Abstract
Rationale
Drug use during adolescence is associated with an increased propensity for drug dependency during adulthood. Therefore, the effects of adolescent exposure to nicotine on adult behavioral responsiveness to nicotine are of particular importance.
Objectives
The objective of the current study was to determine if adolescent nicotine exposure would enhance behavioral sensitivity and development of sensitization to nicotine during adulthood.
Materials and Methods
Male Wistar rats were assigned to one of three groups that received subcutaneous (s.c.) injections of nicotine (0, 0.25, or 0.5 mg/kg) in the home cage for 12 consecutive days during adolescence, PD 31–42. Starting on PD 80, distance traveled, rearing, and stereotypy were recorded in locomotor activity chambers each day for 10 days, following s.c. injections of 0, 0.25, or 0.5 mg/kg nicotine. One week later, a final challenge session took place during which rats were injected with 0.5 mg/kg nicotine.
Results
Rats exposed to nicotine during adolescence displayed a greater locomotor response to a novel environment than saline-treated rats. Adolescent nicotine treatment also resulted in context-independent sensitization to the acute locomotor activating properties of nicotine, including distance traveled and stereotypy, as measured on the first day of adulthood nicotine exposure. Adolescent nicotine-treated rats displayed increased sensitivity to repeated nicotine exposures during adulthood, compared to adolescent saline-treated rats, as measured by distance traveled, rearing, and stereotypic behaviors. Finally, rats treated with nicotine only during adolescence were more sensitive to a final nicotine challenge during adulthood than rats treated with nicotine only previously during adulthood.
Conclusions
Overall, the results suggest that adolescent nicotine treatment predisposes adult rats to develop increased behavioral sensitivity to chronic nicotine treatment and to be more sensitive to the initial effects of nicotine.
Keywords: adolescence, nicotine, locomotor activity, sensitization
1. Introduction
Adolescence is a unique developmental period that is characterized by landmarks such as physical growth, behavioral maturation, and neuronal development. Behaviorally, adolescence also includes the emergence of profound novelty- and sensation-seeking, which, in both humans and laboratory animals, has been associated with drug use (Martin et al. 2002; Cain et al. 2005). The initiation of tobacco use during adolescence is of great concern, because it can result in long-term consequences including an increased risk of progression to use of other illegal drugs (Kandel et al. 1992), and a decreased probability of smoking cessation (Chen and Millar 1998). Neurocircuitry that plays a key role in the rewarding and motor effects of nicotine undergoes changes during the critical adolescent maturational period (Spear 2000, Chambers et al. 2003), and these changes may make the adolescent brain differentially susceptible to the long-lasting effects of nicotine. Additionally, nicotinic receptor binding across many brain regions is greater in adolescent rats than their adult counterparts (Doura et al. 2008). Therefore, age-dependent changes within the brain’s nicotinic system may be involved in ontogenic differences in response to nicotine.
Overall, studies in rats have indicated that adolescents may be more sensitive to the reinforcing effects of nicotine than adults. Nicotine-induced place preference conditioning occurs in adolescent rats but not adult rats (Vastola et al. 2002; Belluzzi et al. 2004). Adolescent rats have been shown to intravenously self-administer more infusions of nicotine than their adult counterparts over a 4-week self-administration paradigm (Levin et al. 2003). Age-dependent behavioral cross-sensitization has also been reported; adolescent rats chronically treated with nicotine showed an increased locomotor response to amphetamine administration compared to naïve animals, whereas adult rats did not (Collins et al. 2004).
In laboratory animals, repeated administration of nicotine results in behavioral sensitization (Domino 2001; Adriani et al. 2003; Berg and Chambers 2008). It has been suggested that behavioral sensitization represents a neuroadaptive process that also underlies motivational sensitization as a core process in drug addiction (Robinson and Berridge 1993; Vanderschuren and Kalivas 1993). The behavioral sensitization produced by repeated drug administration can persist for weeks or months, or perhaps permanently, as a result of these neuroadaptations. Faraday et al. (2003) demonstrated that nicotine’s activity-increasing effects in adulthood were greater in rats with prior nicotine exposure during adolescence, compared to adulthood prior exposure. Because locomotor activity in that study was recorded during the adolescent injection series, the increased locomotor sensitization that was observed in adult rats may have been context-dependent. Indeed, the environmental context in which a drug is delivered is a major factor in the development of behavioral sensitization (Robinson and Berridge 1993; Pierce and Kalivas 1993). In the current study, adolescent treatments occur in the home cage rather than the behavioral testing apparatus, which allows for assessment of pharmacological (context-independent) sensitization during the initial adulthood nicotine exposure in the locomotor activity chambers.
