Abstract
When devising methods to sample Aedes aegypti (L.) eggs from naturally-occurring containers to investigate selective oviposition, failure to take into account certain aspects of Ae. aegypti behavior can bias study inferences. In Iquitos, Peru, we tested three assumptions related to designing Ae. aegypti oviposition field studies, as follows: 1) lining containers with paper as an oviposition substrate does not affect oviposition; 2) diurnal egg-laying activity peaks in the late afternoon or early evening, and there is little oviposition during midday; and 3) the gonotrophic cycle length of wild females averages from 3 to 4 d. When wild females were presented with containers lined and unlined with paper toweling, the presence of paper increased oviposition in plastic and metal containers, but had no effect in cement containers. Recording the number of eggs laid by Ae. aegypti every 2 h throughout the day delineated a bimodal diurnal oviposition pattern, with a small morning peak, decreased activity during midday, and a predominant peak in the late afternoon and evening from 16:00 to 20:00 h. Daily monitoring of captive individual F0 females revealed that the gonotrophic cycle length was typically 3– 4 d for the Iquitos population. These findings will be used to adjust field study design to 1) account for sampling eggs using paper toweling, and 2) determine the time of day and number of days over which to sample Ae. aegypti eggs. We explored how failure to consider these behaviors could potentially bias field assessments of oviposition preferences.
Keywords: Aedes aegypti, oviposition, sampling bias, vector control
Current Aedes aegypti (L.) surveillance and control practices rely on several assumptions regarding the mosquito’s oviposition behavior. For example, sensitivity of ovitraps for detecting Ae. aegypti presence and efficacy of lethal ovitraps for reducing adult populations may be overestimated if female preference for alternative containers is greater than anticipated (Sithiprasasna et al. 2003, Harrington et al. 2008). Similarly, targeted source reduction is based on the assumption that females egg-saturate all available oviposition sites, such that removal of a portion of containers will result in a net reduction in the adult population (Focks and Alexander 2006). If oviposition sites are not egg saturated, targeted container removal may encourage females to oviposit in previously unoccupied sites, so that less than expected population reduction will be achieved. Because of the central importance of oviposition site selection for Ae. aegypti surveillance and dengue prevention strategies, detailed studies are warranted on where wild females deposit their eggs and how they adapt to targeted control measures that aim to reduce without eliminating all oviposition containers.
To infer mosquito egg-laying choices, egg counts typically are compared among oviposition sites differing in a variety of biotic (Blaustein and Kotler 1993, Kiflawi et al. 2003) and/or abiotic attributes (Heard 1994, Harrington et al. 2008). To avoid potential biases, designing a program to sample Ae. aegypti eggs from naturally-occurring containers in the field requires consideration of multiple facets of oviposition behavior. First, egg collection techniques should ideally have no impact on oviposition. If collection techniques differentially affect oviposition rates depending on container type, such effects should be quantified and accounted for when applied to field populations. Second, to minimize disturbing and potentially altering behavior of egg-laying females, eggs should be collected from containers during a time of low diurnal oviposition activity. Third, to obtain representative data on oviposition choices, eggs should be collected on several continuous days for a period equivalent to the average gonotrophic cycle length. Because Ae. aegypti exhibit overlapping generations (Southwood et al. 1972), a shorter sampling period may result in some female preferences being missed, whereas a longer sampling period may cause certain females to be overrepresented.
Collecting Aedes mosquito eggs directly from natural and artificial containers is difficult to achieve without destroying the eggs or oviposition sites. Lining the inside surface of containers with seed germination paper has proven to be an effective method to collect eggs in the field with minimal disturbance to oviposition sites (Steinly et al. 1991, Harrington et al. 2008). Whether the presence of paper affects oviposition rates in different container types has yet to be documented despite evidence from laboratory and field studies that egg-laying Ae. aegypti are influenced by substrate texture (O’Gower 1963, Fay and Perry 1965, Chadee et al. 1995).
Although diurnal Ae. aegypti oviposition activity previously has been investigated, variation between the laboratory and field, as well as among different field populations, makes predicting Ae. aegypti behavior difficult for a specific location. Early laboratory experiments demonstrated that Ae. aegypti oviposition followed a regular circadian rhythm, with low activity throughout the day leading to a clearly defined peak in the late afternoon (Haddow and Gillett 1957). In Kenya, McClelland (1968) observed a bimodal pattern with a small morning peak, low activity during midday, and a predominant peak in late afternoon and early evening. In Trinidad, West Indies, Chadee and Corbet (1987) reported different diurnal oviposition periodicities depending on the season. A pattern consistent with that seen in Kenya emerged during the wet season, whereas during the dry season, oviposition occurred throughout the day, gradually leading to a peak in late afternoon. This trend of oviposition throughout the day, culminating in a late afternoon or evening peak, was also observed in Thailand by Harrington et al. (2008) irrespective of season.
