One of the unfortunate side consequences of evidence-based medicine (EBM) is that sometimes there is no escaping how small the benefit of any individual treatment may seem. In the “days of the giants”, doctors saved lives; now we can consult league tables that bloodlessly inform us how many patients—10, 50 or 200—must receive a treatment to avert even a single bad outcome1, EBM’s venerable number needed to treat (NNT).
The NNT is, of course, based only on average treatment effects. A limitation of EBM is that we study groups of patients, while we treat individuals. This mismatch can be partially addressed by the use of statistical models to calculate the important risks that determine a more “personalized” probability of benefit. When this is done, it turns out, the degree of benefit for an intervention in any typical patient is often even less than the overall average2. This is because benefits are determined by the baseline risk, which is usually highly skewed, such that a few high-risk patients may account for most of the benefit, while most patients have baseline risks (and therefore potential benefit) considerably less than the average3. This is true particularly when the overall risk of the outcome of interest is low, since there is a lower bound for risk at 0%, creating a “floor effect”.
In this issue of Circulation: Cardiovascular Quality and Outcomes, Sussman et al4 describe the “personalized” risks and benefits of aspirin therapy for primary prevention of cardiovascular diseases, a rather extreme exemplar of the pattern described above. NNT to prevent a single ischemic event for any typical patient in a primary prevention population can easily exceed 1000 per year, but may vary substantially depending on individualized risk.
Because the benefits of aspirin are small in the primary prevention setting, and the risks of bleeding are non-negligible—especially for older patients who have the most to gain—not only do individual benefits vary depending on personalized risks of cardiovascular disease (CVD) and personalized risk of bleeding but, the authors show, that what constitutes a rational decision for most patients (i.e. maximizes utility) is highly sensitive to patient preferences. Their main finding is that for many patients the potential net benefit for primary prevention is so small that they should not bother to take an aspirin if aspirin-taking is, to them, a bother. Only about 1/3 of patients between age 35 and 80 eligible for primary prevention seem likely to obtain sufficient benefit to warrant the therapy when taking an aspirin has a disutility of 1%.
It is widely recognized that the benefits of aspirin for primary prevention are dependent on a patient’s baseline risk of CVD, and the major guidelines have incorporated risk estimation in a way that is broadly consistent with the analysis presented by Sussman et al5, 6. However, because Sussman et al explicitly consider that aspirin-taking might be associated with some variable patient-specific disutility independent of bleeding complications, their main conclusion is that a single threshold is inappropriate. Rather, to decide on whether or not a patient should take an aspirin requires a robust discussion of its benefits and harms, including not only individualized risk assessment for CVD and for major bleeding complications, but also the elicitation of patient preferences.
The idea that patients should be involved in their medical decisions may not seem radical. However, clinicians often find the prospect of involving patients in shared decision making during a short primary care visit daunting. The clinicians are often challenged in their ability both to readily estimate risk and to discuss probabilities with patients. To overcome this, risk calculators have been developed to estimate individualized risk in real time (including several different calculators for CVD risk7, 8, 9). The information can then be used in decision aids-- electronic or paper-based--that help communicate the individualized risks and benefits in a clear way that permits the patient to make an informed decision. A key component in this paradigm is the willingness of both patients and clinicians to actively engage in the conversation; for the patients to express their preferences and for the clinician to respect them. Previous studies of shared decision making using personalized risk have shown that involving patients improves knowledge, medication adherence, and satisfaction10, 11.
