1016/j.theriogenology.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Nagai T. Health status and productive performance of somatic cell cloned cattle and their offspring produced in Japan. J Reprod Dev. 2008;54:6–1. doi: 10.1262/jrd.19090. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Nagai T. Death losses due to stillbirth, neonatal death and diseases in cloned cattle derived from somatic cell nuclear transfer and their progeny: a result of nationwide survey in Japan. Anim Sci J. 2009;80:233–238. doi: 10.1111/j.1740-0929.2009.00640.x. [DOI] [PubMed] [Google Scholar]