Variation has been reported in the duration of Ae. aegypti’s gonotrophic cycle. Laboratory observations (Christophers 1960), as well as findings from mark-release-recapture studies in Thailand (Sheppard et al. 1969, Pant and Yasuno 1973) and Tanzania (McClelland and Conway 1971), indicated that females require 3– 4 d to mature a batch of eggs after feeding on blood. In contrast, a more prolonged gonotrophic cycle from 3 to 7 d was estimated by mark-release-recapture in Puerto Rico (Morrison et al. 1999). Because physiological processes in Ae. aegypti are dependent on temperature and relative humidity (Clements 1992, Focks et al. 1993), inconsistencies in behavior may be related to meteorological differences among locations and/or seasons.
The current study was carried out to test the three following assumptions regarding Ae. aegypti oviposition behavior in preparation for designing an egg-sampling research program: 1) lining containers with strips of paper does not differentially affect oviposition; 2) diurnal egg-laying activity peaks in the late afternoon and/or early evening, and there is little oviposition during midday; and 3) gonotrophic cycle length of wild females averages 3– 4 d. In preparation for a larger subsequent oviposition study, we discuss how failure to account for the behaviors that underlie these assumptions could potentially bias a field assessment of oviposition preferences.
Materials and Methods
Study Location
Our study was conducted in the city of Iquitos (73.2°W, 3.7°S, 120 m above sea level), along the banks of the Amazon River in the Department of Loreto, Northeastern Peru. Iquitos is an isolated city of ≈380,000 people and has been described in detail in prior studies (Getis et al. 2003, Schneider et al. 2004, Morrison et al. 2010).
Meteorological and Astronomical Data
For the days on which experiments took place, daily air temperature, relative humidity, and rainfall data were attained from a National Oceanic and Atmospheric Administration meteorological station located at the Iquitos airport ~6 km from the city center (National Climatic Data Center, United States Department of Commerce 2009). Timings of sunrise and sunset in Iquitos throughout the year were obtained from the Naval Oceanography Portal website (United States Naval Observatory 2009).
Substrate Preference
Textured brown paper towel (Kimberly-Clark, Dallas, TX) was used in our study rather than seed germination paper for practical reasons; it was easier to cut and less expensive. To test the impact of paper toweling on oviposition, we compared counts of eggs laid per day in containers made of plastic, metal, and cement that were lined or not lined with strips of paper towel. Plastic (buckets, tubs, bowls, cups, etc.) and metal (55-gallon drums, pots, pans, paint cans, etc.) were the first and second most abundant container materials in Iquitos, respectively (Morrison et al. 2004). Although plastic and metal containers varied widely in size and shape, we elected to use plastic buckets (27 cm diameter × 28 cm height) and metal pots (31.5 cm diameter × 32 cm height) because they were present in most Iquitos homes. Cement containers, while not as common, were also of interest because they tended to be large tanks with the potential to produce many immature Ae. aegypti. To maintain consistency in container size and shape in this experiment, we used metal pots that we lined on the inside with cement (27.5 cm diameter × 28.5 cm height). Eggs were collected daily at a cemetery over 34 continuous days during August and September 2007 and in the open courtyard of our field laboratory for 26 continuous days during September and October 2007. Our field laboratory, a house located in the central part of the city, was typical of an Iquitos household in that adult Ae. aegypti were consistently present.
At each location, two blue plastic buckets, two gray metal pots, and two gray cement-lined metal pots were set out and filled to one-third capacity with tap water. Containers of the same material were paired 0.5 m apart, and the distance between container pairs was ≈1 m. All six containers were set in close proximity in an attempt to standardize microclimate conditions (temperature, relative humidity, solar exposure, etc.) and to reduce the effects of container placement on oviposition. One container of each pair was randomly selected to be lined with a strip of paper towel. Halfway through the experiment, the paper treatment was switched and the alternate container was lined to identify any effect of container position.
Containers were checked for eggs daily between 09:00 and 11:00 h. If eggs were present in lined containers, the paper was exchanged. Papers with eggs were dried, and eggs were counted under a dissecting microscope at ×20 magnification. A subset of eggs was hatched once per week, and individuals were reared to adults to confirm their identity as Ae. aegypti. For containers without paper, eggs were counted on the inside surface of the container using a flashlight and magnifying glass. The count was repeated in unlined containers until the same number was confirmed a second time. Eggs were then wiped off the inside surface of plastic and metal containers with a paper towel or scrubbed off cement containers with a toothbrush. Containers were cleaned and water changed every 2–3 d to minimize differences in water quality.
These data were analyzed with a generalized estimation equation to account for nonnormality in egg counts and autocorrelation among observations from the same container using the geepack package in R version 2.8.1 (Halekoh et al. 2006, R Development Core Team 2008). An autoregressive association matrix was used because observations were made at regular 24-h intervals (Zuur et al. 2009). The main effects of container material (plastic, metal, or cement), paper, location (cemetery or field laboratory), position (which container in pair was lined with paper), and time, as well as the interaction between container material and paper, were examined. A Wald test was applied to identify nonsignificant predictor variables. To validate the final model, Pearson residuals were plotted against fitted values and all variables.
Diurnal Oviposition Periodicity
Timing of oviposition by wild Ae. aegypti was recorded for 6 d in July and 10 d in August 2007, and for 6 d in May 2009. In the open courtyard of the field laboratory, five black plastic tubs (40 cm diameter × 20 cm height) were set out, filled to two-thirds capacity with tap water, and lined with strips of brown paper towel to collect eggs. Egg laying was monitored outdoors because containers in Iquitos are far more likely to be colonized by Ae. aegypti if located outside than inside (Morrison et al. 2004).