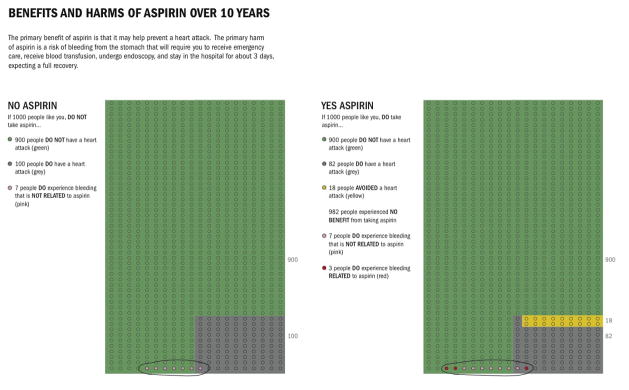
Recent literature suggests that using graphical displays and natural frequencies that show the population and how many will be affected and not affected by the outcome with and without treatment is a successful method of risk communication12, 13. These methods reduce the impact of framing, whereas graphs that show only the numerator appear to inflate the perceived risk and may induce risk-averse behavior. For example, the Figure shows how a graphical display using natural frequencies might be presented to patients with a 10-year cardiovascular risk of 10%. The decision aid to present the benefits of aspirin can also be used to present the risk of aspirin from bleeding. Thus patients can weigh the tradeoffs and make an informed decision.
Figure.
Benefits and Harms of Asprin Over 10 Years
In this case, the clinician can present the information as follows:
“Of 1000 people like you, 100 will and 900 will not experience a heart attack in the next 10 years and about 7 will have a stomach bleed. If all 1000 decided to take aspirin, those 900 not destined to have a heart attack will not get one; 18 of those 100 destined to have a heart attack will now avoid one thanks to the aspirin, and 82 will experience a heart attack in the next 10 years, despite using aspirin regularly, also now 10 will have a stomach bleed, 3 of which occur because of the aspirin.”
This framing allows the patients to consider the absolute risks and benefits of initiating aspirin, deliberate and come to a shared decision with their provider that best reflects their values and preferences.
Back in the real world, the prospects for such shared decision making do not seem especially bright given past performance. Patients are often misinformed about the risks and benefits of therapy and, in particular, often grossly overestimate the benefits of treatments14, 15, 16, 17. For example, among patients undergoing percutaneous coronary intervention (PCI) for non-acute coronary artery disease, the vast majority of patients are convinced that the procedure is likely to save their life or prevent a heart attack18, despite evidence that it does not19. If patients are so ill informed about a major medical procedure that requires signed informed consent, then what hope do we have about the even more common decisions made in the primary care setting, like whether to take a baby aspirin?
Consider too what the incentives are. Previous work has shown that incorporating decision aids within a routine clinical encounter increases the visit length by about 3–4 minutes20. While this has also been shown to lead to a more satisfying encounter, for both the patient and the physician,10, 21 such non-reimbursable discussions over routine medical decisions would clearly hamper throughput. Indeed, physicians may have a financial stake in having misinformed patients who systematically over-estimate treatment benefits. This is obviously true for invasive procedures such as PCI, where better informed patients might more frequently opt for medical management, but also true when quality measures reward low benefit treatments that a fully informed and wholly rational patient might just as well decide to forgo. For many patients in whom the individualized risks and benefits of a therapy are similar, quality indicators based on overall population-wide benefits that measure the proportion of patients on a given therapy can incentivize either excluding the patient from decision making altogether or engaging in arm-twisting rather than the deliberative process described above.
While EBM may seem designed to move the practice of medicine away from the caprice of physician judgment to the unambiguous authority of data, for many clinical decisions the data unambiguously tell us that there is no clearly best decision and it turns out that the ultimate authority is—by necessity—often the patients themselves; that is, particularly where benefits are small (or the balance of risk and benefits is highly dependent on optimal adherence), the main determinant of the best clinical decision are the values and preferences of the patient considered in the presence of adequate knowledge transfer. Moving away from population-based results to individualized decision making requires a change in culture and a change in clinician incentives, including the development of performance measures that encourage shared decision making. In doing so, it is critical to recognize that for many decisions—e.g. whether a 47 year old woman should get a mammogram, whether a 80 year old man with a Hgb A1C of 8.2 should be prescribed an additional agent, whether a 77 year old man without risk factors who had a second colonoscopy 10 years ago should get a third colonoscopy, and whether a patient should take an aspirin for primary prevention—the evidence tells us that the actual outcome of the conversation may be less important than the quality of the discussion.