The present experiment expands earlier findings by investigating additional activity measures, namely rearing and stereotypy. Rearing, or standing upright on the back legs, is considered to be an exploratory behavior (Crusio 2001) and an indication of general CNS excitability (Lat 1963). It has also been postulated to reflect emotional components of behavior, such as anxiety (Gironi Carnevale et al. 1990). Stereotypy is defined as absence of locomotion and intense sniffing or licking/biting in a restricted area of the environment. Because psychostimulants such as amphetamine and cocaine elicit this behavior, it may be related to increases in dopaminergic activity. However, the neurobiological underpinnings of these behavioral different measures may be distinct (Ksir 1994; Conti et al. 1997).
The present study was conducted to test the hypothesis that adolescent exposure to nicotine would result in elevated behavioral sensitivity and enhanced development of sensitization to the locomotor activating effects of nicotine during adulthood. For clarity, “sensitivity” is defined as the behavioral response to a given dose of nicotine. “Sensitization” is displayed when a behavioral response to a given dose of nicotine is greater than a previous behavioral response to that same dose of nicotine. The objective of this study was to investigate whether adolescent nicotine exposure results in long-lasting effects on nicotine-modulated behaviors.
2. Materials and Methods
2.1 Animals and adolescent nicotine exposure
Male Wistar rats (Harlan, Indianapolis, IN) arrived at the laboratory at postnatal days (PD) 23–25. They were housed 4 to a cage (later adjusted to pair-housed when body weights reached approximately 300 g) on a 12-hour reverse light/dark cycle (lights off at 0900 hours) with food and water available ad libitum. Beginning at PD 30, animals received once daily subcutaneous injections of 0, 0.25, or 0.5 mg/kg nicotine ((−)-1-Methyl-2-(3-pyridyl)pyrrolidine (+)-bitartrate salt, Sigma-Aldrich), for 12 consecutive days. Nicotine doses were based on several previous studies (including Adriani et al. 2006; Belluzzi et al. 2004; Berg and Chambers 2008) and calculated as the weight of the base, and were dissolved in a sterile 0.9% saline solution and delivered at an injection volume of 1 ml/kg. Animals were weighed, injected, and placed back into the home cage. Each animal received the same dose of nicotine (or saline) across all 12 days. Following this peri-adolescent injection protocol, animals were handled only during the weekly cage changes from PD 42 until the beginning of locomotor activity experiments at PD 80.
2.2 Behavioral testing
Locomotor activity recording sessions took place in plexiglass recording chambers (43.2 × 43.2 × 30.5 cm), which were each equipped with 16 infrared beam transmitters spanning the x and y axes of the field, plus another 8 transmitters on the z axis (Med Associates, Inc., St. Albans, VT). Transmitter and detector arrays for translational motion were located 5 cm from the chamber floor and spaced 2.5 cm apart. Data were collected using the Activity Monitor 5.0 software (Med Associates, Inc., St. Albans, VT), which was configured to distinguish between small, quick, repeated movements (stereotypic counts), rearing (vertical counts), and translational locomotion (distance traveled).
Starting on PD 80, locomotor behavior was assessed over ten 2-hour sessions (1 session per day) occurring Monday through Friday of two consecutive weeks. Each 2-hour locomotor recording session consisted of a 1-hr baseline phase (to control for any differences in response to being placed in the chamber) and a 1-hr post-injection phase. Activity data were collected in 10-min blocks. Immediately after each 1-hr baseline period ended, animals received an injection of nicotine (0, 0.25 or 0.5 mg/kg, s.c.) according to random assignment within each adolescent nicotine treatment group. Experimental group setup is depicted in Fig. 1. Each adult animal received the same dose of nicotine (or saline) across all ten days of the locomotor activity study.
Fig. 1. Schematic diagram of experimental groups.

During adolescence (left column), rats received one of three treatments (saline, 0.25, or 0.5 mg/kg nicotine) daily for 12 days. During adulthood (middle column), each now-grown group of rats was subdivided into three groups, and received one of three treatments (saline, 0.25, or 0.5 mg/kg nicotine) for 10 days of behavioral testing. Groups that received the final nicotine challenge are shown in the right column. Group sizes in each phase of the study are indicated.
One week after the conclusion of the adult locomotor study, at PD 98, a single challenge session took place, during which animals received an injection of 0.5 mg/kg nicotine and locomotor activity was recorded as previously described. The groups that were included in this final challenge session were the groups that had received either saline in adolescence (so that their only nicotine exposure was in adulthood), or saline in adulthood (so that their only nicotine exposure was in adolescence), as shown in Fig. 1. Experimenter error prevented some of the rats from properly undergoing this final nicotine challenge session, so the n values for this final part of the study are slightly lower.
2.3 Statistical analysis
Analysis for the activity data consisted of a repeated measures ANOVA with between-subject variables of adolescent nicotine group or adult nicotine group and repeated measures of session. Roy’s Largest Root was used to correct the violations of the assumptions of the repeated measures ANOVA test, namely sphericity and heterogeneity of variance. Where appropriate, post hoc analyses (Newman-Keuls) were used to determine which groups were significantly (p < 0.05) different from each other.