Because female Ae. aegypti lay the majority of their eggs during the day (Haddow and Gillett 1957, McClelland 1968, Chadee and Corbet 1987, Harrington et al. 2008), we recorded the number of eggs laid in all five tubs at 2-h intervals from 06:00 to 22:00 h. Eggs present at 06:00 h were laid between 22:00 h the previous evening and 06:00 h. If eggs were found, the paper liner was changed and eggs counted, as described above.
Data were analyzed separately by year. Egg counts were summed across all five tubs at each collection point. The arithmetic mean was calculated over replicate days to obtain a measure of central tendency for the number of eggs laid during each 2-h period. Total egg counts for each 2-h period were analyzed by χ2 test to determine whether females exhibited periodicity in egg laying.
Gonotrophic Cycle Length
F0 generation Ae. aegypti were individually monitored for up to 21 d to determine length of the gonotrophic cycle. Ten females collected from the field as pupae were followed in September 2007. Because the number of field-collected pupae was limiting, 35 females reared in the laboratory from field-collected eggs were followed in October 2007.
Field-collected eggs were allowed to embryonate in a moist chamber for 48 h and then hatched by immersion in hay infusion overnight (Munstermann 1997). The next morning, larvae were transferred at a density of 100 larvae/L to 1-liter plastic containers filled with bottled drinking water. Because tap water in Iquitos contains microorganisms, bottled drinking water was used to standardize all mosquito-rearing procedures. Larvae were fed a 1:1 mixture of fish meal and wheat flour (days 0 and 1, 20 mg/liter; day 2, 30 mg; day 3, 40 mg; days 4 and 5, 60 mg). Larvae were reared indoors and exposed to ambient conditions, that is, natural light and temperature.
Field-collected and laboratory-reared pupae were transferred to individual plastic vials (2.5 cm diameter × 7 cm height) filled to one-half capacity with bottled drinking water for emergence in the laboratory. Within 12 h of emergence, adult females were mouth aspirated into individual 1-pint paper cartons with mesh lids. Each carton contained an unmated adult male that had emerged 2 d prior and a small plastic cup (5 cm diameter × 6 cm height) filled to two-thirds capacity with tap water and lined with brown paper towel for oviposition. Each carton was covered with a wet paper towel and an inverted plastic bag to maintain high humidity and kept in a shaded area in the field laboratory courtyard.
Females were offered human blood from an arm placed on top of the mesh lid for 10 min daily between 15:00 and 17:00 h and then visually examined for engorgement. Because wild female Ae. aegypti living in close association with humans feed frequently on human blood and rarely on plant carbohydrates (Edman et al. 1992, Van Handel et al. 1994, Scott et al. 2000a), mosquitoes were not given access to sugar. Cups were inspected daily for eggs before blood feeding the females. If eggs were present, mosquitoes were temporarily transferred to an empty carton by mouth aspirator while the paper was exchanged. Number of eggs, blood feeding, and mortality were recorded daily.
Female Ae. aegypti often take multiple blood meals before developing a batch of eggs (McClelland and Conway 1971, Scott et al. 1993) and may lay their eggs from a single batch in installments over several days (Christophers 1960), which makes it difficult to clearly distinguish when one gonotrophic cycle ends and the next begins. In this study, we defined the gonotrophic cycle as starting with the initial blood meal, or the first postoviposition blood meal, and ending with the last installment of the resulting egg batch. Eggs laid on consecutive days were considered installments of the same egg batch and attributed to the same gonotrophic cycle (Gillett 1962). Eggs laid more than 1 d apart with an intervening blood meal were attributed to separate gonotrophic cycles. Females that died without laying any eggs were excluded from analyses.
Because data on gonotrophic cycle length could not be transformed to meet the assumption of normality, they were rank transformed and analyzed by repeated measures analysis of variance (ANOVA) for effects of female origin or gonotrophic cycle number using PROC MIXED in SAS 9.2 (SAS Institute 2008). Not all females completed the same number of gonotrophic cycles, so repeated measures ANOVA was carried out on rank-transformed data (Conover 1999) as a means to generalize the Friedman test to an unbalanced repeated measures design.
The University of California, Davis, Institutional Review Board determined that this experiment did not meet the criteria for human subject research, and thus, did not require human subjects approval.
Results
Meteorology and Astronomy
Average minimum and maximum daily temperature, mean temperature, and mean relative humidity remained consistent in Iquitos among study periods (Table 1). Average daily rainfall ranged from 3.2 ± 3.8 mm during our observations on oviposition periodicity in May 2009 to 13.2 ± 29.1 mm when we monitored gonotrophic cycle length in September 2007. More comprehensive climate data for Iquitos during the years 2007 and 2009 are presented in Supplemental Materials (Table S1).
Table 1.
