SUMMARY
Specific information about how telomerase acts in vivo is necessary for understanding telomere dynamics in human tumor cells. Our results imply that under homeostatic telomere length-maintenance conditions only one molecule of telomerase acts at each telomere during every cell division and processively adds ~60 nt to each end. In contrast, multiple molecules of telomerase act at each telomere when telomeres are elongating (non-equilibrium conditions). Telomerase extension is less processive during the first few weeks following the reversal of long-term treatment with the telomerase inhibitor GRN163L, a time when Cajal bodies fail to deliver telomerase RNA to telomeres. This result implies that processing of telomerase by Cajal bodies may affect its processivity. Overexpressed telomerase is also less processive than the endogenously expressed telomerase. These findings reveal two major distinct extension modes adopted by telomerase in vivo.
Keywords: Telomerase, telomere, cancer, cajal body, hTR, hTERT
INTRODUCTION
Linear eukaryotic chromosomes are capped by telomeres, a functional complex consisting of repetitive DNA and associated proteins. Telomeres maintain chromosome integrity by preventing DNA degradation, end-to-end fusion and illegitimate recombination (Blackburn, 2001; de Lange, 2005). Because of the unidirectional nature of DNA polymerases and additional processing events, human telomeres shorten at ~50–200 base pairs per cell division (Harley et al., 1990). Eventually, critically short telomeres trigger replicative senescence or apoptosis(Palm and de Lange, 2008). Most tumor cells and germ line cells overcome this proliferative limit by activating telomerase, a ribonucleoprotein with reverse transcriptase activity that catalyzes de novo repeat addition using sequences in the integral telomerase RNA (hTR/hTERC) as a template(Bodnar et al., 1998; Greider and Blackburn, 1989; Kim et al., 1994).
In human cancer cells, telomere length is maintained at a steady state (e.g. telomere addition and loss are in homeostatic balance). Under length-maintenance conditions, telomerase adds ~50–60 nt to each telomere end during every cell cycle to counteract intrinsic telomere shortening rates (Zhao et al., 2009). However, the molecular characteristics of telomerase extension on a single telomere are unknown. Telomerase complexes isolated from human cells are modestly processive in vitro, capable of adding several telomeric repeats to a given primer in a single recruitment event (Chen and Greider, 2003). This extension processivity can be synergistically enhanced by heterodimers of POT1 and TPP1, components of a six-protein shelterin complex at telomeres (Wang et al., 2007). In the presence of an optimal concentration of POT/TPP1, telomerase processively adds an average of 48 nt of telomeric repeats (Latrick and Cech, 2010), suggesting that one round of telomerase recruitment and extension might be sufficient to account for the ~60 nt of synthesis seen at individual telomeres in cancer cells under maintenance conditions. Experimental demonstration in vivo, however, is lacking.
Under nonequilibrium conditions, in which telomere length has been experimentally manipulated, there is a general consensus that telomerase preferentially extends the shortest telomere (Hemann et al., 2001; Ouellette et al., 2000; Samper et al., 2001). While telomerase action is nonprocessive on S. cerevisiae telomeres with normal length, the processivity is increased at critically short telomeres (Chang et al., 2007). This enhancement of telomerase processivity is proposed to rapidly elongate critically short telomeres. Telomerase does not act on every telomere in each cell cycle in S. cerevisiae (Teixeira et al., 2004). In human cancer cells in which telomerase does act on every telomere, the mechanism underlying the preferential extension of a particularly short telomere remains to be elucidated.
Telomerase extension is coupled with telomere replication in S phase (Zhao et al., 2009). Both hTR and hTERT have been found associated with telomeres during S phase (Jady et al., 2006; Tomlinson et al., 2006). Assembly of catalytically active telomerase requires the chaperones Hsp23 and Hsp90 (Forsythe et al., 2001), and also requires association with Cajal bodies before it can be delivered to hTR foci at telomeres and produce telomere elongation (Cristofari et al., 2007; Venteicher et al., 2009). Two components of the telomeric shelterin complex, TIN2 and TPP1 have been identified as factors required for recruiting hTR foci to telomeres (Abreu et al., 2010). In contrast to these observations in human cells, mouse telomerase RNA (mTR) does not localize to Cajal bodies but resides in different nuclear foci, but nonetheless is found in foci on a subset of telomeres during replication (Tomlinson et al., 2010). The nature of this association of hTR foci with telomeres during S phase remains to be determined.
The low abundance of telomerase in human cancer cells and its transient binding to telomeres have presented a significant challenge in studying telomerase action in vivo. In this work, we investigated telomerase extension under different conditions using techniques that allow us to examine telomere elongation during a single cell cycle. Our results suggest that under telomere length-maintenance conditions, every telomere was elongated by a single telomerase molecule that processively added ~60 nt to the 3’ overhang (processive action). Reduction of hTERT levels by shRNA demonstrated that telomerase abundance was limiting and reducing levels of telomerase reduced the fraction of extended ends without apparently affecting processivity. The absence of non-extended ends under conditions where telomerase is not in excess suggests a telomerase/telomere prepositioning step that ensures that all ends get extended but only by a single molecule. When telomeres are lengthening (recovery after telomeres had been artificially shortened or after overexpression of an exogenous telomerase) telomeres appear to be elongated as a result of multiple telomerase molecules acting on each end to produce longer extensions (distributive action). Long term treatment with the telomerase inhibitor GRN163L (Imetelstat) resulted in the disappearance of hTR/telomere co-localization without changing total Cajal body number or hTR/Cajal body co-localization. This lack of co-localization persisted during the first few weeks following removal of the inhibitor, corresponding to a period in which telomerase activity was restored but telomerase processivity was greatly reduced, suggesting that Cajal bodies are modifying telomerase to make it more processive. These observations demonstrate two major modes of telomerase action, processive and distributive, and reveal additional steps (prepositioning of telomerase at telomeres under length-maintenance conditions and the modification of processivity in Cajal bodies) in telomerase action, and expand our understanding of the multiple mechanisms regulating the ability of telomerase to maintain telomeres in tumor cells.