Acknowledgments
This work was partially funded by grants UL1 RR025752 and R01 NS062153, both from the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: None
Reference List
- 1.Table of NNTs. [Accessed March 23, 2011];Bandolier Journal. 1998 April 1; Available at: URL: http://www.medicine.ox.ac.uk/bandolier/band50/b50-8.html.
- 2.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007 September 12;298(10):1209–12. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 3.Ioannidis JP, Lau J. The impact of high-risk patients on the results of clinical trials. J Clin Epidemiol. 1997 October;50(10):1089–98. doi: 10.1016/s0895-4356(97)00149-2. [DOI] [PubMed] [Google Scholar]
- 4.Sussman JB, Vijan S, Choi H, Hayward RA. Individual and population benefits of daily aspirin therapy: A proposal for personalizing national guidelines. Circulation: Cardiovascular Quality and Outcomes. 2011 doi: 10.1161/CIRCOUTCOMES.110.959239. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009 March 17;150(6):396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 6.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002 July 16;106(3):388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 7.ARIC CHD Risk Calculator. [Accessed March 23, 2011.];Atherosclerosis Risk in Communities Study. 2011 Available at: URL: http://www.aricnews.net/riskcalc/html/RC1.html.
- 8.Framingham Risk Score. [AccessedMarch 23, 2011];Framingham Heart Study. 2011 Available at: URL: http://hp2010.nhlbihin.net/atpiii/calculator.asp?usertype=prof.
- 9.Ridker PM, Buring JE, Rifai N, Cook NR. [AccessedMarch 23, 2011];Reynold's Risk Score. 2011 Available at: URL: http://www.reynoldsriskscore.org/
- 10.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007 May 28;167(10):1076–82. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 11.Montori VM, Shah ND, Pencille LJ, Branda ME, Van Houten HK, Swiglo BA, Kesman RL, Tulledge-Scheitel SM, Jaeger TM, Johnson RE, Bartel GA, Melton LJ, Wermers RA. Use of a decision aid to improve treatment decision in osteoporosis. The OSTEOPOROSIS CHOICE randomized trial. American journal of medicine. 2011 doi: 10.1016/j.amjmed.2011.01.013. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Ancker JS, Senathirajah Y, Kukafka R, Starren JB. Design features of graphs in health risk communication: a systematic review. J Am Med Inform Assoc. 2006 November;13(6):608–18. doi: 10.1197/jamia.M2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carling CL, Kristoffersen DT, Montori VM, et al. The effect of alternative summary statistics for communicating risk reduction on decisions about taking statins: a randomized trial. PLoS Med. 2009 August;6(8):e1000134. doi: 10.1371/journal.pmed.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel CA, Levy LC, Mackenzie TA, Sands BE. Patient perceptions of the risks and benefits of infliximab for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1–6. doi: 10.1002/ibd.20283. [DOI] [PubMed] [Google Scholar]
- 15.Habib SB, Sonoda L, See TC, Ell PJ, Groves AM. How do patients perceive the benefits and risks of peripheral angioplasty? Implications for informed consent. J Vasc Interv Radiol. 2008;19:177–81. doi: 10.1016/j.jvir.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16:515–21. doi: 10.1200/JCO.1998.16.2.515. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer D, Egleston B. How patients with diabetes perceive their risk for major complications. Eff Clin Pract. 2000 Jan–Feb;3(1):7–15. [PubMed] [Google Scholar]
- 18.Rothberg MB, Sivalingam SK, Ashraf J, et al. Patients' and cardiologists' perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med. 2010 September 7;153(5):307–13. doi: 10.7326/0003-4819-153-5-201009070-00005. [DOI] [PubMed] [Google Scholar]
- 19.Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous Coronary Interventions for Nonacute Coronary Artery Disease: A Quantitative Twenty-Year Synopsis. Lancet. 2009 March 14;373(9667):911–8. doi: 10.1016/S0140-6736(09)60319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nannenga MR, Montori VM, Weymiller AJ, et al. A treatment decision aid may increase patient trust in the diabetes specialist. The Statin Choice randomized trial. Health Expect. 2009 March;12(1):38–44. doi: 10.1111/j.1369-7625.2008.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009 September 28;169(17):1560–8. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]