3. Results
3.1 Day 1 Pre-injection Activity: Novelty Response
The possibility that adolescent exposure to nicotine would influence spontaneous locomotor behavior during the initial period of exposure to the novel activity chamber was examined. A one-way ANOVA indicated a significant main effect of adolescent nicotine treatment (F2,60 = 7.684, p < 0.001). Post hoc analysis indicated that the groups that had received 0.25 or 0.5 mg/kg nicotine during adolescence showed significantly greater distance traveled in the novel environment than the adolescent saline-treated group (Fig. 2a).
Fig. 2. Behavioral response to novel environment in adulthood.
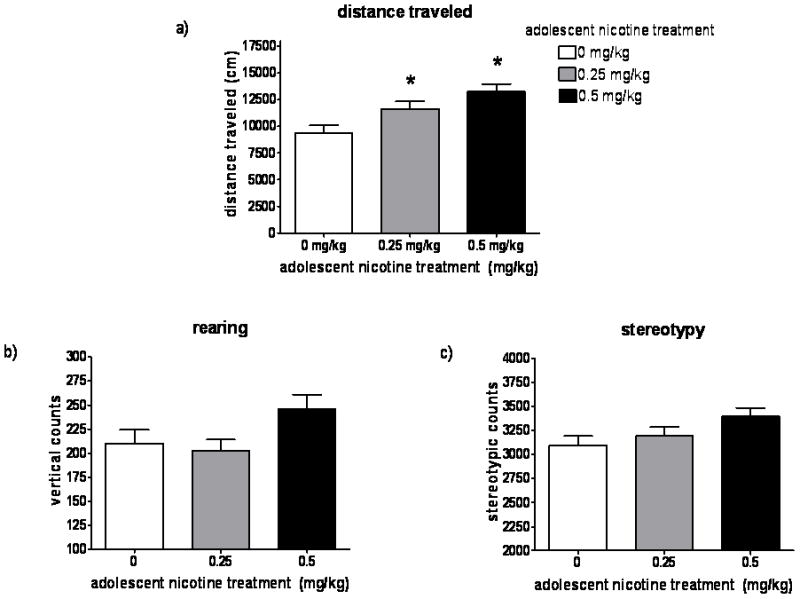
Locomotor activity of adult rats during the 1-hr baseline period in the activity chamber, immediately prior to the first adulthood nicotine treatment (n = 19–22 per group): a) distance traveled; b) rearing; and c) stereotypy. Values are given as mean ± SEM. * Indicates significant (p < 0.05) difference from saline group.
Analysis of the rearing counts in the 1 hour pre-injection period in the novel environment showed an effect of adolescent nicotine treatment that approached significance (p = 0.054) (Fig. 2b). No differences were observed between groups in the number of stereotypic behaviors exhibited in the novel environment (Fig. 2c).
3.2 Effects of Adolescent Nicotine Treatment on Adulthood Nicotine-induced Locomotor Activity
3.2.1 Distance traveled
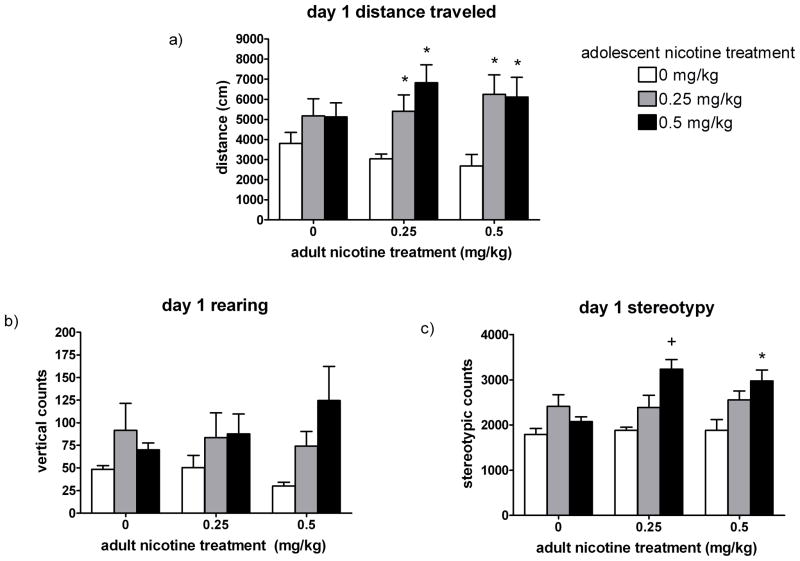
First, the effects of initial (Day 1) adulthood nicotine treatment were examined (Fig. 3a). Because the adolescent nicotine exposure occurred in the homecage, behavioral sensitization to this initial adulthood nicotine injection could be considered context-independent. A two-way ANOVA performed on the Day 1 distance traveled revealed a significant main effect of adolescent treatment (F2,54 = 10.23, p < 0.001). For the initial adulthood injection of 0.25 or 0.5 mg/kg nicotine, significant differences in distance traveled were observed based on differing adolescent treatments (F2,19 = 6.479 and 4.333, p = 0.007 and 0.028, respectively), with adult nicotine treatment producing an approximately 2-fold greater stimulation in the groups exposed to nicotine during adolescence compared to the adolescent saline-treated rats. Adolescent nicotine treatment did not have an effect on the activity observed following an adulthood saline injection (F2,15 = 1.182, p = 0.334).