RESULTS
Telomerase action is processive in human cancer cells
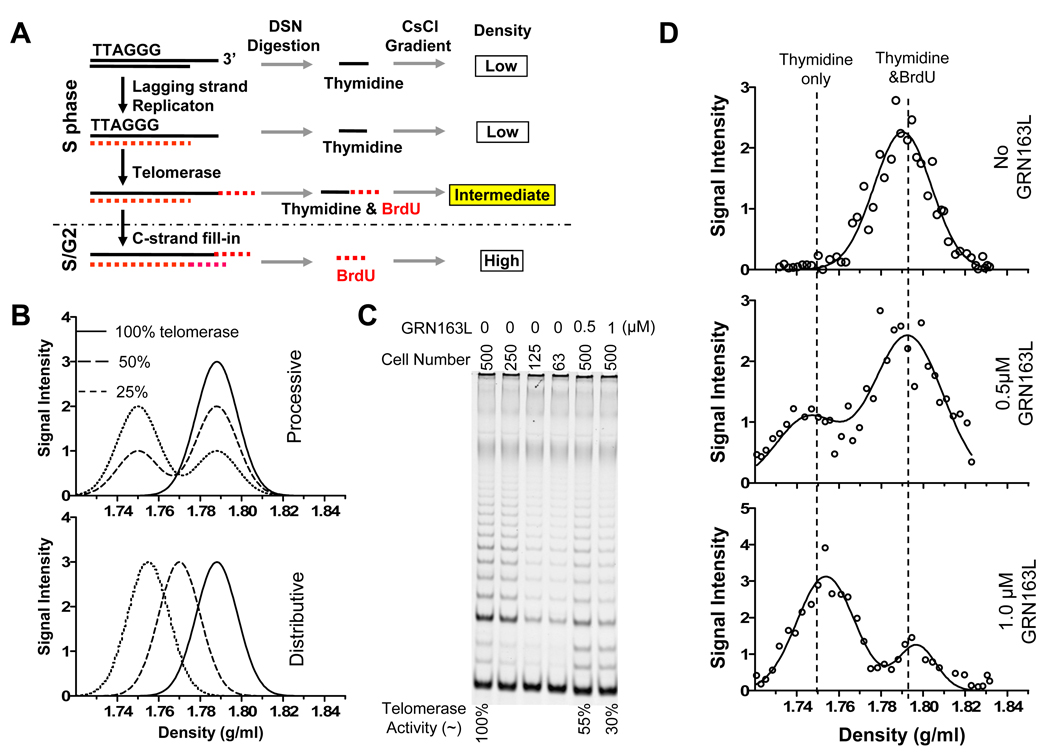
Telomerase action during a single S phase in the presence of BrdU can be examined by first purifying telomeres replicated by lagging strand synthesis on a first CsCl gradient, and then analyzing the density of their overhangs on a second CsCl gradient (Fig. 1A). While unextended overhangs (thymidine only) band at low density, the overhangs extended by telomerase incorporate BrdU in the new synthesized telomeric repeats and shift to a higher density (intermediate density) depending upon how many repeats are added. The ratio of intermediate versus low density overhangs indicates the fraction of telomeres extended by telomerase. The density of the intermediate peak is proportional to the ratio of BrdU to thymidine in the overhangs (supplementary Fig. S1A). Therefore the average length of telomerase addition products (BrdU containing repeats) can be calculated by combining the BrdU/thymidine ratio and the total lagging overhang length. Using this approach we found that under telomere length-maintenance conditions in human H1299 adenocarcinoma and Hela cervical carcinoma cells, telomerase extends most telomere ends by adding ~60 nt to each overhang during every cell cycle (Zhao et al., 2009).
Figure 1.
Telomerase action is processive in H1299 cells. (A) Strategy to study telomerase action on lagging daughter telomeres. Extended lagging overhangs have higher density than unextended overhangs because of the incorporation of BrdU by telomerase. (B) Predicted results for processive and distributive manner of telomerase action when 50% and 75% of activity was inhibited. (C) TRAP assay for telomerase activity in the cells with and without GRN163L treatment. 0.5 and 1.0 µM of GRN163L were used to obtain approximately 50% and 70% of inhibition. (D) CsCl overhang assay of telomere extension in H1299 cells with and without treatment of GRN163L that partially inhibit telomerase activity. See also supplementary Fig. S1.
The action of vertebrate telomerase in vivo was examined to determine whether the extension of each telomere represented the result of a single processive event by one molecule (processive) versus repetitive additions of smaller amounts by a multiple telomerase molecules (distributive). The telomerase inhibitor GRN163L, a synthetic lipid-conjugated 13-mer oligonucleotide with an extremely high affinity for the template region of hTR (Herbert et al., 2005), binds essentially irreversibly to the active site template and blocks activity (Herbert et al., 2002). If telomerase action is processive the inactivation of individual telomerase molecules by a partially inhibitory dose will decrease the fraction of extended ends without affecting the length of the extension products. However, if many telomerase molecules each add a small amount to each end (distributive action), a decreased fraction of active molecules would initially reduce the size of the extension products and would only affect the fraction of extended ends at high levels of inhibition. These two possibilities can be distinguished based on the predicted change in the density of lagging overhangs on CsCl gradients (Fig. 1B).
Treatment with 0.5 or 1 µM GRN163L reduced telomerase activity in H1299 lung adenocarcinoma cells by roughly 50% and 70%, respectively (Fig. 1C). Cells growing in different concentrations of GRN163L were synchronized at G1/S and released into S for 4 hours (middle of S phase) in the presence of BrdU. In the absence of GRN163L, no thymidine-only containing lagging overhangs were present in H1299 lung adenocarcinoma cells labeled for four hours with BrdU, indicating that essentially 100% of the telomeres had been elongated by telomerase (Fig. 1D top). In the presence of increasing inhibition by GRN163L, the density of the extended ends did not change; the fraction of ends at the intermediate density decreased while the fraction of unlabeled ends increased (Fig. 1D middle and bottom). These results are not consistent with multiple molecules producing the extension and indicate that a single molecule of telomerase processively adds the full amount to each end. Our results do not address whether or not this single molecule is a dimer or multimer or whether the same molecule is extending both leading and lagging daughter telomeres.
In H1299 cells the intermediate density peak is half-way between the thymidine only and fully BrdU substituted overhangs, implying an equal number of thymidine and BrdU containing repeats (supplementary Fig. S1A). The total overhang size of lagging daughter telomeres was approximately 130 nt (supplementary Fig. S1B). Lagging strand overhangs prior to telomerase elongation were thus about 65 nt (thymidine-only segment) and telomerase addition produced the remaining 65 nt (BrdU substituted segment). H1299 telomeres shortened at a rate of about 55 bp/PD (base pairs per population doubling) when the majority of telomerase activity (>90%) is inhibited (supplementary Fig. S1C, D). The addition of ~65 nt to every telomere end by telomerase is roughly consistent with the amount that would need to be added to counteract telomere shortening.
The generality of this observation was examined in Hela cervical carcinoma cells. Only about 70% of Hela cells express telomerase at any given time (Bryan et al., 1998), and 70% of telomere ends were extended by telomerase during each cell cycle (Fig. 2C top). Similar to H1299 cells, inhibition by GRN163L resulted in a decrease in the fraction of extended ends without changing their density (Fig. 2C middle). The extended fraction was roughly proportional to the fraction of active molecules (Fig. 2 A, C, and supplemental Fig. S2 A , B) for both Hela and H1299, suggesting that the number of active telomerase molecules was limiting. The rate of telomere shortening in 0.25 and 0.5µM GRN163L (Fig. 2D and supplementary Fig. S2B) was inversely-proportional to the fraction of extended telomeres.
Figure 2.
