Abstract
Biologically motivated mathematical models are important for understanding the mechanisms of radiation-induced carcinogenesis. Existing models fall into two categories: (1) short-term formalisms, which focus on the processes taking place during and shortly after irradiation (effects of dose, radiation quality, dose rate and fractionation), and (2) long-term formalisms, which track background cancer risks throughout the entire lifetime (effects of age at exposure and time since exposure) but make relatively simplistic assumptions about radiation effects. Grafting long-term mechanisms on to short-term models is badly needed for modelling radiogenic cancer. A combined formalism was developed and applied to cancer risk data in atomic bomb survivors and radiotherapy patients and to background cancer incidence. The data for nine cancer types were described adequately with a set of biologically meaningful parameters for each cancer. These results suggest that the combined short–long-term approach is a potentially promising method for predicting radiogenic cancer risks and interpreting the underlying biological mechanisms.
INTRODUCTION
Biologically based mathematical models which have the potential to predict radiation-induced cancer risks caused by modern radiotherapy protocols allow the risks of second cancers to be estimated and minimized during treatment plan optimization. This task is becoming increasingly important because of the increasing number of younger individuals undergoing radiation therapy and improving survival times after treatment. For example, the 5-y relative survival rate for prostate cancer in the USA has increased from ∼67 to almost 98 % and the mean age at diagnosis decreased from 72 to 69 over the past few decades(1, 2). Ten-year survival rates have also improved substantially over the same time.
Patients exposed to radiotherapy as children are probably inherently more sensitive to radiation-induced carcinogenesis than adults and have a longer life expectancy. Consequently, radiotherapy-induced second cancers are a particularly important issue for childhood cancer survivors. Relative risks of cancer in exposed children can be very high, on the order of 10–100 after typical radiotherapy treatments(3).
Many retrospective epidemiological studies of second-cancer risks after radiation therapy have been conducted(3–11). However, radiotherapy treatment techniques are changing quite rapidly, especially in terms of escalating treatment dose, altered dose fractionation and altered normal-tissue dose distributions such as from intensity-modulated radiation therapy(12–14). Radiation-induced second cancers typically develop after a long latency period of a decade or more following exposure(15, 16). For these reasons, risks estimated based on decades-old radiotherapy methods cannot generally be directly applied to modern or prospective protocols. This problem can potentially be solved by developing mathematical models which can predict the second-cancer risk of any given radiotherapy protocol tusing target organ dose distributions (dose–volume histograms (DVHs)). Such models can also provide insight into the underlying mechanisms of radiation carcinogenesis and, as argued, represent a useful initial step towards the reduction of radiotherapy-induced second-cancer risks.
Many radiation carcinogenesis models have been proposed and used over the past several decades(17–35). Some can be called short-term models, meaning that they focus on processes such as cell killing, mutagenesis and chromosome aberrations which occur during and shortly after irradiation(30, 34, 36–38). They typically provide a detailed dose–response relationship for the selected endpoints, but do not directly address the complexity of those processes that take place before exposure and many years to decades after exposure, up to the time when cancer develops. The short-term models help to understand the effects of dose/fluence, radiation quality, dose rate and dose fractionation.
A very different approach is employed in long-term models(17, 20, 21, 24, 39), which encompass the entire lifetime, e.g. tracking the kinetics of pre-malignant cell clones. They concentrate on background carcinogenesis processes, but generally treat radiation exposure as a simple (e.g. linear as function of dose) modulation of the background rates. The long-term models help to understand the effects of age at exposure, time since exposure and modulation of background cancer rates.
Because radiation-specific effects are treated simplistically by most long-term models, predictions from such models are typically limited to exposure conditions where a simple dose–response relationship holds. In situations where this relationship is more complex, such as high fractionated doses to organs located in proximity to the radiotherapy target volume, current long-term models have limited utility. Conversely, the detailed dose–responses produced by short-term models can be used to estimate cancer risks only by considering the effects of factors such as background risks, age at exposure and time since exposure, which are not directly taken into account by short-term formalisms. A unified approach of integrating short- and long-term methods is needed, where a detailed initial dose–response for pre-malignant cell numbers is produced over a wide range of radiation doses, and changes to the shape of this dose–response during the latency period before the development of cancer are also analysed in detail.