Fig. 3. Response to initial adulthood nicotine treatment.
Locomotor activity during the 1 hour following the first adulthood nicotine treatment (n = 6–8 per group): a) distance traveled; b) rearing; and c) stereotypy. Adolescent nicotine treatment is indicated by legends; adult nicotine treatment is indicated by x-axis labels. Values are given as mean ± SEM. Significant differences indicate expression of context-independent sensitization to nicotine. * Indicates significant (p < 0.05) difference from adolescent saline group within a particular adult nicotine treatment. + Indicates significant (p < 0.05) difference from both other adolescent groups within a particular adult nicotine treatment.
A repeated measures ANOVA on the daily distance traveled by all rats across the 10 days of the study revealed a significant Day x Adolescent Treatment x Adult Treatment interaction (F36,486 = 2.495, p = 0.02). The significant 3-way interaction was then decomposed by holding each adolescent treatment constant.
A repeated measures ANOVA on the adult locomotor activity data collected from rats treated with saline during adolescence did not reach significance, but showed a trend toward the expected overall effect of adult nicotine treatment (Fig. 4a). A priori hypothesis asserted that nicotine should increase locomotor activity across days; therefore, individual ANOVAs were performed. However, examining distance traveled on individual days revealed no significant differences between the 3 injection conditions, although between-group differences on Day 9 approached significance (F2,16 = 3.388, p = 0.059).
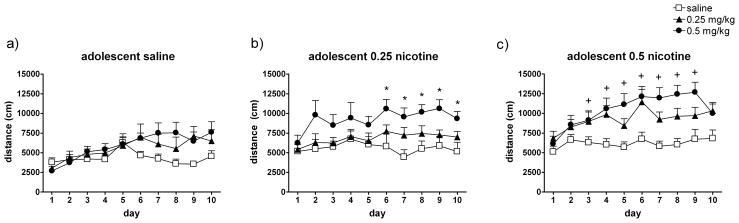
Fig. 4. Adolescent nicotine treatment results in dose-dependent enhanced sensitization to the ambulatory activity-increasing effects of nicotine during adulthood.
The mean (± SEM) distance traveled across all 10 test days by adult rats that received (a) saline during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–7/group); (b) 0.25 mg/kg nicotine during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–8/group), or (c) 0.5 mg/kg nicotine during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–8/group). Symbols and legend indicate treatment administered during the adulthood 10-day locomotor activity tests. * Indicates that the 0.5 mg/kg nicotine adult-treated group was significantly (p < 0.05) different than saline. + Indicates that the 0.25 mg/kg and 0.5 mg/kg nicotine adult-treated groups were significantly different from saline.
A repeated measures ANOVA on adult distance traveled by rats treated with 0.25 mg/kg nicotine during adolescence (Fig. 4b) revealed a significant effect of Day (F9,171 = 4.482, p = 0.011) and a Day x Adult Treatment interaction that approached significance (F18,171 = 2.67, p = 0.058). Post hoc analyses revealed that the adults receiving 0.5 mg/kg nicotine showed significantly greater locomotor activity than saline controls and 0.25 mg/kg nicotine on days 6 through 10.
A repeated measures ANOVA on adult distance traveled by rats treated with 0.5 mg/kg nicotine during adolescence (Fig. 4c) revealed a significant effect of Day (F9,171 = 4.901, p = 0.008). Post hoc analyses revealed that the adults receiving 0.25 mg/kg or 0.5 mg/kg nicotine showed significantly greater LMA than saline controls on days 4 through 9.
3.2.2 Rearing
On the first day of the adulthood phase of the study, adolescent nicotine treatment did not significantly influence rearing behavior following the initial adulthood injection (Fig. 3b). However, this was likely due to the relatively high between-subject variability, as trends towards sensitization by both doses of adolescent nicotine were observed.
A repeated measures ANOVA on the daily rearing counts by all rats across the 10 days of the study revealed a significant Day x Adult Treatment interaction (F18,486 = 2.398, p = 0.001), as well as a significant main effect of Adolescent Treatment (F2,43 = 9.724, p < 0.001). Each adolescent treatment was then held constant to examine the effects of adult nicotine treatment on rearing.