Daily air temperature, relative humidity, and rainfall in Iquitos, Peru, during study periods
| Experiment | Avg min. daily temp °C ± SD | Avg max. daily temp °C ± SD | Mean temp °C ± SD | Mean RH % ± SD | Avg daily rainfall mm ± SD |
|---|---|---|---|---|---|
| Substrate preference | |||||
| Aug.–Sept. 2007 (34 d) | 21.7 ± 0.6 | 32.6 ± 1.6 | 25.8 ± 1.0 | 80.2 ± 4.0 | 11.9 ± 25.9 |
| Sept.–Oct 2007 (26 d) | 21.9 ± 0.6 | 33.2 ± 1.4 | 26.2 ± 0.8 | 79.4 ± 3.6 | 12.2 ± 29.7 |
| Periodicity | |||||
| July 2007 (6 d) | 20.1 ± 2.6 | 31.8 ± 2.6 | 24.6 ± 1.8 | 80.7 ± 6.0 | 11.2 ± 17.9 |
| Aug. 2007 (10 d) | 21.1 ± 1.3 | 32.3 ± 0.9 | 25.7 ± 0.7 | 79.4 ± 4.2 | 5.1 ± 8.4 |
| May 2009 (6 d) | 22.2 ± 0.7 | 31.6 ± 1.3 | 25.8 ± 0.8 | 85.4 ± 3.3 | 3.2 ± 3.8 |
| Gonotrophic cycle length | |||||
| Sept. 2007 (21 d) | 21.8 ± 0.4 | 33.1 ± 1.2 | 26.1 ± 0.7 | 79.2 ± 2.6 | 13.2 ± 29.1 |
| Oct. 2007 (21 d) | 22.2 ± 0.9 | 32.3 ± 1.5 | 25.4 ± 1.3 | 83.3 ± 5.2 | 11.8 ± 16.6 |
Timing of sunrise and sunset shifted by ≈30 min throughout the year. Sunrise occurred between 05:29 and 0:600 h, whereas sunset occurred between 17:43 and 18:14 h.
Substrate Preference
Oviposition by Ae. aegypti was affected by container material, paper, and time. There was no effect of position (which container of the pair was lined with paper, Wald P = 0.276) or location (cemetery versus field laboratory, Wald P = 0.310). Fig. 1 shows the mean number of eggs laid per day (±SE) by wild Ae. aegypti in plastic, metal, and cement containers, subdivided into those lined and unlined with paper. In the absence of paper, females laid fewer eggs on metal (β = −1.33, P < 0.001) and plastic containers (β = −1.20, P < 0.001) compared with cement containers. The effect of paper on oviposition depended on container material. Although paper had no effect on egg counts in cement containers (P = 0.536), the presence of paper led to increased oviposition in metal (β = 1.32, P = 0.004) and plastic containers (β = 0.81, P = 0.083). Overall, more eggs were deposited over time as the experiment progressed (β = 0.013, P = 0.002).
Fig. 1.
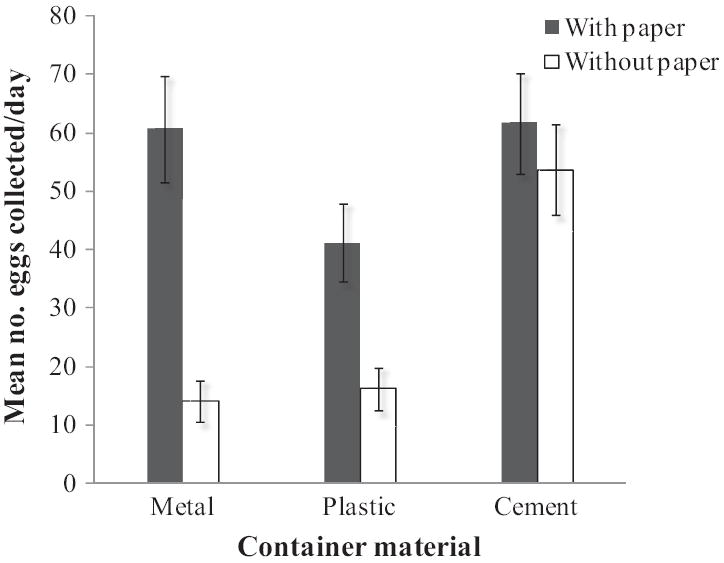
Mean number of eggs laid per day (±SE) by wild Ae. aegypti in containers made of metal, plastic, and cement. For each type of material, females were given the choice between containers lined with paper versus unlined. No significant differences in egg counts were found because of container position (which container in pair was lined with paper, Wald P = 0.276) or container location (cemetery versus field laboratory, Wald P = 0.310). Data shown are combined with respect to position and location.
Diurnal Oviposition Periodicity
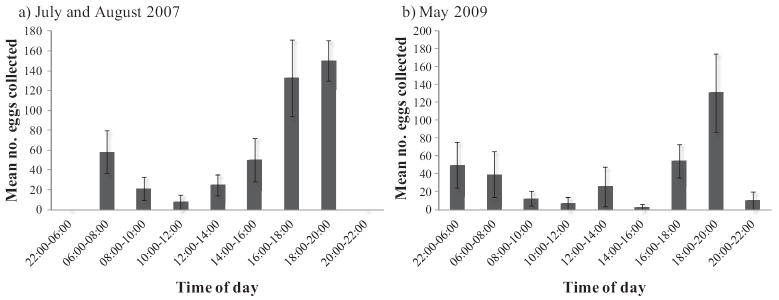
During July and August 2007, 7,118 Ae. aegypti eggs were collected (daily mean = 445 ± 57 SE). Field populations of Ae. aegypti exhibited significant variation in the number of eggs laid among time periods (χ2 = 13,274.71, df = 8, P < 0.001), with a diurnal bimodal pattern (Fig. 2a). Oviposition began in the morning with a small peak from 06:00 to 08:00 h (13% of total eggs collected), decreased during midday with the fewest eggs laid from 10:00 to 12:00 h, and then increased again with the largest peak (64% of total eggs collected) occurring in the evening from 16:00 to 20:00 h. No eggs were laid overnight from 22:00 to 06:00 h.