METHODS
Model assumptions
The unified short–long-term formalism discussed here belongs to the class which can be called initiation, inactivation and repopulation (iir) models(30, 34, 36, 38). It was described in detail previously(40, 41). It assumes that the target cells for radiation carcinogenesis are organ-specific stem cells, which reside in specialized stem cell niches or compartments. The number of such niches per organ and the number of stem cells per niche are homeostatically regulated. Radiation can kill stem cells, causing them to lose the ability to generate a clone (inactivation). Surviving cells respond by compensatory proliferation, attempting to restore pre-irradiation stem cell numbers (repopulation). Radiation can also alter normal stem cells (e.g. by causing mutations in tumour suppressor genes or other critical areas of the genome), moving these altered cells into a pre-malignant state. This phenomenon is called initiation, and it can also occur spontaneously during normal ageing, with some low probability per unit time. These three processes of iir comprise the short-term component of the formalism.
Long-term models, into which the short-term mechanisms are embedded, describe timescales of years or decades. Once an initiated stem cell is produced in a given stem cell niche, either spontaneously or due to radiation exposure, this cell can give rise to a pre-malignant clone. The clone can die out by stochastic extinction due to spontaneous cell death or due to cell killing by radiation. If the clone survives, it will take over the stem cell niche relatively quickly (in less than a year) because pre-malignant stem cells are assumed to have a net growth advantage over their normal counterparts (e.g. because pre-malignant cells are more resistant to apoptosis and less dependent on exogenous growth factors for proliferation). The stem cell niche will then become fully pre-malignant, i.e. filled with pre-malignant cells.
The number of pre-malignant cells per pre-malignant niche may be greater than the number of normal stem cells in a normal niche, but it is nevertheless assumed to be regulated by homeostatic mechanisms. Radiation exposure can weaken these regulatory mechanisms, allowing pre-malignant niches to grow larger in the period after irradiation. This process is called promotion.
A pre-malignant clone can gradually spread beyond the niche in which it originated, either by invading and taking over adjacent niches, or by division (splitting) of the original niche and growth of each of the daughter niches to full size. These processes result in a net clonal expansion with approximately exponential kinetics on the timescale of multiple years and decades.
The model assumes that any pre-malignant cell in any clone has a certain small probability per unit time of becoming a fully malignant cell, eventually capable of giving rise to clinical cancer. This process is called malignant transformation. It is assumed to be unaffected by radiation, but is affected by the patient's age: at older ages, the carcinogenic potential of pre-malignant cells decreases. Such a decline can be caused by age-dependent loss of stem cell function, stem cell niche function or both. It is consistent with the evidence of declining cancer risks at very old ages in both animals and humans(28, 29). Eventual cancer risk is assumed to be proportional to the number of pre-malignant cells, shifted by a lag time (e.g. 10 y) needed for a fully malignant cell to grow into a clinically detectable tumour.
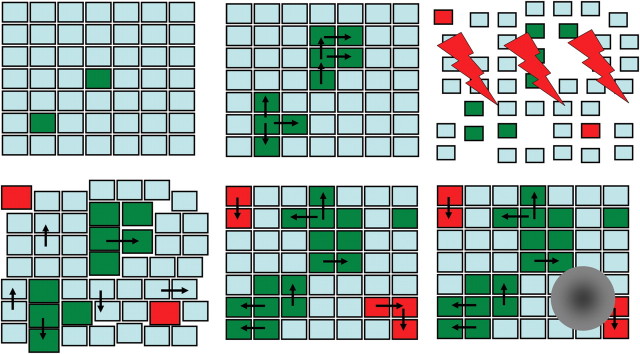
The processes of promotion, clonal expansion and transformation comprise the long-term component of the formalism. Model assumptions regarding the proposed stem cell kinetics, both short- and long-term, are shown in Figure 1.
