Abstract
The purpose of this study was to examine the efficacy of a chemotherapeutic drug, doxorubicin (DOX), loaded in pH-sensitive micelles poly(l-histidine) (Mn:5K)-b-PEG (Mn:5K) micelles. The micelles were designed to target the acidic extracellular pH of solid tumors. Studies of pH-dependent cytotoxicity, growth rate of the tumor, pharmacokinetics and biodistribution were conducted. In vitro DOX uptake upon A2780 cells by incubating the cells in a pH 6.8 complete medium at a concentration of 20 μg DOX/ml in the micelle formulation was more than five times that of pH 7.4 condition for initial 20 min. In vivo pharmacokinetic data showed that AUC (area under concentration curve) and half life time (t1/2) (plasma half life) of DOX in the pH sensitive micelles increased about 5.8- and 5.2-fold of free DOX in phosphate buffered saline (PBS), respectively. It appeared that DOX in the pH-sensitive micelles preferentially accumulated in the tumor site. The distributions at 12 h post injection in other organs including liver, kidney, spleen, lung and heart were not significantly different from those of DOX in PBS at a 6 mg DOX/kg dose. The in vivo test of anti-tumor activity was performed with human ovarian carcinoma A2780 which was subcutaneously xenografted in female nu/nu athymic mice. The pH-sensitive micelle formulation significantly retarded tumor growth rate without serious body weight loss. The triggered drug release by the reduced tumor pH is believed to be a major mechanism of the observed efficacy after passive accumulation of the micelles by EPR effect. This may have resulted in a local high dose of drug in the tested solid tumor.
Keywords: pH-sensitive micelle, poly(l-histidine)-b-PEG, biodistribution, pharmacokinetics, ovarian tumor
Introduction
Cancer chemotherapy is often complicated by toxic side effects of anti-cancer drugs. Despite advances in the development of new anti-tumor drugs, these drugs still possess significant serious side effects that limit the dose size as well as use of the drugs.
Triggered release of a drug from long circulating vesicles at tumor sites while maintaining a minimal release rate during circulation, which may result in a high local dose in tumor sites and less side effects, is a very desirable property in carrier designs for tumor chemotherapy. Approaches to achieve triggering systems include magnetic (Pardoe et al. 2003), thermal (Needham et al. 2000), and ultrasonic activation (Rapoport et al. 2004) for enhanced drug release from the carriers, which were specifically designed to respond to external signals or energy. The external activation could be useful for the tumors whose location is well-identified and which are accessible by external signal or energy sources. In addition it would be technically difficult to restrict the activation only in tumor regions and thus the external activation does not distinguish the blood compartment and tumor cells.
The difference in pH between solid tumors and normal tissue properties has been long recognized. For instance, the average extracellular pH values of human solid tumors xenografted in mice fall below 7.0. The difference in pH is small but apparent as a natural signal of solid tumors for triggered release. Some approaches have described drug release by the cleavages of chemical bonds, which conjugate a drug to polymers, by endosomal pH (Kataoka et al. 2000, Park 2002). However, chemical bond cleavage is not currently applicable for a triggered release by such small difference in extracellular pH.
It has been difficult to develop effective pH-sensitive formulations responding to tumor extracellular pH due to the lack of a proper pH-sensitive functional group in the physiological pH range (Sikic et al. 1997, Tanigawara 2000). Recently, Bae’s group reported new self-assembled pH-sensitive polymeric nanocarriers. The carriers were modified with acidic sulfonamide and characterized for enhanced drug release and internalization into cells at tumor pH (Na and Bae 2002, Han et al. 2003, Na et al. 2003). These self-assembled nanocarriers or micelles switched their surface properties from hydrophilic to hydrophobic by deionization of sulfonamide group at tumor pH, resulting in distribution and reorganization of the self-assembly structures. This caused enhanced drug release and interactions with cells for internalization. The shortcoming of the system was that the carriers became more rigid and aggregated at tumor pH which may cause delayed elimination of the carriers from the tumor sites or the body. On the other hand, a mixed polymeric micelle system, consisting of poly(l-histidine) and poly(dl-lactide) for core and PEG for shell, responded to acidic tumor pH or early endosomal pH by ionization of histidyl groups and destabilization of the structure. The mixed micelle was more stable at pH 7.4 than poly(l-histidine)/PEG micelle. The detailed micelle characteristics and pH-dependent stability were reported (Lee et al. 2003a). Folate receptor mediated endocytosis and early endosomal pH targeting suggested the feasibility to overcoming mutidrug resistance in vitro (Lee et al. 2003b) and in vivo (Lee et al. 2005).