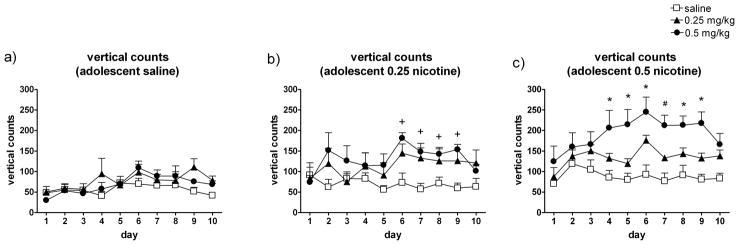
A repeated measures ANOVA on the adult rearing counts in rats treated with saline during adolescence revealed no significant effects of adult nicotine treatment (Fig. 5a). In the rats treated with 0.25 mg/kg nicotine during adolescence, a similar ANOVA revealed a significant Day x Adult Treatment interaction (F18,171 = 2.03, p = 0.012) (Fig. 5b). Post hoc analyses revealed that the groups treated with 0.25 or 0.5 mg/kg during adulthood showed significantly more rearing behavior than the saline-treated adults on days 6–9. A repeated measures ANOVA on the adult rearing counts in rats treated with 0.5 mg/kg nicotine during adolescence revealed a significant main effect of Adult Treatment (F9,171 = 6.573, p = 0.007), as well as a Day x Adult Treatment interaction that approached significance (F18,171 = 1.597, p = 0.06) (Fig. 5c). Post hoc analysis revealed that on days 4–9, the 0.5 mg/kg nicotine group demonstrated significantly more rearing behavior than the other two groups. On day 7, all three groups were significantly different from each other.
Fig. 5. Adolescent nicotine treatment results in dose-dependent enhanced sensitization to the rearing-increasing effects of nicotine during adulthood.
The mean (± SEM) rearing counts by adult rats across all 10 test days by rats that received (a) saline during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–7/group); (b) 0.25 mg/kg nicotine during adolescence (amd saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–8/group), or (c) 0.5 mg/kg nicotine during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–8/group). Symbols and legend indicate treatment administered during the adulthood 10-day locomotor activity tests. * Indicates that the 0.5 mg/kg nicotine adult-treated group was significantly (p < 0.05) different than saline. + Indicates that the 0.25 mg/kg and 0.5 mg/kg nicotine adult-treated groups were significantly different than saline. # Indicates that all three groups are different from each other.
3.2.3 Stereotypic behavior
A two-way ANOVA performed on the Day 1 stereotypy counts revealed a significant main effect of adolescent treatment (F2,54 = 13.65, p < 0.001), indicating that rats that had received 0.5 mg/kg nicotine during adolescence demonstrated significantly more stereotypic behavior (F2,19 > 5.36, p < 0.014) in response to both the 0.25 mg/kg and 0.5 mg/kg nicotine dose during adulthood than the adolescent saline or 0.25 mg/kg nicotine groups (Fig. 3c).
A repeated measures ANOVA on the daily rearing counts by all rats across the 10 days of the study revealed a significant Day x Adult Treatment interaction (F18,486 = 5.02, p > 0.0001), as well as a significant main effect of Adolescent Treatment (F2,54 = 16.81, p < 0.001). Each adolescent treatment was held constant to examine the effects of adult nicotine treatment on stereotypic behaviors.
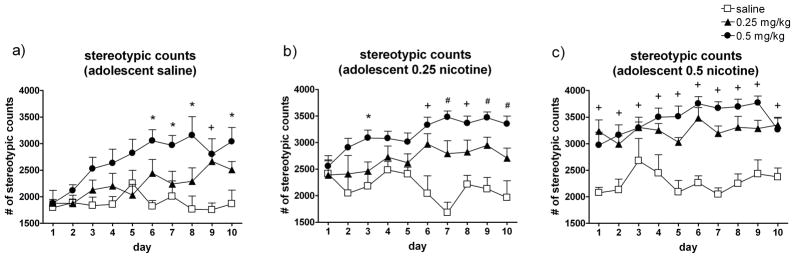
Among the rats treated with saline during adolescence, a significant Day x Adult Treatment interaction was revealed by a repeated measures ANOVA (F18,144 = 2.39, p = 0.002) (Fig. 6a). Post hoc analyses indicated that the rats receiving daily injections of 0.5 mg/kg nicotine demonstrated a greater number of stereotypic movements than saline-treated rats on days 6–10.
Fig. 6. Adolescent nicotine treatment results in dose-dependent enhanced sensitization to the stereotypy-increasing effects of nicotine during adulthood.
The mean (± SEM) stereotypy counts by adult rats across all 10 test days by rats that received (a) saline during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–7/group); (b) 0.25 mg/kg nicotine during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–8/group), or (c) 0.5 mg/kg nicotine during adolescence (and saline, 0.25 mg/kg, or 0.5 mg/kg nicotine in adulthood, n = 6–8/group). Symbols and legend indicate treatment administered during the adulthood 10-day locomotor activity tests. * Indicates that the 0.5 mg/kg nicotine adult-treated group was significantly (p < 0.05) different than saline. + Indicates that the 0.25 mg/kg and 0.5 mg/kg nicotine adult-treated groups were significantly different than saline. # Indicates that all three groups are different from each other.