Figure 1.
A representation of model assumptions. Each small square represents a stem cell niche. Cyan squares are normal stem cell niches, green squares are spontaneously initiated pre-malignant niches and red squares are pre-malignant niches initiated by radiation. The order of panels (left to right) represents a time sequence: two niches are spontaneously initiated and grow by invasion of adjacent niches (black arrows). Radiation (lightning symbols) reduces the number of cells in all niches (squares become smaller) and eliminates some niches entirely (gaps). It also initiates two new niches (red squares). After exposure, killed cells are replaced by compensatory proliferation of surviving cells (gaps disappear, initial normal niche sizes are restored) and pre-malignant niches expand in size (promotion, shown by larger green squares). Over time, promotion is reversed (green squares return to default size), but invasion of normal niches by pre-malignant ones continues and spontaneous initiation continues as well. Eventually, malignant transformation occurs in one of the pre-malignant niches and a tumour is formed (grey mass).
Mathematical implementation
These assumptions are implemented mathematically in a mixed deterministic–stochastic formalism, which was described in the previous papers(40, 41). The long-term processes, i.e. the pre-malignant stem cell dynamics before irradiation and years–decades after irradiation until the development of cancer, are described by deterministic equations, mainly to reduce the number of adjustable parameters. The short-term processes, i.e. the dynamics during radiotherapy and the stem cell population recovery period a few weeks–months after exposure, are modelled stochastically. Here, the stochastic approach was used because cell inactivation (killing) is extensive during radiotherapy, making stochastic extinction of many pre-malignant clones a very real possibility which should not be neglected.
Consequently, the number of pre-malignant cells (and hence the cancer risk) is estimated as follows: (1) deterministic long-term equations are used to track the average expected number of pre-malignant niches from birth until radiation exposure. (2) This number is used as the input for stochastic equations, which estimate the effects of radiation, e.g. a multi-fraction radiotherapy protocol. At the end of this step, the average number of surviving pre-malignant stem cell niches is calculated, and the promoting effects of radiation on the number of pre-malignant cells per niche are also included. (3) The results serve as the input for deterministic long-term equations, which are used until old age.
Four model parameters can be derived from background cancer incidence and therefore do not directly depend on radiation: spontaneous stem cell initiation and subsequent malignant transformation (a, units = time−2), pre-malignant niche replication (b, units = time−1), stem cell ageing (c, units = time−2) and the lag time L from the appearance of the first malignant cell until the development of cancer. Seven other parameters describe the effects of radiation: initiation (X, units = time dose−1), promotion (Y, units = dose−1), homeostatic regulation of the number of pre-malignant stem cells per niche (δ, units = time−1), the carrying capacity for the number of pre-malignant stem cells in a niche (Z, units = cells niche−1), the stem cell radiation inactivation constants (α, units = dose−1 and β, units = dose−2) and the maximum net stem cell repopulation rate (δ, units = time−1). Not all of these parameters are needed when only the relative risk of radiation-induced cancer is estimated—in this case, parameters a and c cancel out(40, 41). Further simplification is possible if the irradiation protocol involves only a single acute dose instead of a series of doses—in that case, there is no proliferation during exposure, eliminating λ. For relatively low doses (<1 Gy), cell killing can also be neglected, eliminating α, β and Z. Consequently, depending on the situation, the number of adjustable parameters can be substantially reduced.
RESULTS AND DISCUSSION
The combined short–long-term formalism was applied to three sets of data for a total of nine solid cancer types (breast, lung, stomach, thyroid, pancreatic, bladder, brain, colon and rectal): (1) background cancer incidence (from US Surveillance, Epidemiology and End Results (SEER) database), (2) radiogenic cancer risks in Japanese atomic bomb survivors and (3) radiotherapy-induced second-cancer risks from a variety of second-cancer studies. A single set of parameters was used to fit all data for each cancer type. The results were presented in two previous papers(40, 41).