Fig. 2.
Periodicity in Ae. aegypti oviposition activity during (a) July and August 2007 and (b) May 2009. Number of eggs laid every 2 h is expressed as an arithmetic mean (±SE).
During May 2009, 1,998 eggs were collected (daily mean = 333 ± 59 SE). Oviposition periodicity was again evident (χ2 = 3,060.62, df = 8, P < 0.001), with the largest peak in egg laying (56% of total eggs) occurring from 16:00 to 20:00 h (Fig. 2b). Similar to July and August 2007, few eggs were collected daily from 10:00 to 12:00 h. The most striking difference between the two study periods was that oviposition continued late into the night during May 2009, with 15% of total eggs laid overnight from 22:00 to 06:00 h.
Gonotrophic Cycle Length
Nine of 10 females collected from the field as pupae and 24 of 35 females reared from field-collected eggs oviposited. Fig. 3 shows the number of gonotrophic cycles completed by these 33 females. Because only a single female reared from field-collected eggs survived to the sixth gonotrophic cycle, data from the sixth cycle were deleted and analyses were limited to the first five gonotrophic cycles for both groups of females.
Fig. 3.
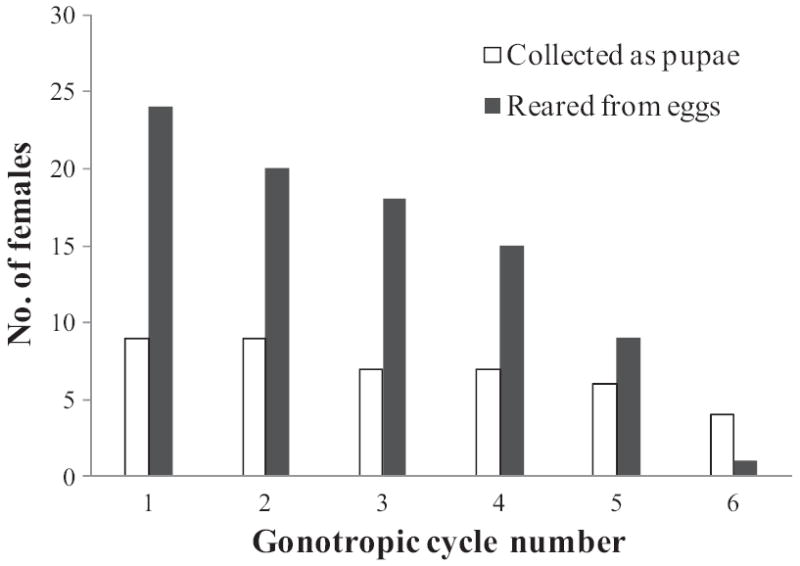
Number of F0 generation female Ae. aegypti completing each gonotrophic cycle. Captive females were offered a blood meal once per day and given constant access to an oviposition cup.
Least square means (±SE) for gonotrophic cycle length are shown in Fig. 4. Gonotrophic cycle length did not differ by female origin (ANOVA, F = 3.40; df = 1, 31; P = 0.075) or gonotrophic cycle number (ANOVA, F = 1.07; df = 4, 83; P = 0.375). Females from Iquitos required an average of 3.8 d to complete their gonotrophic cycle.
Fig. 4.
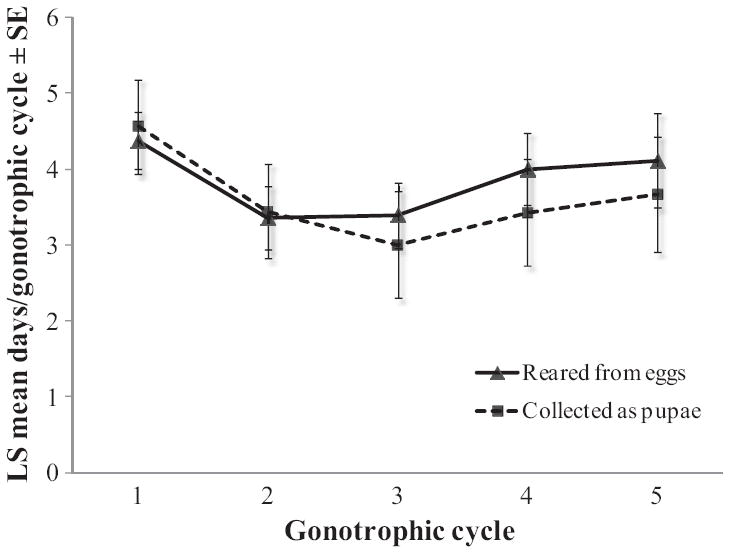
Gonotrophic cycle length of F0 Ae. aegypti. Least square means (±SE) are shown separately for females collected as pupae versus eggs and for each gonotrophic cycle.