This study aims to target triggered release solely by extracellular pH of sensitive solid tumors using poly(l-histidine)/PEG based polymeric micelles. It is further anticipated that the pH-sensitive micelle formulations will avoid or minimize the road-block effect of the carriers because the enhanced release is attributed by destabilization of the micelle core (carrier accumulation in the vicinity of tumor vasculature after extravasation) (Ishida et al. 2001) for subsequent administration. In this study, we evaluate pharmacokinetics and biodistribution of doxorubicin (DOX) loaded pH-sensitive polymeric micelles in the ovarian A2780 tumor-bearing mice, the efficacy of triggered release as well as their anti-tumor activity.
Materials and methods
Materials
RPMI-1640, Na2B4O7, NaN3, triethylamine (TEA), ethylenediamine tetraacetic acid (EDTA), DOX·HCl and Daunomycin·HCl (for HPLC internal standard) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). TEA, glutaraldehyde, methanol, chloroform, isopropyl alcohol and dimethylsulfoxide (DMSO) were provided from J. T. Baker (Deventer, The Netherlands). Solvable was purchased from NEN/ Dupont (USA). Penicillin–streptomycin, Tris–HCl (pH 8.4), fetal bovine serum (FBS), Sorensen’s buffer, 0.25% (w/v) trypsin–0.03% (w/v) EDTA solution, phosphate buffer solution (PBS) were purchased from Gibco Co. (Uxbridge, UK). Cell counting kit-8 was purchased from Dojindo Molecular Technologies, Inc. (Gaithersburg, MD, USA). Poly(l-histidine)/PEG block copolymer was synthezized in house as previously described in a separate report (Lee et al. 2003a). Briefly, after l-histidine (His) was derivatized by introducing a carbobenzoxy (CBZ) group to the α-amino group, the amino group in the imidazole ring of Nα-CBZ-l-histidine was protected with a dinitrophenyl (DNP) group. Nα-CBZ-Nim-DNP-l-histidine was then transformed to N-carboxy anhydride (NCA) form by thionyl chloride. The NCA was initiated with isopropylamine for ring-opening polymerization. The purified poly(Nim-DNP-His) was coupled with carboxylated PEG to yield diblock copolymers. The polymer was then deprotected by thiolysis with 2-mercaptoethanol to yield polyHis(Mn:5K)/PEG(Mn:5K).
DOX loaded pH-sensitive micelle
DOX loaded polyHis/PEG micelle formulation was prepared as described before (Lee et al. 2003a,b). Briefly, DOX·HCl was stirred with excess TEA (2 × DOX·HCl) in DMSO overnight to obtain DOX base. The block polymer (50 mg) was dissolved in 10 ml of DMSO and mixed with a DOX base solution (10 mg DOX base in 10 ml DMSO) and stirred for 3 h. The solution was transferred to a preswollen dialysis membrane (Spectra/Por molecular weight cut-off 15,000) and dialyzed against NaOH–Na2B4O7 buffer solution (pH 9.0) for 24 h at 4°C. The medium was exchanged several times and the content in the dialysis tube was subsequently lyophilized. The amount of entrapped DOX was determined by HPLC after dissolution in 10% DMSO in methanol. DOX analaysis was performed using a Supelco LC-18 column (250 × 4.6 mm i.d., 5 μm particle size) and Hitachi HPLC instrument (D-6000 interface; L-4200H UV–VIS detector; F-1080 Fluoresence Detector; L-6200A intelligent pump and AS-2000 Autosampler). The mobile phase was acetonitrile–0.1% NaH2PO4 solution (40:60 v/v) adjusted to pH 3.0 with H3PO4; the flow rate was 1.0 ml/min. DOX was eluted at 6.2 min and detected by a fluorescence detector with excitation wavelength at 480 nm and emission wavelength at 550 nm. The drug loading efficiency of micelles was in the range of 75–85%. The DOX loaded pH-sensitive micelles is termed “DOX micelle” hereafter.
Particle size distribution by Zetasizer
The particle size of the micelle (0.1 g/l) was measured by Zetasizer. Photon correlation spectroscopy (PCS) was conducted with a Zetasizer 3000 (Malvern Instruments, USA) with He–Ne laser beam at a wavelength of 633 nm at 25°C on a fixed scattering angle of 90°. The mean particle sizes of the DOX micelle were 95 ± 12 nm.