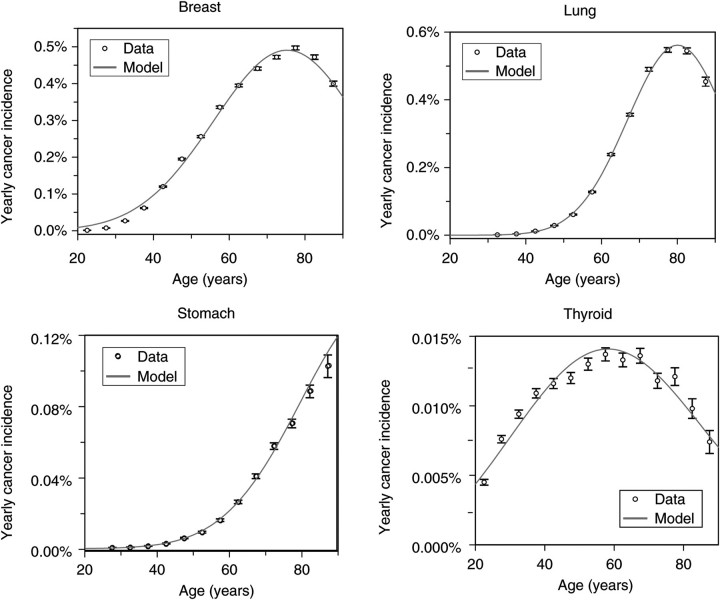
Here, in Figures 2–4, the results are shown only for four cancers: female breast, male lung, male stomach and gender-averaged thyroid. These particular types were chosen because they represent different patterns of risk behaviours.
Figure 2.
Best-fit model predictions for US background incidence (from the SEER database) for some analysed cancer types (female breast, male lung, male stomach and gender-averaged thyroid). Error bars represent 95 % confidence intervals.
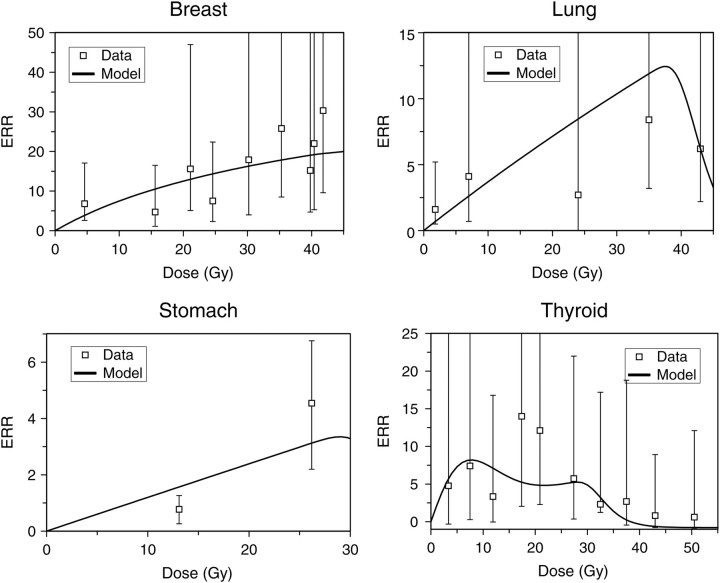
Figure 4.
Best-fit model predictions for high-dose fractionated radiotherapy ERRs for the same cancer types as in the previous figures. The error bars represent 95 % confidence intervals, and the points were taken from the literature(8–11, 43).
Spontaneous breast cancer incidence rises quickly with age throughout most of life, but then peaks and turns over at around age 80 (Figure 2). A similar trend is seen for lung cancer, where the rate of initial rise in incidence is even steeper. In both cases, the rapid increase in incidence is attributed to clonal expansion of pre-malignant cells (e.g. by invasion of adjacent niches by pre-malignant niches), and the turnover at old age is attributed to ageing of pre-malignant stem cells in all niches. Stomach cancer incidence possibly also turns over, but this may occur at very old ages (>90) for which the data are less accurate. Thyroid cancer incidence, on the other hand, peaks at a much younger age of 60.