Processive extension of telomere by telomerase in Hela cells. (A) Dose-response curve for the inhibition of telomerase activity by GRN163L (assayed by TRAP). ±SD of three independent experiments. (B) q-PCR determination of mRNA level of hTERT in clones 4 and 10 after sh-hTERT virus infection and drug selection. (C) CsCl overhang assays of telomere extension on lagging daughter in normal Hela cells (top) and in cells with telomerase partially inhibited by GRN163L (middle) or sh-hTERT (bottom). The Y-axis represents an arbitrary scale within each experiment that best displays the variation between the different fractions. (D) Summary of data on telomerase action and telomere shortening in response to partial inhibition of telomerase by GRN163L. (E) Summary of data on telomerase action and telomere shortening in response to knockdown of hTERT. It is worth noting that both telomerase activity assayed by TRAP and qPCR of hTERT mRNA are both PCR based and of limited precision, and thus minor differences between the data in 2D and 2E are not significant. See also supplementary Fig. S2.
In addition to GRN163L-mediated telomerase inhibition, we also examined the effects of reducing telomerase by depleting hTERT levels using shRNA in Hela cells. Two clones with about 50% reduction of telomerase mRNA levels (Fig. 2B) were examined for their effects on telomere extension. The decrease in telomerase changed the ratio of extended versus unextended overhangs but not the length of the extension products (Fig. 2C bottom panel, Fig. 2E and supplementary Fig. S2C), reinforcing the conclusion that telomerase action is processive and further suggesting that one and only one extension event occurs on each telomere.
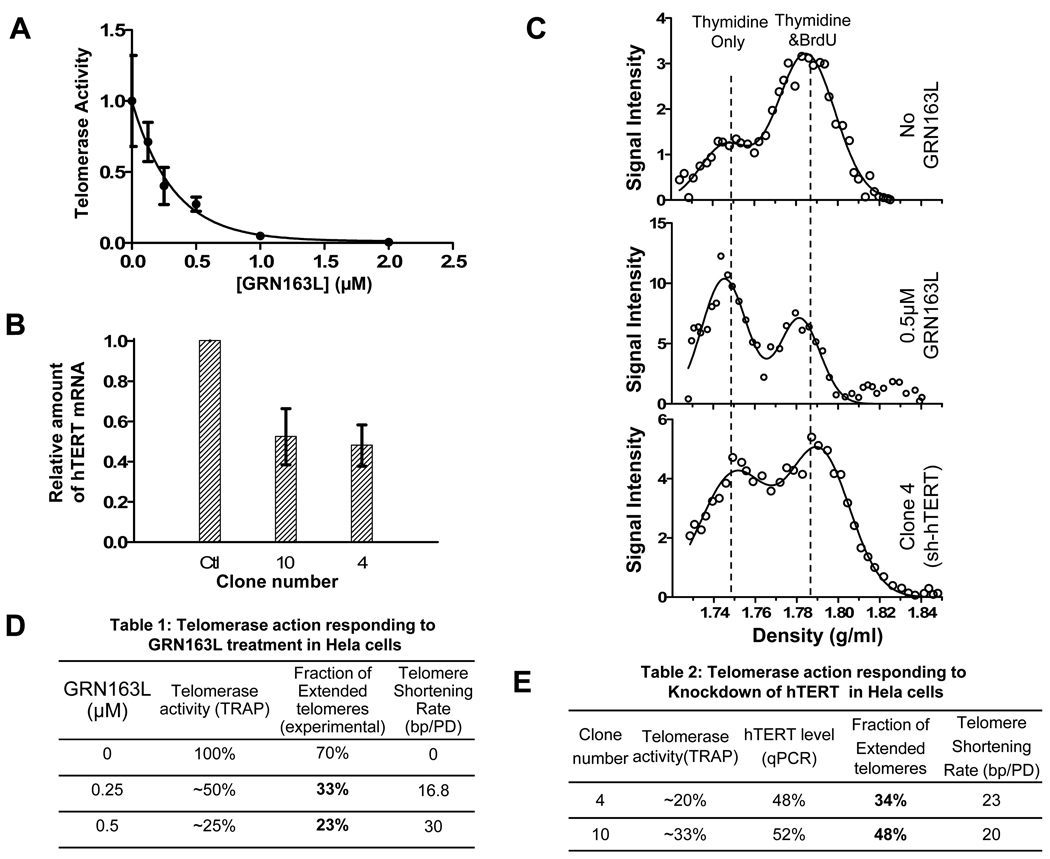
More than one round of telomerase extension occurs on each telomere when telomerase is overexpressed in Hela cells
The number of catalytically active telomerase molecules in human cancer cells is low, ranging from 50 to several hundred per cell ((Cohen et al., 2007) and our unpublished data), and limiting for telomere length homeostasis (Cristofari and Lingner, 2006; McChesney et al., 2000). Overexpressing hTERT in Hela cells produced a 10-fold increase in TRAP activity and elongated telomeres at 120 nt/PD (Fig. 3 A , B). The intermediate peak of telomerase extension products in these cells banded at a density of 1.808, indicating that 73% of each overhang was BrdU substituted. With 60 nt due to the initial thymidine containing overhang (generated by lagging DNA replication) , the size of the BrdU containing telomerase extension products would be about 160 nt to produce the overall 73% fraction. This is roughly equal to the observed telomere elongation rate (120 nt) plus that required for compensating for the rate of telomere shortening during each division (44 nt) (supplementary Fig. S2B).
Figure 3.
Distributive extension of telomere by telomerase in Hela cells with overexpressed hTERT. (A) A partial inhibition of telomerase activity by GRN163L in cells with overexpressed telomerase. Telomerase activity was assayed by TRAP. (B) Telomeres are elongated by overexpressed telomerase. Telomere length was assayed by TRF. (C) CsCl overhang assay showing telomere extension in cells with overexpressed telomerase (top) and inhibitory effect of GRN163L on telomerase action (bottom).
Treating hTERT-overexpressing Hela cells with 0.5 µM GRN163L inhibited telomerase activity to about the level of normal Hela cells (Fig. 3A). In contrast to the results under length maintenance conditions (Fig. 2), this inhibition resulted in a shift of the extension products to a lower CsCl denstity with only a small decrease of the fraction of ends that had been extended (Fig. 3C). This indicates that overexpressed telomerase is acting in a distributive fashion (Fig. 1B), where several different telomerase molecules act on each end during this period of telomere elongation. In addition, the shifted peak of the extension products (40 nt) after GRN163L inhibition is less than that observed with endogenous telomerase (Fig. 1 & Fig. 2), suggesting an altered processivity of the overexpressed exogenous enzyme. Due to technical limitations, we are unable to formally determine how many telomerase molecules were involved in the additions at each telomere.
Taken together, this data indicates that under telomere length maintenance conditions each telomere is extended once and only once and there is not enough excess telomerase available for a second round of extension before the open configuration closes to make the end inaccessible. Overexpression of hTERT either modifies the open configuration or simply provides enough extra telomerase so that multiple rounds of extension by different molecules can occur.