In vitro flow cytometry
A2780 mt ovarian cancer cells were cultured in 75 mm cell culture flasks using RPMI 1640 media supplemented with 10% FBS, 0.4% nistatin, 1.2% insulin, 1.2% Penicillin–streptomycin. Media was changed every other day. Incubator was maintained at 5.0% CO2 and 36.5°C. The cells (1 × 106 cells/well) were seeded in a 6-well plate and incubated overnight and harvested by 0.2% (w/v) trypsin–0.1% (w/v) EDTA solution. A measure of 2 ml DOX micelles with a 20 μg/ml DOX concentration in medium was introduced to each well and incubated for 20 min. The cells were trypsinized and washed three times with PBS solution and then fixed with 2.5% glutaradehyde. After filtering through a nylon mesh, cell fluorescence was measured by flow cytometry (FACSCAN, Becton Dickinson) described in detail in Gao et al. (2004).
Cytotoxicity
The A2780 cells (5 × 104 cells/ml) growing in flasks were harvested by 0.2% (w/v) trypsin–0.1% (w/v) EDTA solution. Two-hundred microlitres of cells in RPMI 1640 complete medium were seeded in a 96-well plate and incubated for one day. The stock DOX solution (6 mg/ml, pH 7.4) was diluted to prepare the micelle solutions with various DOX concentrations (10–0.0001 μg/ml) for cell cytotoxicity tests. Each solution was diluted again with to RPMI 1640 cell culture complete medium. The pH of the culture medium was adjusted pH 7.4 and 6.8 with 0.1 N HCl. The cells were washed three times with PBS (pH 7.4) after 72 h incubation at pH 7.4 and 6.8 complete medium. Two hundred microlitres of medium containing 20 μl of Cell Conuting Kit-8 (Dojido, Gaithersburg, MD, USA) solution were added to each well and the plate was incubated for an additional 4 h. The absorbance of each well was read with a microplate reader (Model 680 microplate reader, Bio-Rad) using a test wavelength of 450 nm.
Pharmacokinetics
To examine the pharmacokinetics of DOX micelles in the mice body, DOX in PBS solution and the DOX micelles were injected by i.v. through the lateral tail vein in mice. At given time intervals (5, 30 min, 1, 2, 4, 12 and 24 h) post-injection, the mice were anesthetized by methoxyflurane. Blood was collected via cardiac puncture or main belly artery, placed in microtubes with 10 μl 50 U/ml heparin. The whole blood (0.8 ml) was transferred to a tube, containing 200 μg daunomycin as the internal standard and 0.01 mol silver nitrate was added to DOX sample to prevent DOX from binding to DNA (Murdter et al. 1998). Triple extraction was performed after adding chloroform/isopropyl alcohol (3:1 v/v). The solutions were frozen overnight, thawed, and centrifuged at 16,000g for 10 min. The organic phase was removed and the resulting solutions were evaporated to dryness and re-dissolved in a mobile phase solution (methanol/isopropyl alcohol/sorensen’s buffer = 2:2:6 v/v/v). These samples were analyzed (at excitation wavelength = 480 nm and emission wavelength = 560 nm) using a HPLC system. HPLC of DOX was performed using a Supelco LC-18 column (250 × 4.6 mm i.d., 5 μm particle size) and Hitachi HPLC instrument (D-6000 interface, F-1080 Fluoresence Detector; L-6200A intelligent pump, and AS-2000 Autosampler). The non-comparmental pharmacokinetic parameters including the area under the drug concentration time curve (AUC), t1/2, clearance (CL), volume of distribution of drug (Vd) and mean retention time (MRT) were calculated using the trapezoidal rule (Gihaldi and Perrier 1982). The data between the different formulations were compared for statistical significance by the one-way analysis of variance (ANOVA).
Biodistribution
DOX micelles and DOX PBS solution were injected through the tail vein of the tumor bearing mice with the total dose of DOX 6 mg/kg. Animals were sacrificed 12 h i.v. post injection then tumor, liver, kidney, spleen, lung and heart were excised, dried by filter paper, digested and extracted with trypsin, and fixed with 2.5% glutaraldehyde. After filtering through nylon mesh, cell fluorescence was measured by flow cytometry (Gao et al. 2005). Fluorescence histograms were recorded with a Facscan (Beckton Dickinson) flow cytometer and analyzed using CellQuest softerware supplied by the manufacturer. Minimums of 10,000 events were analyzed to generate each histogram.