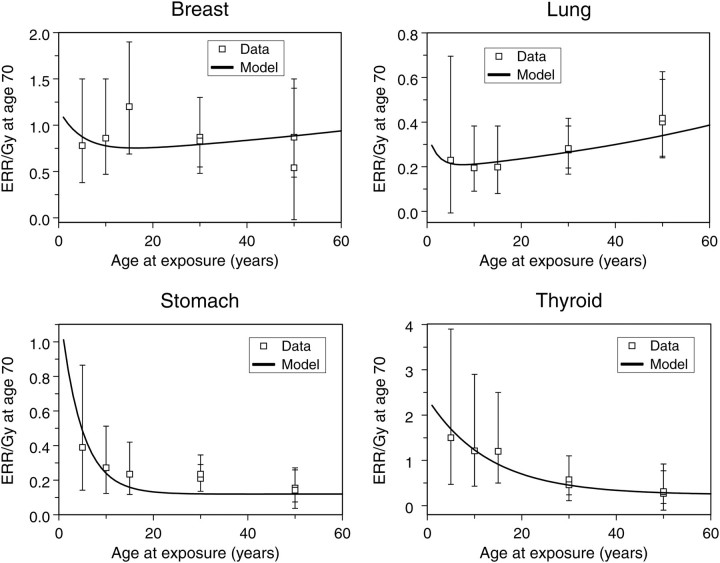
The estimated excess relative risks per unit of radiation dose (ERR/Gy) in Japanese atomic bomb survivors (Figure 3) also display different cancer type-specific patterns as a function of age at exposure. For example, stomach and thyroid cancer ERRs decrease with age at exposure, for lung cancer the ERR appears to increase and for breast cancer it is approximately stable with age at exposure. Notable differences between cancer sites can also be seen in risks at high radiotherapeutic doses (Figure 4).
Figure 3.
Best-fit model predictions for ERR/Gy estimates from Japanese atomic bomb survivors, as the function of age at exposure, for the same cancer types as in the previous figure. The points and error bars (95 % confidence intervals) are taken from Preston et al.(42).
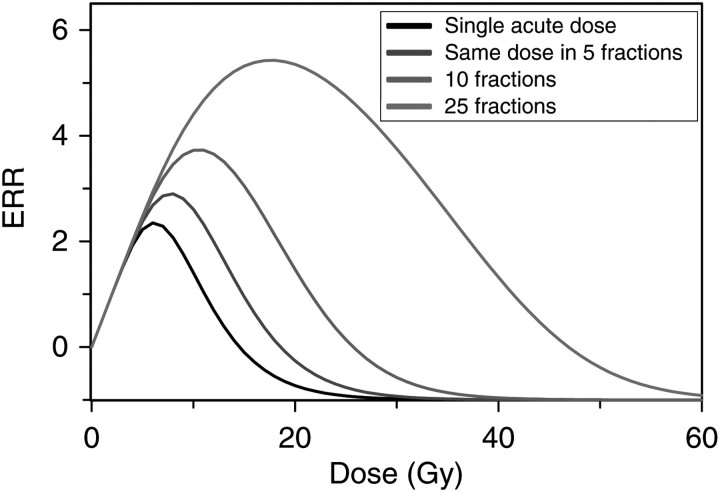
Dose fractionation is predicted to have a substantial effect on model-based estimates of second-cancer risks (Figure 5). Combined with the cancer type-specific risk differences shown in earlier figures, this suggests that in order to obtain useful predictions of second-cancer risk, one must know not only the cumulative DVH for the protocol of interest, but also the fractionation regimen and target organ-specific biological parameters. For example, for a breast radiotherapy protocol, one would need parameters for the lung, which is the main organ at risk for radiotherapy-induced cancer in this case.