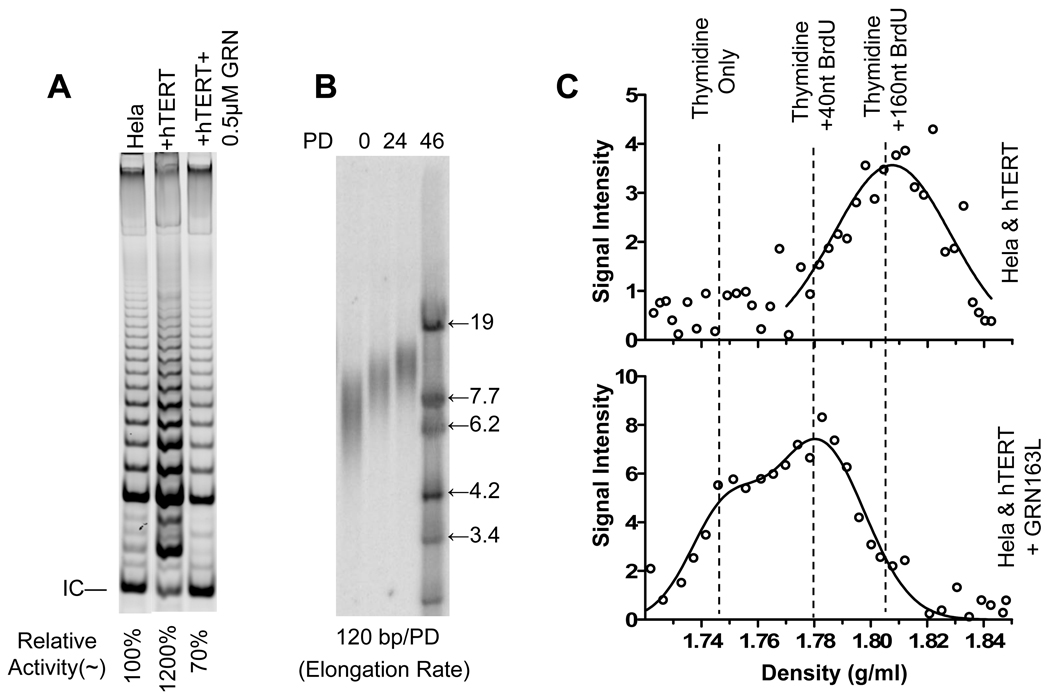
Multiple telomerase molecules act on artificially shortened telomeres
Overexpression of the hTERT cDNA is a physiologically abnormal method to elongate telomeres. Telomere elongation using only the endogenous telomerase occurs following removal of a telomerase inhibitor in tumor cells in which telomeres have been shortened during prolonged treatment with the telomerase inhibitor (Herbert et al., 1999). After 59 population doublings (PDs) in 2 µM GRN163L, the average length of Hela telomeres dropped from 6.5 kb to about 4 kb (Fig. 4A). After removing GRN163L, telomeres grew back to their original size over about 40 PD, indicating that telomerase had been extending the ends more than during length-maintenance conditions. Telomerase activity in cells released from GRN163L treatment returned to the same level as untreated Hela cells within one week (less than 5 PDs), and remained constant thereafter (Fig. 4B and our unpublished data). In contrast to normal Hela cells where only 70% telomeres were extended (Fig. 2C), all of the telomeres (100%) were elongated by telomerase during a single cell cycle in cells 22 PD following the removal of GRN163L (Fig. 4C top). This is consistent with the disappearance of the fraction of Hela cells transiently not expressing telomerase that is present in the parental cultures with longer telomeres. The density of the intermediate peak increased from 50% BrdU at maintenance to 67% BrdU. The total overall length for lagging overhangs in these cells was 200 nt (Fig. 5B), demonstrating that telomerase had added ~135 nt (200×67%) to a thymidine containing initial overhang of ~65 nt at each telomere. In response to the decreased number of active telomerase molecules in the presence of GRN163L (supplementary Fig. S3), the intermediate peak shifted to lower density (Fig. 4C), indicating distributive action in which multiple molecules of telomerase act on single telomere (Fig. 1B).
Figure 4.
Distributive extension of telomere by endogenous telomerase in Hela cells with artificially shortened telomeres. (A) Telomere shortening induced by GRN163L treatment and gradual recovery in length after a removal of drug. (B) Telomerase activity rapidly returns to normal level and remains steady thereafter. (C) CsCl overhang assays of telomerase extension in cells with artificially shortened telomeres 22 PD after release (top). Telomerase action was assayed on CsCl gradients (middle and bottom) in different inhibitory concentrations of GRN163L. See also supplementary Fig. S3.
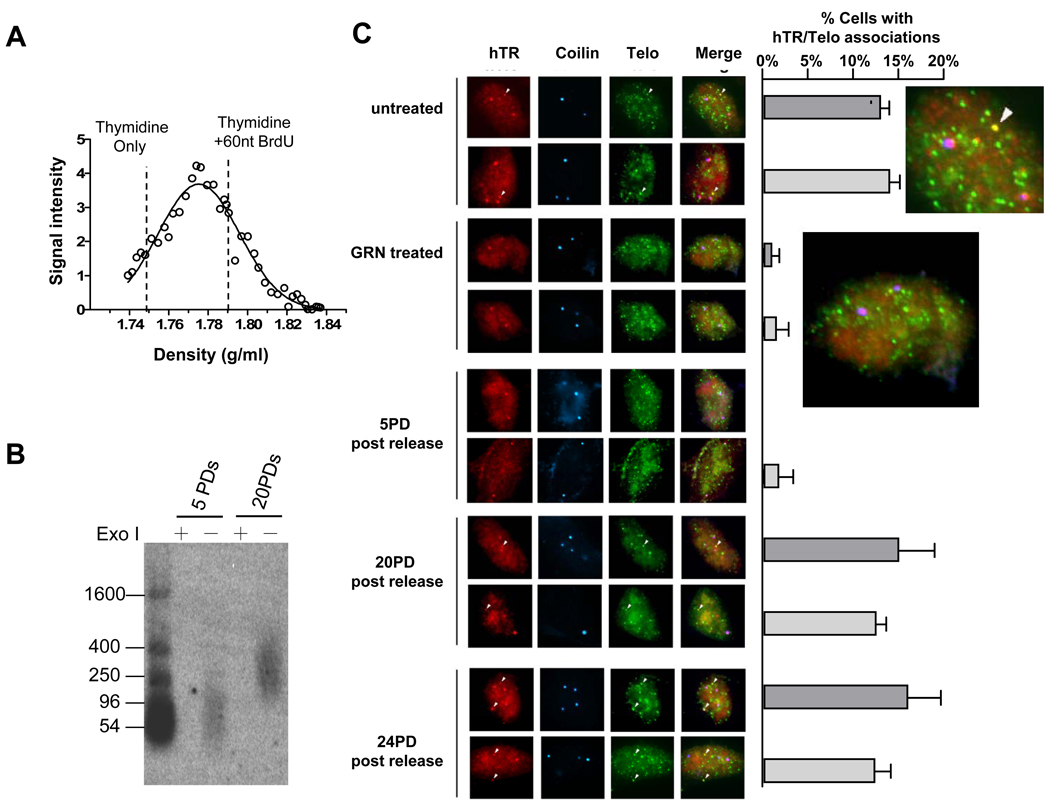
Figure 5.