Anti-tumor activity
Five to six week old female nu/nu mice (BALB/c mice) were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were accommodated in an autoclaved micro-isolator cage housed in appositive pressure containment rack and maintained under the guidelines of an approved protocol from the University of Utah Institutional Animal Care and Use Committee. They were randomly assigned to experimental and control groups of five or seven animals each. The xenografts of human overian A2780 carcinoma were developed by subcutaneously implanting 2 × 106 A2780 cells in the right rare flank from cell culture in the nude mice, as described previously (Gao et al. 2005). When the tumor volume reached around 50–100 mm3, animals were treated four times at 3-day intervals with either DOX dissolved PBS or encapsulated in pH sensitive polyHis/PEG micelles. Both formulations were injected intravenously via mice tail vein at the dose of 3 mg/kg through 25G5/8 needles.
The tumor inhibition activity was assessed with the tumor volume size, which was calculated by the following equation: V = (w)2 × (l )/2, where (w) and (l ) are width and length of the tumor measured by a caliper. The body weight was measured simultaneously as a factor of side effect from formulation.
Results and discussion
We hypothesized that polyHis/PEG based pH-sensitive micelles are destabilized in neutral or slightly acidic environment, resulting in triggered DOX release followed by enhanced drug permeation into tumor cells due to high concentration gradients, while maintaining a low DOX release rate during circulation in the blood compartment. Fluorescence histograms of A2780 cells incubated with pH 7.4 and 6.8 media at an identical concentration of DOX loaded in the micelles for initial 20 min are presented in Figure 1A. Three cell population-profile patterns were similar but the profiles were shifted to higher fluorescence intensity regions when the cells were treated with the DOX micelle formulation at two different pHs (normal blood pH and tumor extracellular pH) at DOX 20 μg/ml for 20 min. The DOX fluorescence intensity of the cells treated at pH 6.8 appeared approximately five times higher than at pH 7.4, confirming higher DOX availability to the cells at pH 6.8 from the beginning of treatment.
Figure 1.

(A) Fluorescence histograms of A2780 cells incubated with free DOX or DOX loaded in polyHis/PEG pH-sensitive micelles. The DOX concentration in the incubation media was maintained constant at 20 μg/ml. For the micelle formulation, DOX (20 μg) was incorporated in 125 μg polyHis/PEG micelles/ml solution. Cells incubated in pH 6.8 medium (bold lines), pH 7.4 medium (dotted lines) and control plain medium without DOX (for fluorescence background, regular lines). All cells were incubated for 20 min after adding the formulations; (B) The cytotoxicity of DOX PBS and DOX polyHis/PEG micelles at pH 7.4 and 6.8 after 48 h incubation with varying the concentration of DOX and the block polymer.
This observation prompted us to micelle cytotocixity at pH 7.4 and 6.8. The viability results of A2780 are presented in Figure 1B. The viability, when treated with the DOX micelles at 1 μg/ml DOX concentration for 72 h, was 72% at pH 7.4 but 36% at pH 6.8. The cells at pH 7.4 were still killed due to DOX released from the micelle or cellular uptake of the intact micelles most probably by fluid phase pinocytosis. Although IC50 at pH 7.4 is located out of the concentration range tested in this study, it is apparent that the ratio of IC50 values at two pHs may be somewhere in a 20–200 range.
DOX concentration profiles in the plasma after i.v. administration of free DOX and the DOX micelles are shown in Figure 2. The DOX micelles showed a remarkably prolonged DOX profile in the blood; however, free DOX was rapidly eliminated from the blood compartment. For the DOX micelle formulation, the DOX concentration combined by DOX in the micelles and released DOX was retained >33% of the initial total DOX concentration in the blood up to 4 h, and >10% of the blood DOX level even at 24 h, while DOX PBS solution exhibited 5% of the initial blood concentration after 4 h. The free DOX concentration after 8 h was not detectable in our measurement condition (detection limit: 50 ng/ml). The effective elimination half-life t1/2 of the micelle formulation was five-times longer than DOX PBS solution (10.9 vs. 2.1 h), while the total CL was decreased (1818.2 vs. 311.9 ml min−1 kg−1). PEG graft on the surface of micelles seems to be responsible for the increased circulation time in the blood. Also the mean residence time (MRT) (2.2 vs. 12.7 h) and the VD at steady state (Vdss) (3998.4 vs. 3956.0 l/kg) were calculated for the DOX micelles and DOX PBS solution.