Discussion
When devising methods to sample Ae. aegypti eggs from naturally-occurring containers to investigate selective oviposition, failure to account for certain aspects of the mosquito’s behavior can bias study inferences. In this study, we gathered site-specific data on substrate preference, diurnal periodicity, and gonotrophic cycle length that can be used to minimize bias in the design of an egg-sampling program. Our results from Iquitos, Peru, differed from some findings from other locations, underscoring the importance of validating key assumptions for different locations and field populations.
Lining containers with paper as a transportable oviposition substrate facilitated collecting Ae. aegypti eggs in the field (Steinly et al. 1991, Harrington et al. 2008). Our assumption, however, that paper does not differentially affect the number of eggs laid by wild Ae. aegypti in different container types proved untrue. Lining smooth-walled metal and plastic containers with textured paper toweling provided a rougher surface, which we suspect resulted in increased oviposition in these containers (Fig. 1). This is consistent with previous studies demonstrating that Ae. aegypti prefer to lay eggs on rough substrates (O’Gower 1963, Fay and Perry 1965, Chadee et al. 1995). Although containers differed in color (metal and cement-lined pots were gray, and plastic buckets were blue), this did not explain the observed pattern of oviposition. In the absence of paper, metal and plastic containers received comparable numbers of eggs, whereas cement containers, having the roughest texture, received significantly more eggs.
The presence of paper, therefore, introduced a systematic bias by increasing Ae. aegypti oviposition in containers composed of smooth materials. Container material could no longer be examined as a selection criterion for oviposition because oviposition surfaces were made artificially homogeneous. Investigators using paper to collect eggs can account for this limitation by either disregarding container material in analyses, or adjusting analyses based on ratios of eggs laid during pilot studies in different container types without paper.
The assumption that most oviposition in Iquitos occurs in the late afternoon or early evening, with little activity during midday, was confirmed (Fig. 2). Our results were broadly consistent with reports of Ae. aegypti oviposition patterns in the laboratory (Haddow and Gillett 1957, Chadee 2008) and the field (McClelland 1968, Chadee and Corbet 1987, Corbet and Chadee 1990). In contrast to Iquitos, Ae. aegypti laid eggs throughout the day in Thailand (Harrington et al. 2008) and during the dry season in Trinidad, West Indies (Chadee and Corbet 1987), leading to a broad, diffuse peak around sunset. Differences in the diurnal pattern of mosquito oviposition between locations or seasons may be the result of temperature, humidity, and/or rainfall (Corbet 1963, Chadee and Corbet 1987). Because our data were collected during only 3 mo, no inferences could be made regarding seasonal changes in the timing of oviposition. Unlike in Thailand (Harrington et al. 2008) and Trinidad (Chadee and Corbet 1987), however, rain falls throughout the year in Iquitos (see detailed data in Supplemental Materials Table S1). Because of consistency in air temperature and relative humidity (National Climatic Data Center 2009), we did not expect large seasonal fluctuations in oviposition patterns. Timing of peak egg-laying activity remained relatively constant throughout the year, with maximum oviposition during the hours closest to sunset.
A shortcoming of our study was that microclimate factors (air temperature, relative humidity, water temperature, and light intensity) were not measured at the individual container level. Although conditions recorded at the airport were indicative of general climate trends in Iquitos, variation in microclimate between containers or between study periods would have provided additional valuable information for data analysis.
Because changing oviposition substrates in containers can disturb females, the timing and frequency of egg sampling can potentially affect Ae. aegypti oviposition patterns (Chadee 2008). Studies of other egg-laying species have demonstrated that interference by experimenters can cause females to reject or avoid oviposition sites (Cooley et al. 1986, Castilla and Swallow 1995). In Iquitos, very few eggs were laid during midday from 10:00 to 12:00 h, indicating that sampling containers for eggs during this time window would minimize disruption and subsequent alteration of oviposition behavior.
Our estimates of gonotrophic cycle length were consistent with other laboratory findings (Christophers 1960) and results from mark-release-recapture studies in Thailand (Sheppard et al. 1969, Pant and Yasuno 1973) and Tanzania (McClelland and Conway 1971). In Puerto Rico, a longer gonotrophic cycle from 3 to 7 d was estimated by mark-release-recapture (Morrison et al. 1999). Lower ambient temperature leads to slower blood meal digestion and a longer gonotrophic cycle (Focks et al. 1993, Scott et al. 2000b). The average maximum and minimum daily temperatures (±SD) recorded in Puerto Rico by Morrison et al. (1999) (27 ± 2°C and 19 ± 2°C, respectively) were cooler than those in Iquitos during our experiment (Table 1), consistent with the idea that differences in ambient temperature influenced the observed differences in gonotrophic cycle length. It should be noted that holding mosquitoes captive in the laboratory was a limitation in our study design. Females were confined to a small space and given the opportunity to feed on blood only once per day.