Less processive extension is associated with an absence of hTR foci on telomeres. (A) Reduced telomere extension (lower density of lagging overhangs on CsCl gradient) in cells released for 5 PD from GRN163L treatment. (B) Size of lagging overhang length for the cells released from GRN163L for 5 and 20 PDs. (C) Fluorescence micrographs (left panel) showing the intra-nuclear localization of hTR (red), telomeres (green), and Cajal bodies (visualized by immunofluorescence via coilin antibodies, blue) in cells treated as indicated. Plots (right panel) show the mean percentage of cells with hTR-telomere co-localizations observed in 5–15 fields of cells ± standard error. The results of two separate experiments are shown (dark grey and light grey). See also supplementary Fig. S4.
Less processive telomerase is associated with less hTR co-localizing to telomeres
The above data was obtained 22 PD following GRN163L reversal, when telomeres were elongating. However, we did not observe telomere elongation at earlier PD (Fig 4A) despite rapidly restored telomerase activity (by 5 PDs, Fig. 4B). During this initial period telomerase extension products banded at a density of 1.775, which represents 35% BrdU substitution. Total lagging daughter overhangs were about 85 nt (Fig. 5B), thus telomerase had added only 30 nt (85×35%) to each telomere. Although we don’t know how many telomerase molecules participated in this 30 nt addition, it is clear that under these conditions telomerase is much less processive than in normal Hela cells. Since Cajal bodies are involved in the processing and positioning of telomerase at telomeres(Cristofari et al., 2007; Jady et al., 2006; Tomlinson et al., 2006) ,we examined whether changes in Cajal body or hTR positioning might be involved in this changed telomerase behavior.
Co-localization of hTR with one or more telomeres was found in ~13% of normal unsynchronized Hela cells (Fig. 5C). Treatment with GRN163L resulted in the progressive loss of hTR/telomere co-localization, dropping to ~6% after one week and ~2% after two weeks, where it remained at all times analyzed up to 12 weeks (Fig. 5C and Supplemental Fig. S4). No change was found in either the number of Cajal bodies/cell or the non-telomeric co-localization of hTR with Cajal bodies. This very low level of co-localization of hTR foci to telomeres was also present in cells newly released from GRN163L (5 PDs after release). The rapid inhibition of telomerase by GRN163L and its rapid recovery following GRN163L removal suggests that the slow change in co-localization is not directly related to the actual inhibition of telomerase by GRN163L. The number of hTR foci co-localizing with telomeres returned to normal by 20 PD, which coincides with the time that telomerase extension products changed from being short (~85) to ~200 nt (Fig. 5B) and telomeres began elongating (Fig. 4B). It has been reported that TCAB1 and TPP1 are directly involved in Cajal body mediated delivery of hTR/telomerase onto telomeres (Abreu et al., 2010; Venteicher et al., 2009). We examined the levels of TCAB1 and TPP1 during this period. TPP1 displayed no change in expression upon GRN163L treatment. There was a minor ~15% decrease of TCAB1 in early released cells and a minor ~20% increase in late released cells (Fig. S4).
DISCUSSION
Telomerase extension occurs at most telomeres during a single cell cycle in human cancer cells (Zhao et al., 2009). In this study, our data indicates that under telomere length-maintenance conditions the extension on each telomere is carried out by one molecule of telomerase that processively adds ~60 nt to the end in both H1299 and Hela cells. This demonstrates that human telomerase is processive in vivo. The mechanism of telomerase action changes when telomeres elongate (either following overexpression of hTERT or after reversing the inhibition telomerase that produced shortened telomeres). Rather than increasing the processivity of telomerase so that each molecule adds many more repeats, telomerase becomes distributive, with multiple molecules acting in succession to produce greater numbers of extensions. Based on the behavior of telomerase in yeast (Chang et al., 2007) many authors have speculated that telomerase processivity is increased on short mammalian telomeres. Our results establish this is not the case. However, multiple differences (length, rates of shortening per division, fraction of extended ends, timing of replication during S phase) make mammalian telomere length control very different from that in yeast. Our results demonstrate that processivity is not increased on very short telomeres, but that the mode of elongation changes from a processive to a distributive one. These results elucidate different mechanisms of telomerase action under steady state and non-equilibrium conditions. In a cell under non-equilibrium conditions, containing both very long or maintenance-length and some much shorter telomeres, telomerase may be extending the maintenance-length telomeres with a single processive molecule while using multiple molecules in succession to preferentially elongate the very short telomeres. Mutational analysis has identified domains that affect the processivity of telomerase in vitro, but the ability of wild-type telomerase to exhibit changes in processivity in vivo has not been previously demonstrated in mammals. We also find that the endogenous telomerase is transiently less processive after cells have been released from long-term exposure to the telomerase inhibitor GRN163L, and also that exogenous overexpressed telomerase is less processive.
Processivity and hTR foci
The reduction in processivity that occurs following the reversal of long-term GRN163L inhibition (Fig. 5A) is accompanied by changes in Cajal body-mediated trafficking of telomerase. Although no change in Cajal body number per cell or co-localization of hTR with Cajal bodies was seen, there was a large reduction in the number of hTR foci that co-localized with telomeres. The mechanism by which GRN163L produces this change is unclear, but it must be indirect since it requires several weeks while the direct inhibition of telomerase action by GRN163L in culture only requires a few days. Similar effects have been seen following depletion of TCAB1, the factor that brings telomerase to Cajal bodies. TCAB1 depleted cells show a decrease in hTR/telomere co-localization that is associated with telomere shortening (Venteicher et al., 2009). This is consistent with our observation of a decrease in telomerase processivity following a reduction in hTR-telomere co-localization, so that it is now insufficient to compensate for the rate of telomere shortening. However, an estimated 90% knockdown of TCAB1 only resulted in a 30% reduction of hTR at telomeres (Venteicher et al., 2009). Although we observed a small (~15%) decrease in TCAB1 expression in cells newly released from GRN163L and a similarly small increase (~20%) after 20 PD (Fig. S4), we do not believe these small changes are sufficient to explain the very large (6-fold) reduction of hTR at telomeres that we observed. This suggests the existence of additional factors in addition to TCAB1 that contribute to the regulation of telomerase localization to telomeres and its extension processivity.
Telomerase activity in extracts was rapidly restored within a few days of GRN163L removal but the decrease in processivity persisted for several weeks (Fig. 4B and Fig. 5A). The timing of the return to a normal processivity coincided with the time that hTR foci reappeared (Fig. 5B,C). It is unclear why delivery of hTR foci to telomeres should affect processivity. In vitro, telomerase processivity is increased by interactions with both TPP1 and Pot1 (Latrick and Cech, 2010), and TPP1 is required for hTR co-localization at telomeres (Abreu et al., 2010). However, we observed no change of the expression level of TPP1 upon treatment with GRN163L (Fig. S4), suggesting that in addition to facilitating specific TPP1/Pot1 interactions that might affect processivity, there may be posttranslational modifications, post-transcriptional modifications (hTR), conformational changes, or associations with other factors that occur in Cajal bodies that affect the processivity of the telomerase delivered to telomeres in hTR foci. These changes may be removed or altered by extraction and thus not affect the in vitro activity or processivity of telomerase in cell lysates.