Figure 2.

The blood concentration-time profiles of DOX after i.v. administration of DOX PBS and the DOX micelles. Each formulation was administered to mice (female, n = 3) at a DOX dose of 6 mg/kg.
DOX biodistribution was assessed by flow cytometry, based on the intracellular fluorescence intensity of DOX of various organs. The advantage of this technique is that it does not require organ homogenization and allows to measure intracellular drug concentration, while other methods that require tissue homogenization do not discriminate the location of DOX either in insterstitium or inside cells.
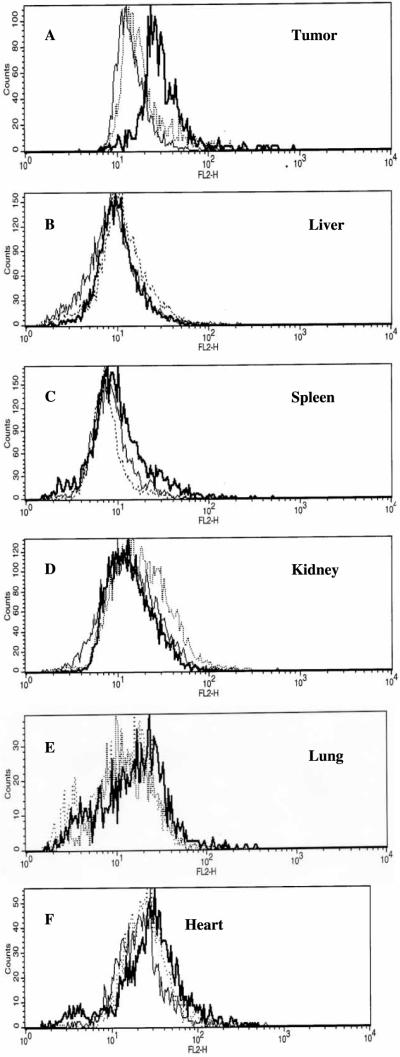
The intracellular DOX fluorescence in the liver, kidney, spleen, lung and heart cells remained close to the autofluorescence of the corresponding control cells when the DOX micelles and DOX PBS solution were intravenously administered at 6 mg/kg DOX concentration, indicating a low uptake by cells for the given DOX concentration as shown in Figure 3B–F. However, the DOX fluorescence intensity in tumor cells was significantly higher for the DOX micelles than for DOX PBS and control as shown in Figure 3 A (the regular line is for control; the dotted line for DOX PBS; and the bold line for DOX micelle).
Figure 3.
Fluorescence histograms of (A) tumor; (B) liver; (C) kidney; (D) spleen; (E) lung and (F) heart cells in mice post 12 h after i.v. injection of the formulations. DOX PBS (regular lines), DOX micelles (bold lines) and control (dotted lines). The DOX dose size of each formulation was 6 mg/kg.
These pharmacokinetic and biodistribution results strongly suggest that the DOX micelle formulation resulted in better accumulation in A2780 tumor with minimal distribution to other organs after 12 h than DOX PBS formulation. This may be attributed to longer plasma half-life of DOX micelle formulation and triggered release of DOX by tumor pH after extravasation.
The A2780 cells xenografted in nude mice were used for the study of the tumor growth inhibition in vivo. The results of the anti-tumor activity of the DOX loaded micelles injected intravenously on days 0, 3, 6 and 9 in nude mice bearing s.c. A2780 cells are shown in Figure 4A. The DOX micelle group significantly inhibited the growth of A2780 xenografts in nude mice via the four i.v. injections when compared to free DOX treatment after 5 weeks ( p < 0.01 compared with free DOX). The body weight change curve shows that mice weight loss was less than 5% by treating with both DOX PBS and the DOX micelles, as shown in Figure 4B. This may be related to the low dose size used in the study. The treated mice started recovering their body weights soon after injections were stopped.
Figure 4.