A repeated measures ANOVA on the adult stereotypic counts in rats treated with 0.25 mg/kg nicotine during adolescence revealed a significant Day x Adult Treatment interaction (F18,171 = 5.02, p > 0.0001) (Fig. 6b). Post hoc analyses showed that as early as day 3, the rats treated with 0.5 mg/kg nicotine demonstrated more stereotypy than the other groups. On days 6 and 8, the rats receiving either one of the nicotine doses showed more stereotypic behavior than the saline group; and on days 7, 9, and 10, all three groups were significantly different from each other.
Finally, a repeated measures ANOVA on the adult stereotypic counts in rats treated with 0.5 mg/kg nicotine during adolescence revealed a significant Day x Adult Treatment interaction (F18,171 = 1.796, p = 0.029) (Fig. 6c). For all 10 days of the experiment, the rats receiving 0.25 or 0.5 mg/kg nicotine demonstrated significantly more stereotypic behavior than the saline group.
3.3. Final nicotine challenge session
One week following the conclusion of the adult locomotor activity testing phase, rats were challenged with a single injection of 0.5 mg/kg nicotine and were monitored for one hour in the activity chambers. The purpose of this final challenge session was to compare persistent behavioral sensitization to nicotine between rats that were previously exposed to nicotine during adolescence and those that were previously exposed during adulthood, in order to examine age-dependent differences in development of sensitization. Among rats that were treated with saline during adulthood (and thus were only exposed to differing nicotine doses during adolescence) (Fig. 7a), those groups that had received 0.25 or 0.5 mg/kg nicotine during adolescence showed significantly greater distance traveled in response to the challenge than those with no prior nicotine exposure (F2,7 = 4.641, p = 0.05). Among rats treated that were treated with saline during adolescence (and thus were only exposed to differing nicotine doses during adulthood) (Fig. 7b), only the group that had received the highest nicotine dose during adulthood demonstrated elevated locomotor activity in response to the challenge dose (F1,8 = 4.948, p = 0.05).
Fig. 7. Nicotine exposure only during adolescence confers persistent sensitization to the ambulatory activity-increasing effects of a single adulthood nicotine challenge, compared to nicotine exposure only during adulthood.
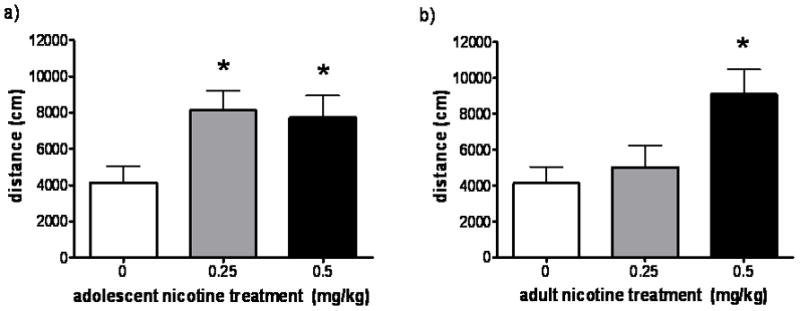
Distance traveled in the 1 hour following a final nicotine challenge of 0.5 mg/kg for all rats (n = 5–7 per group). This challenge was given 1 week after the conclusion of the 2-week adulthood locomotor activity study. Panel a) shows rats that received saline during adulthood, so that their only nicotine exposure prior to the challenge was during adolescence (adolescent treatments indicated on x-axis). Panel b) shows rats that received saline during adolescence, so that their only nicotine exposure prior to the challenge was during adulthood (adult treatments indicated on x-axis). Values are given as mean ± SEM. * Indicates significant (p < 0.05) difference from saline group.
Group differences in rearing and stereotypic behaviors (data not shown) in response to the final nicotine challenge were less remarkable. In both behaviors, no age-dependent differences in persistent sensitization were observed.
4. Discussion
The aim of the present study was to test whether adolescent exposure to nicotine would result in alterations in adulthood behavioral sensitivity to the same drug. The primary findings of this study are that adolescent nicotine exposure results in 1) an elevated ambulatory response to a novel environment in adulthood, 2) enhanced initial sensitivity to nicotine-induced ambulatory and stereotypy behavior during adulthood, and 3) enhanced development of behavioral sensitization to the ambulatory, stereotypy-, and rearing-inducing properties of nicotine during adulthood in a dose-dependent manner. Additionally, a final nicotine challenge session revealed that prior nicotine exposure during adolescence resulted in greater sensitivity to the ambulatory activity-inducing effects of single adulthood nicotine injection than prior nicotine exposure only during adulthood. The findings reported here extend previous work showing that nicotine exposure during adolescence yields persistent changes in the neurobiological substrates underlying nicotine-induced locomotor activity (Faraday et al. 2003, Adriani et al. 2006), and add a number of important elements. First, three different measures of locomotor activity are assessed: distance traveled, rearing, and stereotypy. Secondly, the study design allowed for a systematic comparison of adulthood behavioral nicotine sensitivity between rats that had previous nicotine exposure either only in adulthood or only in adolescence. Finally, the administration of adolescent nicotine treatments in the homecage allowed for investigation of context-independent sensitization in response to the first nicotine treatment in adulthood.