Figure 5.
The effect of dose fractionation on predicted ERR. As the same total radiation dose is split into fractions (one fraction per day, with gaps on weekends), thereby protracting it over a longer time, predicted cancer risk grows because cell repopulation during prolonged exposure partially compensates for cell killing by radiation. Plausible parameter values guided by model fits to available data were used.
CONCLUSIONS
The microdosimetry community has learned to do very sophisticated short-term modelling, but grafting on long-term mechanisms is badly needed for modelling cancer. This grafting-on appears possible and practical. The first attempts at combining short- and long-term models seem quite promising, allowing prediction of radiotherapy-induced second-cancer risks based on data from Japanese atomic bomb survivors and older epidemiological second-cancer studies. The results obtained after applying this model to data suggest that the approach represents an improvement over previous approaches. Further development of more realistic biologically based mathematical models which integrate both short- and long-term processes is desirable and should enhance both predictive power and mechanistic understanding of radiogenic cancers.
FUNDING
This work was supported through NIH grants U19-AI67773 and P01CA049062, and NASA grant NSCOR04-0014-0017/NNJ04HJ12G/NNJ06HA28G.
REFERENCES
- 1.Farkas A., Schneider D., Perrotti M., Cummings K. B., Ward W. S. National trends in the epidemiology of prostate cancer, 1973 to 1994: evidence for the effectiveness of prostate-specific antigen screening. Urology. 1998;52:444–448. doi: 10.1016/s0090-4295(98)00242-8. discussion 448–449 doi:10.1016/S0090-4295(98)00242-8. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Tiwari R. C., Murray T., Ghafoor A., Samuels A., Ward E., Feuer E. J., Thun M. J. Cancer statistics, 2004. CA Cancer. J. Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. doi:10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Neglia J. P., et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J. Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. doi:10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 4.Boice J. D., Jr, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J. Natl Cancer Inst. 1987;79:1295–1311. [PubMed] [Google Scholar]
- 5.Boice J. D., Jr, Lubin J. H. Occupational and environmental radiation and cancer. Cancer Causes Control. 1997;8:309–322. doi: 10.1023/a:1018496919324. doi:10.1023/A:1018496919324. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi A. K., et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J. Natl Cancer Inst. 2007;99:1634–1643. doi: 10.1093/jnci/djm201. doi:10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 7.Travis L. B., Curtis R. E., Boice J. D., Jr, Platz C. E., Hankey B. F., Fraumeni J. F., Jr Second malignant neoplasms among long-term survivors of ovarian cancer. Cancer Res. 1996;56:1564–1570. [PubMed] [Google Scholar]
- 8.Travis L. B., et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J. Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. doi:10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 9.Travis L. B., et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J. Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 10.Travis L. B., et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. doi:10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen F. E., et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J. Natl Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. doi:10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 12.Yu C. X., Amies C. J., Svatos M. Planning and delivery of intensity-modulated radiation therapy. Med. Phys. 2008;35:5233–5241. doi: 10.1118/1.3002305. doi:10.1118/1.3002305. [DOI] [PubMed] [Google Scholar]