The number of catalytically active telomerase molecules per tumor cell has been estimated to be less than 100 (Cohen et al., 2007), while the number of telomere ends following replication in an aneuploid cancer cell with 70–80 chromosomes is several hundred. One role for Cajal bodies may be to coordinate the successive delivery of this limited number of molecules to different telomeres during S-phase (Cristofari and Lingner, 2006; Jady et al., 2006). The decreased processivity we observed following reversal of long-term exposure to GRN163L suggests that passage through Cajal bodies and delivery to telomeres may have additional effects.
Because of its high specificity and efficiency in inhibition of telomerase activity both in vitro and in vivo, GRN163L (Imetelstat) is now in phase II clinical trials (Roth et al., 2010). If similar effects on hTR localization/processivity occur in patients, it has significant implications on dosing schedules. The requirements for achieving adequate drug levels to continuously suppress telomerase activity may be much greater than that needed to simply sustain the lack of hTR/telomere co-localization. Preclinical in vitro studies have found that telomere length rapidly recovers following removal of inhibition, suggesting that 4–5 days of telomerase inhibition followed by 2–3 days of telomere elongation would result in very little overall shortening. However, if lack of telomerase co-localization is sustained for a few extra days, recovery of telomerase activity between doses would be ineffective in elongating telomeres and the rate of shortening would only show minor differences under a less frequent dosing schedule.
Processivity and distributive action in cells overexpressing hTERT
Many molecules of telomerase successively elongate telomeres in cells soon after the overexpression of telomerase (distributive action). Inhibition by GRN163L results in the amount of extension progressively decreasing, and when a significant fraction of non-extended ends appears the amount added to the remainder is only ~40 nt compared to the ~65 nt added under length-maintenance conditions. The reasons for this decreased processivity of individual molecules remain unresolved. The accuracy of the TRAP assay and the variability of inhibition under different experimental conditions make it unreliable to distinguish whether the actual inhibition achieved by 0.5 µM GRN163L in the cells overexpressing telomerase was 75% versus 90%. If telomerase were inhibited by 75% in this experiment, the probability of a non-extended end would be 75% after one cycle of recruitment, 56% (.752) after two cycles, and 34% (.74) after 4 cycles. If one estimates that the thymidine-only peak in cells overexpressing hTERT and inhibited by 0.5 µM GRN163L (Fig. 3C) represents ~30% of the total, this would be consistent with ~4 different molecules each adding 40 nt to the ends to produce the observed 160 nt extension. Whether these multiple recruitments occur within the same time window of open configuration present before overexpression or whether the massive overexperession of the catalytic subunit is altering the open configuration by interacting with other factors is unknown. We cannot formally exclude the possibility that some of the decreased addition length results from overexpressed telomerase causing degradation of the 3’ end.
Prepositioning of Telomerase at Telomeric Overhangs
Under telomere length maintenance conditions, our data indicates that every telomere is extended by telomerase only once in both H1299 and Hela cells. This observation has important implications for the molecular mechanisms by which telomerase interacts with the protein complex at the 3’ overhang and the nature of the open configuration during which telomerase is capable of extending the end. TRF1 and TRF2 (at least GFP-fusion protein versions) appear to have telomere binding half-lives of seconds to minutes (Mattern et al., 2004), suggesting that telomerase recruited to the telomere by binding to shelterin components may not be stably bound but could be in constant flux, resulting in an increased diffusible local concentration of telomerase at the telomeres. Whether the distribution of telomerase is uniform over the telomere or biased towards the base or terminus (Vega et al., 2003) is unknown in mammals. If telomerase were simply interacting with factors at the end of the telomere based upon diffusion (from a high local concentration due to interaction with telomeric proteins or delivery of multiple telomerase molecules to the double-stranded region of the telomere by Cajal bodies (Abreu et al., 2010)), one would expect a Gaussian distribution of elongation products. A Gaussian distribution of the recruitment of diffusible telomerase to the ends could produce an average of one elongation event per telomere, but it should produce a distribution in which the average might be one but some ends would be extended by more than one molecule and others not at all. This was not observed. Thus special conditions must be present to narrow the distribution to prevent a significant fraction of either non-extended ends or ends extended several times. One possibility is that the time it takes for telomerase to add ~60 nt is long in comparison to the time of the open configuration during which telomerase could be recruited to act on the end. Under these circumstances, the open configuration would have closed before a second telomerase molecule could be recruited to act, and the ability of more than one molecule to act would be minimized. A diffusion-limited recruitment of telomerase could thus yield the observed result as long as the abundance of telomerase in the vicinity of the end of the telomere was in sufficient excess to ensure that virtually every end recruited a molecule. However, the hTERT depletion results (Fig. 2D) demonstrate that the concentration of telomerase was limiting (not in excess) in these experiments. Depletion of hTERT by only ~50% resulted in a roughly 50% decrease in the fraction of ends that were extended. A high enough local concentration of telomerase to ensure that a Gaussian distribution did not result in any detectable non-extended ends should only show extremely small changes resulting from a 50% decrease in the amount of telomerase. Because of these considerations, we propose the working hypothesis that there is a specific prepositioning step at the overhang that ensures that following the completion of replication every end both gets extended but also gets extended by only one molecule of telomerase under length-maintenance conditions. This prepositioning step could be separate from the delivery of multiple telomerase molecules to the telomere by Cajal bodies. It would not distinguish between an active telomerase molecule and one with GRN163L bound to its active site, thus the prepositioning of an inhibited telomerase molecule would prevent a second uninhibited molecule from being subsequently recruited before the open configuration closed. The probability of being able to preposition telomerase would be directly proportional to amount of telomerase present under length homeostasis.
One of several possible models for the prepositioning step is that the closed configuration would have Pot1 sequestering the end in a TRF1/2-Tin2-TPP1-Shelterin complex (Bianchi and Shore, 2008). The open configuration would be initiated by binding of the locally diffusible or a specifically transferred telomerase molecule to this terminal TPP1-Pot1, either in competition with the sequestered end or causing a conformational change that caused the sequestered end to be released. This could produce the observed result if the residency time (the time it took telomerase to elongate the telomere) were longer than the potential time of the open configuration for initiating extension, since all elongating ends would close before a second elongation event could occur. Since our data indicates that only a single molecule of telomerase elongates each end at maintenance, this would imply that telomerase is released from its TPP1-Pot1 interaction after it processively adds ~60 nt. Under length-maintenance conditions, this could provide the signal for closing the open configuration by allowing the freed Pot1 to reestablish sequestration of the end. Many other detailed hypotheses for the nature of these steps are possible, and our working model is only presented as a framework for understanding the concept of prepositioning.