(A) Effects of the DOX micelles and DOX PBS on the growth of A2780 ovarian carcinoma s.c. transplanted in nu/nu mice (female, n = 7). Each formulation was administered four times at three-day intervals (arrows) at a dose of 6 mg/kg; (B) Body weight changes of A2780 tumor bearing nu/nu mice (female, n = 7) treated with saline, DOX PBS, and DOX micelle. Each formulation was administered four times at three-day intervals (arrows) at a dose of 6 mg/kg DOX.
The volume of tumors treated with the pH sensitive micelles was approximately 4.71-fold smaller than that treated with DOX PBS solution after 39 days. This result could be attributed to the triggered release of DOX by tumor extracellular pH (pHe < 7.0) after accumulation of pH sensitive micelle in the tumor sites via the EPR effect. PEGylated carriers are known to be long circulating vehicles in the blood vessel. Hence they are not only prolong drug half-life time but also reduce drug accumulation in the normal tissue. The pH sensitive micelle modified with PEG (5K) greatly improved its solubility and increase circulating time in the blood steam as shown in Figure 2. This property provides a more effective modality in tumor chemotherapy with high local concentrations of the drug at tumor sites, while minimizing release of the drug from micelles to normal tissue during the circulating in the blood (pH 7.4).
In summary the tumor treatment with DOX loaded polyHis/PEG pH-sensitive micelles retarded tumor growth and caused minimal weight loss in mice. The pH sensitive micelles with the triggered drug release mechanism at tumor sites effectively kill sensitive tumor cells and its application may expand to different tumors and different types of anti-cancer drugs.
Acknowledgements
This work was supported by NIH CA101850.
References
- Gao ZG, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm. 2004;1:317–330. doi: 10.1021/mp049958h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Rel. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gihaldi M, Perrier D. Pharmacokinetics. Marcel Dekker; New York: 1982. [Google Scholar]
- Han SK, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: Physicochemical characteristics and pH-dependent aggregation. Colloids Surf A Physicochem Eng Aspects. 2003;214:49–59. [Google Scholar]
- Ishida T, Kirchmeier MJ, Moase EH, Zalipsky S, Allen TM. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta. 2001;1515:144–158. doi: 10.1016/s0005-2736(01)00409-6. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-l-aspartate) copolymer micelles: Their pharmaceutical characteristics and biological significance. J Control Rel. 2000;64:143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate mediated targeting. J Control Rel. 2003a;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Rel. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Lee ES, Shin HJ, Na K, Bae YH. Poly(l-histidine)-PEG Block Copolymer Micelles and pH-induced destabilization. J Control Rel. 2003b;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- Murdter TE, Sperker B, Bosslet K, Fritz P, Kroemer HK. Simultaneous high-performance liquid chromatographic determination of a glucuronyl prodrug of doxorubicin, doxorubicin and its metabolites in human lung tissue. J Chromatogr B Biomed Sci Appl. 1998;709:289–295. doi: 10.1016/s0378-4347(98)00079-6. [DOI] [PubMed] [Google Scholar]
- Na K, Bae YH. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: Characterization, aggregation and adriamycin release in vitro. Pharm Res. 2002;19:681–688. doi: 10.1023/a:1015370532543. [DOI] [PubMed] [Google Scholar]
- Na K, Lee ES, Bae YH. Adriamycin loaded pullulan acetate/sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J Control Release. 2003;87:3–13. doi: 10.1016/s0168-3659(02)00345-0. [DOI] [PubMed] [Google Scholar]
- Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- Pardoe H, Clark PR, St Pierre TG, Moroz P, Jones SK. A magnetic resonance imaging based method for measurement of tissue iron concentration in liver arterially embolized with ferrimagnetic particles designed for magnetic hyperthermia treatment of tumors. Magn Reson Imaging. 2003;21:483–488. doi: 10.1016/s0730-725x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Park TG. Perfusion culture of hepatocytes within galactose-derivatized biodegradable poly(lactide-co-glycolide) scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2002;59:127–135. doi: 10.1002/jbm.1224. [DOI] [PubMed] [Google Scholar]
- Rapoport NY, Christensen DA, Fain HD, Barrows L, Gao Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics. 2004;42:943–950. doi: 10.1016/j.ultras.2004.01.087. [DOI] [PubMed] [Google Scholar]
- Sikic BI, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40:13–19. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- Tanigawara Y. Role of p-glycoprotein in drug disposition. Ther Drug Monit. 2000;22:137–140. doi: 10.1097/00007691-200002000-00029. [DOI] [PubMed] [Google Scholar]