- 13.Cahlon O., Hunt M., Zelefsky M. J. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin. Radiat. Oncol. 2008;18:48–57. doi: 10.1016/j.semradonc.2007.09.007. doi:10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Mendenhall W. M., Amdur R. J., Palta J. R. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J. Clin. Oncol. 2006;24:2618–2623. doi: 10.1200/JCO.2005.04.7225. doi:10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov V. K., Gorski A. I., Tsyb A. F., Ivanov S. I., Naumenko R. N., Ivanova L. V. Solid cancer incidence among the Chernobyl emergency workers residing in Russia: estimation of radiation risks. Radiat. Environ. Biophys. 2004;43:35–42. doi: 10.1007/s00411-003-0223-6. doi:10.1007/s00411-003-0223-6. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga M., Norman J. E., Jr, Asano M., Tokuoka S., Ezaki H., Nishimori I., Tsuji Y. Malignant breast tumors among atomic bomb survivors, Hiroshima and Nagasaki, 1950–74. J. Natl Cancer Inst. 1979;62:1347–1359. [PubMed] [Google Scholar]
- 17.Armitage P., Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer. 1954;VIII:1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis S. B., Luebeck E. G., Hazelton W. D., Moolgavkar S. H. The role of promotion in carcinogenesis from protracted high-LET exposure. Phys. Med. 2001;17(Suppl. 1)):157–160. [PubMed] [Google Scholar]
- 19.Curtis S. B., Luebeck E. G., Hazelton W. D., Moolgavkar S. H. A new perspective of carcinogenesis from protracted high-LET radiation arises from the two-stage clonal expansion model. Adv. Space Res. 2002;30:937–944. doi: 10.1016/s0273-1177(02)00158-8. doi:10.1016/S0273-1177(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich W. F., Cullings H. M., Funamoto S., Paretzke H. G. Promoting action of radiation in the atomic bomb survivor carcinogenesis data? Radiat. Res. 2007;168:750–756. doi: 10.1667/RR0919.1. doi:10.1667/RR0919.1. [DOI] [PubMed] [Google Scholar]
- 21.Little M. P., Li G. Stochastic modelling of colon cancer: is there a role for genomic instability? Carcinogenesis. 2007;28:479–487. doi: 10.1093/carcin/bgl173. doi:10.1093/carcin/bgl173. [DOI] [PubMed] [Google Scholar]
- 22.Little M. P., Wright E. G. A stochastic carcinogenesis model incorporating genomic instability fitted to colon cancer data. Math. Biosci. 2003;183:111–134. doi: 10.1016/s0025-5564(03)00040-3. doi:10.1016/S0025-5564(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 23.Michor F., Iwasa Y., Lengauer C., Nowak M. A. Dynamics of colorectal cancer. Semin. Cancer Biol. 2005;15:484–493. doi: 10.1016/j.semcancer.2005.06.005. doi:10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Moolgavkar S. H. The multistage theory of carcinogenesis and the age distribution of cancer in man. J. Natl Cancer Inst. 1978;61:49–52. doi: 10.1093/jnci/61.1.49. [DOI] [PubMed] [Google Scholar]
- 25.Moolgavkar S. H. Model for human carcinogenesis: action of environmental agents. Environ. Health Perspect. 1983;50:285–291. doi: 10.1289/ehp.8350285. doi:10.2307/3429560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordling C. O. A new theory on the cancer inducing mechanism. Br. J. Cancer. 1953;7:68–72. doi: 10.1038/bjc.1953.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtaki M., Niwa O. A mathematical model of radiation carcinogenesis with induction of genomic instability and cell death. Radiat. Res. 2001;156:672–677. doi: 10.1667/0033-7587(2001)156[0672:ammorc]2.0.co;2. doi:10.1667/0033-7587(2001)156[0672:AMMORC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Pompei F., Polkanov M., Wilson R. Age distribution of cancer in mice: the incidence turnover at old age. Toxicol. Ind. Health. 2001;17:7–16. doi: 10.1191/0748233701th091oa. doi:10.1191/0748233701th091oa. [DOI] [PubMed] [Google Scholar]
- 29.Pompei F., Wilson R. A quantitative model of cellular senescence influence on cancer and longevity. Toxicol. Ind. Health. 2002;18:365–376. doi: 10.1191/0748233702th164oa. doi:10.1191/0748233702th164oa. [DOI] [PubMed] [Google Scholar]