Telomerase action in stem cells
It is important to know whether the mechanisms we have found for telomerase action in cancer cells also apply to stem cells. We thus performed our telomerase action assays using human Embryonic Stem cells (hES). Technical limitations restricted the assays to unsynchronized cells. Similar to what we observed in human cancer cells, telomerase extends most (70%) telomeres during a single cell cycle in hES cells and C-rich Fill-in is uncoupled from telomerase elongation of the G-rich strand (Fig. S5). Although at present we are unable to determine the extension processivity of telomerase in hES cells due to our inability to synchronize the cells without altering their pleuripotency, it appears that the general principles of telomerase regulation are shared by cancer and stem cells.
Major modes of telomerase action
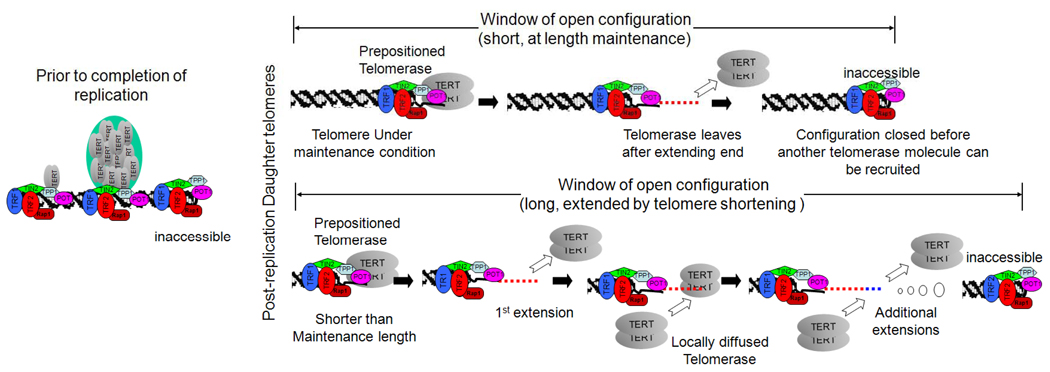
Figure 6 presents a model that summarizes the two major modes of telomerase action under homeostatic versus disequilibrium length conditions. Prior to the completion of replication, multiple telomerase molecules processed by Cajal bodies are delivered to the telomere. Whether these remain in a packaged structure or are dispersed along the length of the telomere is unknown. Passage of the replication fork and processing makes the 3’ overhang accessible for telomerase action. Under length maintenance conditions the end is extended by a single molecule of telomerase that processively adds ~60 nt to the end. A prepositioning step ensures that the frequency of elongation is not solely due to diffusion-limited interactions due to a high local concentration of telomerase but is limited to a single extension reaction before the open configuration closes. When telomeres are shorter than their maintenance size, the length of the open configuration is extended, possibly due to lesser amounts of TRF1 and TRF2 binding to the double stranded regions. Additional telomerase molecules are then able to diffuse to the overhang from high local concentrations and additional extensions take place. Thus, multiple rounds of extension could occur until the closed condition became reestablished. In addition to these two major modes of telomere elongation, we have identified two situations in which the processivity of telomerase is decreased; in cells overexpressing hTERT and following a treatment that interferes with Cajal-body delivery of hTR foci to telomeres. The detailed understanding of the mechanisms underlying all of these different ways in which telomerase acts on telomeres will greatly contribute to our knowledge of how telomerase functions in human cancer cells and what steps might be subject to manipulation for therapeutic purposes.
Figure 6.
Model for telomerase action under steady and non-equilibrium condition. A prepositioned telomerase occupies the overhang at the start of the open configuration, and the open configuration closes before a second molecule can access the end. If the open configuration window is extended, additional molecules can be recruited. See also supplementary Fig. S5.
EXPERIMENTAL PROCEDURES
Cell Culture and GRN163L treatment
Hela cervical carcinoma, H1299 lung adenocarcinoma were cultured at 37°C in 5% CO2 in 4:1 DMEM:Medium 199 containing 10% calf serum (Hyclone, Logan, UT). Hela cells with short telomeres were obtained by continuously treating cells with 2 µM GRN163L (Geron Corp., Menlo Park, CA) for about 3 months.
Cell Cycle synchronization
Hela and H1299 cells were synchronized at G1/S by double thymidine block. Briefly, exponentially growing cells were treated with 2 mM thymidine for 19 hr followed by washing with PBS (3 times). Cells were then cultured in fresh medium for 9 hr, 2 mM thymidine was added for 16 hr, followed by washing with PBS. Cells were released to pre-warmed fresh medium containing 100 µM BrdU for 4 hr, harvested and genomic DNA purified using Blood&Cell culture Midi kit (QIAGENE, Valencia CA). Cells were treated with GRN163L 3 days before synchronization started and the same concentration of GRN163L was included in all medium used for synchronization. Cells were always allowed to attach for 4 hours before GRN163L addition to avoid effects on spreading (Jackson et al., 2007).
shRNA knockdown of hTERT
The retroviral shRNA for human hTERT was generous gift from Dr. Hahn (Masutomi et al., 2003). 15 µg of proviral hTERT shRNA plasmid was cotransfected into 293FT cells with 7.5 µg each of the packaging plasmids pPAX2 and pMD2G using calcium phosphate. Viral supernatants were collected at 24–48, 48–72 and 72–96 hrs, filtered, and used to infect Hela cells.
Detection of expression level of hTERT mRNA
Total RNA was prepared using the RNeasy kit (Qiagen ) according to the manufacturer’s instructions. RNA was reverse transcribed to cDNA by using First-strand cDNA kit (Roche). All Real-Time PCR reactions were performed in a 20 µl mixture containing cDNA preparation, 2× Reaction Buffer (Roche), 4 mM MgCl2, 1 µM of hTERT primers (Forward 5’-GGGGTCACTCAGGACAGC-3’ and Reverse 5’-TCTTGAAGTCTGAGGGCAGTG-3’), 0.2 mM dNTPs mix and 0.1 µl of Universal probe #37. GAPDH was used as internal control for all experiments. The CT value from each template was calculated using the Roche 480 software. The Roche 480 software calculated crossing points (CT) based on the first maximum of the second derivative of the amplification curve of template. Relative expression level of hTERT mRNA was calibrated by GAPDH mRNA to minimize experimental variation.
Overexpression of hTERT into Hela cells
Hela cells were infected with pBabeHygro virus containing the hTERT cDNA. hTERT overexpression was confirmed by PCR and TRAP assay.
Lagging Daughter Overhang Analysis
CsCl gradient separation of leading versus lagging telomeres was performed as described (Zhao et al., 2009). The DSN (Duplex Specific Nuclease)assay on lagging daughters was performed as described (Zhao et al., 2008) with minor modification. Briefly, purified lagging telomere DNA was digested with DSN at 37°C for 2 hr. Overhangs were resolved on 1% denature agarose gel followed by transfer to Hybond-XL+ membrane using capillary transfer. The membrane was air-dried and DNA was fixed by UV cross-linking. A high specific activity C-rich probe (Herbert et al., 2003) was hybridized to the G-rich overhangs at 42 °C.