Nicotine treatment during adolescence resulted in elevated locomotion (distance traveled) during the 1 hr of pre-injection activity recording that took place immediately before the first adulthood injection. Since this was the rats’ first experience in the activity chambers, these data reflect an elevated locomotor response to a novel environment in the rats with a history of adolescent nicotine treatment, suggesting that adolescent nicotine exposure results in persistent alterations in neural circuits regulating novelty-seeking behavior. These findings are consistent with Adriani et al. (2006), who reported elevated spontaneous locomotor activity in a novel environment in adult rats that were pretreated with nicotine during adolescence, but not in adult rats that were pretreated with nicotine during postadolescence.
Adolescent nicotine treatment resulted in differences in locomotor activity induced by the first nicotine injection during adulthood. Both nicotine doses during adolescence resulted in significantly greater distance traveled in response to the initial adulthood dose of 0.25 mg/kg or 0.5 mg/kg nicotine. This observation is consistent with previous results (Faraday et al. 2003), but unique in that the present study demonstrated context-independent sensitization. Additional behavioral measures were also up-regulated following the first adulthood nicotine treatment. There was also a significantly increased stereotypic response on this first adulthood nicotine test day, as well as a non-significant trend toward increased rearings, in the rats treated with 0.5 mg/kg nicotine during adolescence. These data indicate that adolescent nicotine exposure resulted in a long-lasting elevation of behavioral responses to the stimulating effects of nicotine, which might reflect enhanced sensitivity to the reinforcing effects of nicotine. If nicotine exposure during adolescence had similar effects in humans, then increased sensitivity to the reinforcing effects of nicotine would likely promote continued smoking following a return to use in adulthood. Additionally, the fact that adolescent nicotine exposure took place in the home cage indicates that this behavioral sensitization was context-independent, a finding that has been reported in adult rats (Nisell et al. 1996) but not yet in adolescents.
In contrast to the adolescent saline-treated group, rats that received nicotine during adolescence showed significant overall effects and dose-dependent enhancement of response to all three measures of the locomotor-activating properties of nicotine across repeated adulthood nicotine treatments. Overall, the increases in daily distance traveled were more robust and appeared earlier in the 10-day study in the rats that had received 0.5 mg/kg nicotine during adolescence than those that had received 0.25 mg/kg nicotine or saline. A similar phenomenon was observed in the rearing behavior. Rats treated with 0.5 mg/kg nicotine during adolescence showed increased rearing responses to both adulthood doses of nicotine beginning earlier in the adulthood study than the rats previously exposed to 0.25 mg/kg nicotine. Stereotypic behavior also followed a similar pattern. Interestingly, this was the only behavioral measure in which significant adulthood sensitization occurred across days in adolescent-saline-treated rats. In the rats treated with 0.25 mg/kg during adolescence, significant sensitization was observed beginning on treatment day 6. Among the rats treated with 0.5 mg/kg nicotine during adolescence, enhanced sensitivity to the adulthood nicotine doses was apparent on the very first adulthood treatment day, and on every day thereafter.
The final adulthood challenge session allowed for comparison between the persistent effects of adolescent or adult nicotine pre-exposure. In the case of distance traveled, adolescent nicotine treatment in the absence of adulthood nicotine exposure yielded significant sensitization to the challenge dose as compared to nicotine-naïve animals. This demonstrates that nicotine exposure on PD 28–42 was sufficient for the expression of behavioral sensitization to nicotine almost two months later at PD 98. Moreover, of the rats treated with saline during adolescence, only the rats that received 0.5 mg/kg nicotine during adulthood showed a sensitized response to the final challenge dose of nicotine; the rats that received 0.25 mg/kg nicotine during adulthood did not show this sensitized response. This indicates that the low dose of nicotine during adulthood alone was not sufficient to sensitize the rats to the 0.5 mg/kg challenge dose. However, the higher nicotine dose during adulthood yielded a sensitized response to the challenge dose, regardless of adolescent treatment. Taken together, these data indicate that the dose-response for development of a prolonged sensitized locomotor response to a challenge nicotine injection is shifted to the left in adolescent rats relative to adults.