- 30.Sachs R. K., Brenner D. J. Solid tumor risks after high doses of ionizing radiation. Proc. Natl Acad. Sci. USA. 2005;102:13040–13045. doi: 10.1073/pnas.0506648102. doi:10.1073/pnas.0506648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachs R. K., Chan M., Hlatky L., Hahnfeldt P. Modeling intercellular interactions during carcinogenesis. Radiat. Res. 2005;164:324–331. doi: 10.1667/rr3413.1. doi:10.1667/RR3413.1. [DOI] [PubMed] [Google Scholar]
- 32.Schneider U., Walsh L. Cancer risk estimates from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Radiat. Environ. Biophys. 2008;47:253–263. doi: 10.1007/s00411-007-0151-y. doi:10.1007/s00411-007-0151-y. [DOI] [PubMed] [Google Scholar]
- 33.Schollnberger H., Mitchel R. E., Crawford-Brown D. J., Hofmann W. Nonlinear dose–response relationships and inducible cellular defence mechanisms. J. Radiol. Prot. 2002;22:A21–25. doi: 10.1088/0952-4746/22/3a/304. doi:10.1088/0952-4746/22/3A/304. [DOI] [PubMed] [Google Scholar]
- 34.Shuryak I., Sachs R. K., Hlatky L., Little M. P., Hahnfeldt P., Brenner D. J. Radiation-induced leukemia at doses relevant to radiation therapy: modeling mechanisms and estimating risks. J. Natl Cancer Inst. 2006;98:1794–1806. doi: 10.1093/jnci/djj497. doi:10.1093/jnci/djj497. [DOI] [PubMed] [Google Scholar]
- 35.Yakovlev A., Polig E. A diversity of responses displayed by a stochastic model of radiation carcinogenesis allowing for cell death. Math. Biosci. 1996;132:1–33. doi: 10.1016/0025-5564(95)00047-x. doi:10.1016/0025-5564(95)00047-X. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay K. A., Wheldon E. G., Deehan C., Wheldon T. E. Radiation carcinogenesis modelling for risk of treatment-related second tumours following radiotherapy. Br. J. Radiol. 2001;74:529–536. doi: 10.1259/bjr.74.882.740529. [DOI] [PubMed] [Google Scholar]
- 37.Little M. P. A multi-compartment cell repopulation model allowing for inter-compartmental migration following radiation exposure, applied to leukaemia. J. Theor. Biol. 2007;245:83–97. doi: 10.1016/j.jtbi.2006.09.026. doi:10.1016/j.jtbi.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Wheldon E. G., Lindsay K. A., Wheldon T. E. The dose–response relationship for cancer incidence in a two-stage radiation carcinogenesis model incorporating cellular repopulation. Int. J. Radiat. Biol. 2000;76:699–710. doi: 10.1080/095530000138376. doi:10.1080/095530000138376. [DOI] [PubMed] [Google Scholar]
- 39.Heidenreich W. F., Paretzke H. G. The two-stage clonal expansion model as an example of a biologically based model of radiation-induced cancer. Radiat. Res. 2001;156:678–681. doi: 10.1667/0033-7587(2001)156[0678:ttscem]2.0.co;2. doi:10.1667/0033-7587(2001)156[0678:TTSCEM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Shuryak I., Hahnfeldt P., Hlatky L., Sachs R. K., Brenner D. J. A new view of radiation-induced cancer: integrating short- and long-term processes. Part I: approach. Radiat. Environ. Biophys. 2009;48:263–274. doi: 10.1007/s00411-009-0230-3. doi:10.1007/s00411-009-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuryak I., Hahnfeldt P., Hlatky L., Sachs R. K., Brenner D. J. A new view of radiation-induced cancer: integrating short- and long-term processes. Part II: second cancer risk estimation. Radiat. Environ. Biophys. 2009;48:275–286. doi: 10.1007/s00411-009-0231-2. doi:10.1007/s00411-009-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preston D. L., Ron E., Tokuoka S., Funamoto S., Nishi N., Soda M., Mabuchi K., Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 2007;168:1–64. doi: 10.1667/RR0763.1. doi:10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 43.Travis L. B., et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J. Natl Cancer Inst. 1997;89:1429–1439. doi: 10.1093/jnci/89.19.1429. doi:10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]