Based on our estimates of gonotrophic cycle duration in Iquitos, we recommend collecting Ae. aegypti eggs from containers over 3–4 consecutive days at our study site. Because the purpose of our sampling scheme was to draw conclusions representative of the entire population, we aim to reduce sources of sampling bias. Within a population, different habitat selection strategies are likely employed by different individuals (Doligez et al. 2003). The process of selecting a subset of houses in which to collect eggs already limits the number of individual females in the population that can be observed and their options for oviposition sites. Collecting eggs for a time period equivalent to the length of the gonotrophic cycle should yield representative data on oviposition choices. Because Ae. aegypti generations overlap in the field, a shorter collection period would exclude some females, whereas a longer collection period may overrepresent females that are able to complete multiple egg-laying cycles.
These findings were used to design a subsequent, in-depth field investigation of Ae. aegypti oviposition site selection among naturally-occurring containers in Iquitos. We were able to modify our analyses to account for sampling eggs using paper toweling, as well as determine the appropriate time of day and number of days over which to sample Ae. aegypti eggs. By recognizing and reducing potential sources of bias in field study designs, investigators can develop a more accurate understanding of Ae. aegypti behavior in the wild. Insight into oviposition behavior, in particular how females allocate their eggs among different containers and how they adapt to changes in container availability, will help improve Ae. aegypti surveillance and control.
Supplementary Material
Acknowledgments
We thank Gregor J. Devine for facilitating these studies in Iquitos, Peru; Edwin Requena, Claider Valderrama, Hugo Jaba, and Kelly Liebman for assistance with field work; Alicia M. Ellis and Steven T. Stoddard for statistical advice; and William K. Reisen and Anthony J. Cornel for insightful comments on earlier drafts. This work was supported by University of California Jastro-Shields, Hazeltine, and McBeth awards; the Innovative Vector Control Consortium; and Regents of the University of California from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative.
References Cited
- Blaustein L, Kotler BP. Oviposition habitat selection by the mosquito, Culiseta longiareolata: effects of conspecifics, food and green toad tadpoles. Ecol Entomol. 1993;18:104–108. [Google Scholar]
- Castilla AM, Swallow JG. Artificial egg-laying sites for lizards: a conservation strategy. Biol Conserv. 1995;72:387–391. [Google Scholar]
- Chadee DD. The diel oviposition periodicity of Aedes aegypti (L.) (Diptera: Culicidae) in the laboratory: substrate-movement effect. Ann Trop Med Parasitol. 2008;102:259–265. doi: 10.1179/136485908X278739. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Corbet PS. Seasonal incidence and diel patterns of oviposition in the field of the mosquito, Aedes aeypgti (L.) (Diptera: Culicidae) in Trinidad, West Indies: a preliminary study. Ann Trop Med Parasitol. 1987;81:151–161. doi: 10.1080/00034983.1987.11812107. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Corbet PS, Talbot H. Proportions of eggs laid by Aedes aegypti on different substrates within an ovitrap in Trinidad, West Indies. Med Vet Entomol. 1995;9:66–70. doi: 10.1111/j.1365-2915.1995.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti (L.) the yellow fever mosquito. Cambridge University Press; London, United Kingdom: 1960. [Google Scholar]
- Clements AN. The Biology of Mosquitoes, vol. 1: Development, Nutrition and Reproduction. Chapman & Hall; London, United Kingdom: 1992. [Google Scholar]
- Conover WJ. Practical Nonparametric Statistics. Wiley; New York, NY: 1999. [Google Scholar]
- Cooley SS, Prokopy RJ, McDonald PT, Wong TTY. Learning in oviposition site selection by Ceratitis capitata flies. Entomol Exp Appl. 1986;40:47–51. [Google Scholar]
- Corbet PS. Oviposition cycles of certain sylvan culicine mosquitoes (Diptera: Culicidae) in Uganda. Ann Trop Med Parasitol. 1963;57:371–381. doi: 10.1080/00034983.1963.11686189. [DOI] [PubMed] [Google Scholar]
- Corbet PS, Chadee DD. Incidence and diel pattern of oviposition outdoors of the mosquito, Aedes aegypti (L) (Diptera: Culicidae) in Trinidad, W.I. in relation to solar aspect. Ann Trop Med Parasitol. 1990;84:63–78. doi: 10.1080/00034983.1990.11812434. [DOI] [PubMed] [Google Scholar]
- Doligez B, Cadet C, Danchin E, Boulinier T. When to use public information for breeding habitat selection? The role of environmental predictability and density dependence. Anim Behav. 2003;66:973–988. [Google Scholar]
- Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J Med Entomol. 1992;29:1035–1038. doi: 10.1093/jmedent/29.6.1035. [DOI] [PubMed] [Google Scholar]
- Fay RW, Perry AS. Laboratory studies of ovipositional preferences of Aedes aegypti. Mosq News. 1965;25:276–281. [Google Scholar]
- Focks DA, Alexander N. Multicountry study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): analysis of the literature and model development. J Med Entomol. 1993;30:1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69:494–505. [PubMed] [Google Scholar]