To our knowledge, this is the first study to systematically investigate the effects of adolescent and/or adult nicotine exposure on the development of adulthood behavioral sensitization to nicotine, using not only distance traveled but also rearing and stereotypy measures. The observation that nicotine-induced behavioral sensitization was both increased across sessions and prolonged within sessions may shed light on the underlying neurobiological changes produced by the adolescent nicotine pretreatment. A working hypothesis is that drug exposure during adolescence results in long-lasting changes in sensitivity to drugs of abuse, which increases the propensity to use and/or abuse drugs during adulthood. Adolescents have shown differential responses to nicotine when compared with adults in studies of behaviors such as conditioned place preference (Vastola et al. 2002; Beluzzi et al. 2004; Shram and Le 2010), locomotor activity (Faraday et al. 2003; Adriani et al. 2006), as well as in neurobiological studies assessing neuronal structure (McDonald et al. 2007) and nicotinic cholinergic activity (Slotkin et al. 2008). A likely neurobiological substrate underlying these changes in sensitivity is the mesolimbic dopamine system. Given that the mesolimbic dopamine system has been shown to underlie the locomotor stimulant action of nicotine (Clarke et al. 1988), and that dopamine neurons within the mesolimbic system are more sensitive to nicotine-induced long term potentiation during adolescence than adulthood (Placzek et al. 2009), the current study provides additional support for the idea that adolescent nicotine exposure produces persistent neuroadaptations in the mesolimbic dopamine system.
Previously reported findings that nicotinic cholinergic receptor (nAChR) levels are up-regulated in the midbrain of adult rats following adolescent treatment with nicotine may underlie the observations in the current study (Trauth et al. 1999; Abreu-Villaca et al. 2003; Adriani et al. 2003). Neuronal nAChRs have recently been directly linked to mediation of nicotine-stimulated locomotor activity (Gotti et al. 2010). While another recent study concluded that nicotine exposure resulted in less nAChR upregulation in adolescents than adults (Doura et al. 2008), a different group reported increased functionality of existing nAChRs following adolescent nicotine treatment (Kota et al. 2009). Thus, changes in receptor number or receptor function may play a role in the effects observed in the current study.
One possible shortcoming of the current study is the lack of precisely age-matched controls for the comparison of adolescent and adult nicotine effects. However, the inclusion of all three nicotine dose groups in both adolescence and adulthood allows for a cross-wise study design, and the inclusion of a final adulthood nicotine challenge session allows for investigation of the effects of all combinations of adolescent and adult treatments. The results obtained here support the notion that the study design was sufficient to investigate the differential effects of adolescent and adult nicotine exposure on subsequent sensitization to the behavioral effects of nicotine.
In summary, adolescent nicotine exposure results in enhanced sensitization to the locomotor activating effects of nicotine during adulthood, suggesting that drug exposure during adolescence can result in persistent changes in behavior and may enhance vulnerability to the reinforcing effects of nicotine and promote smoking in humans.
Acknowledgments
This work was supported by NIH grants AA07462 and AA10256. The authors would like to thank Susan Conroy for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling, enzyme induction, neurotransmitter-specific effects, regional selectivity, and the role of withdrawal. Brain Res. 2003;988:164–172. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology. 2006;184:382–90. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharm. 2008;45:1201–1207. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–95. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–75. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Marck BT, Matsumoto AM, Dorsa DM, Palmiter RD. Induction of stereotypy in dopamine-deficient mice requires striatal D1 receptor activation. Proc Natl Acad Sci. 2001;98:10451–6. doi: 10.1073/pnas.181356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Clarke PB, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988;246:701–8. [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produced persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Dev Brain Res. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Conti LH, Segal DS, Kuczenski R. Maintenance of amphetamine-induced stereotypy and locomotion requires ongoing dopamine activation. Psychopharmacology. 1997;130:183–8. doi: 10.1007/s002130050227. [DOI] [PubMed] [Google Scholar]
- Crusio WE. Genetic dissection of mouse exploratory behavior. Behav Brain Res. 2001;125:127–132. doi: 10.1016/s0166-4328(01)00280-7. [DOI] [PubMed] [Google Scholar]
- Domino EF. Nicotine induced behavioral locomotor sensitization. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:59–71. doi: 10.1016/s0278-5846(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav. 2003;74:917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Gironi Carnevale UA, Vitullo E, Sadile AG. Post-trial NMDA receptor allosteric blockade differentially influences habituation of behavioral responses to novelty in the rat. Behav Brain Res. 1990;39:187–195. doi: 10.1016/0166-4328(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Carbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–25. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kota D, Robinson SE, Damaj MI. Enhanced nicotine reward in adulthood after exposure to nicotine during early adulthood in mice. Biochem Pharmacol. 2009;78:873–9. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- Ksir C. Acute and chronic nicotine effects on measures of activity in rats: a multivariate analysis. Psychopharmacology. 1994;115:105–9. doi: 10.1007/BF02244758. [DOI] [PubMed] [Google Scholar]
- Lat J. The spontaneous exploratory reactions as a tool for psychopharmacological studies. Proc 2nd Int Pharm Meeting; Prague. 1963. pp. 47–66. [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Schwartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, Omar HA. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adol Psych. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Eppolito AK, Brielmaier JM, Smith LN, Bergstrom HC, Lawhead MR, Smith RF. Evidence for elevated nicotine-induced structural plasticity in nucleus accumbens of adolescent rats. Brain Res. 2007;1151:211–8. doi: 10.1016/j.brainres.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–81. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Age-dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–92. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Bran Res. 2010;206:240–4. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res Bull. 2008;76:152–65. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci and Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 1993;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]