Estimation of Telomerase Activity
The telomeric repeat amplification protocol (TRAP) was used to measure the telomerase activity(Shay and Bacchetti, 1997). Samples were resolved by PAGE and scanned using a Typhoon PhosphorImager scanner system (Molecular Dynamics, GE Healthcare, Piscataway, NJ). The telomerase products (6 bp ladder) and the 36 bp internal control (IC) bands were quantified using the Imagequant software provided by manufacturer. Relative telomerase activity was calculated as the intensity ratio of the TRAP ladder to that of the IC band.
Telomere Length Measurement
The average length of telomeres were measured as described (Zhao et al., 2009).
Fluorescence in situ hybridization (FISH) and Immunofluorescence (IF) Detection of hTR, Telomeres and Cajal Bodies
Detection of hTR and telomeres via FISH was performed essentially as described (Tomlinson et al., 2006). Briefly, 25 ng/coverslip of each of three Cy3 conjugated DNA probes complementary to different regions of human telomerase RNA (17) and 20 ng/coverslip of an Oregon Green conjugated telomere DNA probe (22) was used. Following FISH, Cajal bodies were detected by IF with the primary antibodies against the Cajal-body marker protein, coilin and Cy5 conjugated secondary antibody (Jackson ImmunoResearch laboratories, West Grove, PA) as described (Tomlinson et al., 2006). Cells were mounted in Prolong Gold (Invitrogen) and microscopy was performed using a Zeiss Axioskop 2 Mot Plus fluorescence microscope (Carl Zeiss Microimaging, Thornwood, NY). Images were acquired at 63x (Plan Apochromat objectives, numerical aperture 1.4) using a cooled charge-coupled device ORCA-ER digital camera (Hamamatsu photonics, Bridgewater, NJ) and IPLab Spectrum software (BioVision Technologies, Inc., Exton, PA.)
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by AG01228 from the National Institute on Aging (WEW), CA104676 from the National Cancer Institute (MPT and RMT), the American Federation for Aging Research (YZ) and a Ruth L. Kirschstein National Research Service Award (F31GM087949) for Individual Predoctoral Fellows from the National Institutes of Health (EA). We thank Prof. Songyang Zhou from Baylor College of Medicine for providing the pCMV-TPP1 plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental data including five figures can be found online.
REFERENCES
- Abreu E, Aritonovska E, Reichenbach P, Cristofari G, Culp B, Terns RM, Lingner J, Terns MP. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol. 2010;30:2971–2982. doi: 10.1128/MCB.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Shore D. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol Cell. 2008;31:153–165. doi: 10.1016/j.molcel.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Dunham MA, Reddel RR. Telomere length dynamics in telomerase-positive immortal human cell populations. Exp Cell Res. 1998;239:370–378. doi: 10.1006/excr.1997.3907. [DOI] [PubMed] [Google Scholar]
- Chang M, Arneric M, Lingner J. Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 2007;21:2485–2494. doi: 10.1101/gad.1588807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Greider CW. Determinants in mammalian telomerase RNA that mediate enzyme processivity and cross-species incompatibility. EMBO J. 2003;22:304–314. doi: 10.1093/emboj/cdg024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- Cristofari G, Adolf E, Reichenbach P, Sikora K, Terns RM, Terns MP, Lingner J. Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Mol Cell. 2007;27:882–889. doi: 10.1016/j.molcel.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Forsythe HL, Jarvis JL, Turner JW, Elmore LW, Holt SE. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J Biol Chem. 2001;276:15571–15574. doi: 10.1074/jbc.C100055200. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci U S A. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW, Gryaznov SM. Lipid modification of GRN163, an N3'-->P5' thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24:5262–5268. doi: 10.1038/sj.onc.1208760. [DOI] [PubMed] [Google Scholar]
- Herbert BS, Pongracz K, Shay JW, Gryaznov SM. Oligonucleotide N3'-->P5' phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002;21:638–642. doi: 10.1038/sj.onc.1205064. [DOI] [PubMed] [Google Scholar]
- Herbert BS, Shay JW, Wright WE. Analysis of telomeres and telomerase. Curr Protoc Cell Biol. 2003 doi: 10.1002/0471143030.cb1806s20. Chapter 18 Unit 18 16. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Zhu CH, Paulson V, Watkins L, Dikmen ZG, Gryaznov SM, Wright WE, Shay JW. Antiadhesive effects of GRN163L--an oligonucleotide N3'->P5' thio-phosphoramidate targeting telomerase. Cancer Res. 2007;67:1121–1129. doi: 10.1158/0008-5472.CAN-06-2306. [DOI] [PubMed] [Google Scholar]
- Jady BE, Richard P, Bertrand E, Kiss T. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol Biol Cell. 2006;17:944–954. doi: 10.1091/mbc.E05-09-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Latrick CM, Cech TR. POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 2010;29:924–933. doi: 10.1038/emboj.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA, et al. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- Mattern KA, Swiggers SJ, Nigg AL, Lowenberg B, Houtsmuller AB, Zijlmans JM. Dynamics of protein binding to telomeres in living cells: implications for telomere structure and function. Mol Cell Biol. 2004;24:5587–5594. doi: 10.1128/MCB.24.12.5587-5594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney PA, Aisner DL, Frank BC, Wright WE, Shay JW. Telomere dynamics in cells with introduced telomerase: a rapid assay for telomerase activity on telomeres. Mol Cell Biol Res Commun. 2000;3:312–318. doi: 10.1006/mcbr.2000.0229. [DOI] [PubMed] [Google Scholar]
- Ouellette MM, Liao M, Herbert BS, Johnson M, Holt SE, Liss HS, Shay JW, Wright WE. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Roth A, Harley CB, Baerlocher GM. Imetelstat (GRN163L)--telomerase-based cancer therapy. Recent Results Cancer Res. 2010;184:221–234. doi: 10.1007/978-3-642-01222-8_16. [DOI] [PubMed] [Google Scholar]
- Samper E, Flores JM, Blasco MA. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep. 2001;2:800–807. doi: 10.1093/embo-reports/kve174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell. 2004;117:323–335. doi: 10.1016/s0092-8674(04)00334-4. [DOI] [PubMed] [Google Scholar]
- Tomlinson RL, Li J, Culp BR, Terns RM, Terns MP. A Cajal body-independent pathway for telomerase trafficking in mice. Exp Cell Res. 2010 doi: 10.1016/j.yexcr.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol Biol Cell. 2006;17:955–965. doi: 10.1091/mbc.E05-09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega LR, Mateyak MK, Zakian VA. Getting to the end: telomerase access in yeast and humans. Nat Rev Mol Cell Biol. 2003;4:948–959. doi: 10.1038/nrm1256. [DOI] [PubMed] [Google Scholar]
- Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, Terns MP, Artandi SE. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hoshiyama H, Shay JW, Wright WE. Quantitative telomeric overhang determination using a double-strand specific nuclease. Nucleic Acids Res. 2008;36:e14. doi: 10.1093/nar/gkm1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW, Wright WE. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell. 2009;138:463–475. doi: 10.1016/j.cell.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.