- Gillett JD. Contributions to the oviposition-cycle by the individual mosquitoes in a population. J Insect Physiol. 1962;8:665–681. [Google Scholar]
- Haddow AJ, Gillett JD. Observations on the oviposition cycle of Aedes (Stegomyia) aegypti (Linnaeus) Ann Trop Med Parasitol. 1957;51:159–169. doi: 10.1080/00034983.1957.11685804. [DOI] [PubMed] [Google Scholar]
- Halekoh U, Hojsgaard S, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15:11. [Google Scholar]
- Harrington LC, Ponlawat A, Edman JD, Scott TW, Vermeylen F. Influence of container size, location, and time of day on oviposition patterns of the dengue vector, Aedes aegypti, in Thailand. Vector Borne Zoonotic Dis. 2008;8:415–423. doi: 10.1089/vbz.2007.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard SB. Imperfect oviposition decisions by the pitcher plant mosquito Wyeomyia smithii. Evol Ecol. 1994;8:493–502. [Google Scholar]
- Kiflawi M, Blaustein L, Mangel M. Oviposition habitat selection by the mosquito Culiseta longiareolata in response to risk of predation and conspecific larval density. Ecol Entomol. 2003;28:168–173. [Google Scholar]
- McClelland GAH. Field observations on periodicity and site preference in oviposition by Aedes aegypti (L.) and related mosquitoes (Diptera: Culicidae) in Kenya. Proc R Entomol Soc Lond Ser A. 1968;43:147–154. [Google Scholar]
- McClelland GAH, Conway GR. Frequency of blood feeding in the mosquito Aedes aegypti. Nature. 1971;232:485–486. doi: 10.1038/232485a0. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Costero A, Edman JD, Clark GG, Scott TW. Increased fecundity of Aedes aegypti fed human blood before release in a mark-recapture study in Puerto Rico. J Am Mosq Control Assoc. 1999;15:98–104. [PubMed] [Google Scholar]
- Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, Watts D, Stancil JD, Olson JG, Blair P, Scott TW. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Minnick SL, Rocha C, Forshey BM, Stoddard ST, Getis A, Focks DA, Russell KL, Olson JG, Blair PJ, Watts DM, Sihuincha M, Scott TW, Kochel TJ. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munstermann LE. Care and maintenance of Aedes mosquito colonies. In: Crampton JM, Beard CB, Louis C, editors. Molecular Biololgy of Insect Disease Vectors: A Methods Manual. Chapman & Hall; London, United Kingdom: 1997. pp. 13–20. [Google Scholar]
- National Climatic Data Center, United States Department of Commerce. Online Climate Data Directory. NCDC; 2009. Aug 5, [15 Aug 2009]. Web. ( http://www.ncdc.noaa.gov/oa/climate/climatedata.html) [Google Scholar]
- O’Gower AK. Environmental stimuli and the oviposition behaviour of Aedes aegypti var. queenslandis Theobald (Diptera, Culicidae) Anim Behav. 1963;11:189–197. [Google Scholar]
- Pant CP, Yasuno M. Field studies on the gonotrophic cycle of Aedes aegypti in Bangkok, Thailand. J Med Entomol. 1973;10:219–223. doi: 10.1093/jmedent/10.2.219. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing, version 2.8.1. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- SAS Institute. SAS user’s guide, version 9.2. SAS Institute; Cary, NC: 2008. [Google Scholar]
- Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J Med Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- Scott TW, Clark GG, Lorenz LH, Amerasinghe PH, Reiter P, Edman JD. Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J Med Entomol. 1993;30:94–99. doi: 10.1093/jmedent/30.1.94. [DOI] [PubMed] [Google Scholar]
- Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000a;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000b;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Sheppard PM, Macdonald WW, Tonn RJ, Grab B. Dynamics of an adult population of Aedes aegypti in relation to dengue haemorrhagic fever in Bangkok, Thailand. J Anim Ecol. 1969;38:661–702. [PMC free article] [PubMed] [Google Scholar]
- Sithiprasasna R, Mahapibul P, Noigamol C, Perich MJ, Zeichner BC, Burge B, Norris SLW, Jones JW, Schleich SS, Coleman RE. Field evaluation of a lethal ovitrap for the control of Aedes aegypti (Diptera: Culicidae) in Thailand. J Med Entomol. 2003;40:455–462. doi: 10.1603/0022-2585-40.4.455. [DOI] [PubMed] [Google Scholar]
- Southwood TRE, Murdie G, Yasuno M, Tonn RJ, Reader PM. Studies on the life budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bull WHO. 1972;46:211–226. [PMC free article] [PubMed] [Google Scholar]
- Steinly BA, Novak RJ, Webb DW. A new method for monitoring mosquito oviposition in artificial and natural containers. J Am Mosq Control Assoc. 1991;7:649–650. [PubMed] [Google Scholar]
- United States Naval Observatory. Naval Oceanography Portal: Sun or Moon Rise/Set Table for One Year. [27 Aug 2009];2009 ( www.usno.navy.mil/USNO/astronomical-applications/data-services/rs-one-year-world)
- Van Handel E, Edman JD, Day JF, Scott TW, Clark GG, Reiter P, Lynn HC. Plant sugar, glycogen, and lipid assay of Aedes aegypti collected in urban Puerto Rico and rural Florida. J Am Mosq Control Assoc. 1994;10:149–153. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer; New York, NY: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.