Abstract
High-frequency ultrasound (8–14 MHz) is routinely used to display cutaneous melanomas. Maximum thickness measurement (Breslow index) has been shown to be well correlated to histologic findings for lesions of more than 0.75 mm. Some morphological criteria (strong delineation, hypoechoic texture, homogeneity) have been reported to help differentiate between malignant and benign pigmented blue lesions, but remain insufficient. Vascular ultrasound analysis using Doppler mode provides additional information and showed good specificity for malignancy (90%–100%), but variable sensitivity (34%–100%). Recent advances in ultrasound imaging allow functional evaluation. Likewise, dynamic contrast-enhanced ultrasound using contrast medium injection and specific perfusion and quantification software showed promising results in clinical and preclinical trials for early prediction of tumor response to target treatments.
Keywords: melanoma, ultrasound, dynamic contrast-enhanced ultrasound
Introduction
Melanoma imaging has benefited from significant advances in ultrasound imaging. Indeed, since the 1990s, because of its accessibility, low cost, and the shallowness of lesions, this technique has rapidly become established as an essential continuum to the clinical examination. Today, advances in high-frequency probes allow accurate detection, characterization, and measurement of lesions.
Functional imaging has recently become an important issue in oncology for evaluation of new targeted therapies and antiangiogenic treatments allowing very early assessment of treatment effectiveness. In particular, ultrasound with injection of contrast medium could play an important role alongside other functional imaging modalities, such as positron emission tomography, computed tomography, or magnetic resonance imaging. It is yet to be established which melanomas may be susceptible to treatment with a combination of antiangiogenic agents and chemotherapy, and current treatment monitoring is in need of a reliable functional imaging tool. This paper reviews the role of different ultrasound modalities (from morphologic to vascular assessment) in initial management of primary melanoma and outlines the development and importance of new functional ultrasound modalities for monitoring antiangiogenic or antivascular treatments.
Morphologic analysis and sonometry: Breslow index
It is now recognized that high frequency ultrasound is superior to clinical examination alone for the detection and measurement of superficial lesions. In skin melanoma, the maximum thickness (Breslow index) is considered as the main prognostic factor related to metastatic potential. The Breslow index determines the margins of resection of the primary tumor and the sentinel node biopsy procedure.
The accuracy of measurement of thickness depends on the axial resolution of the ultrasound system. Axial resolution is basically defined as the shortest distance between two targets on the firing axis of the probe that can be separated on the image. The shortest distance (δ) is approximately equivalent to the length of the ultrasonic wave (λ), which is inversely proportional to the frequency of the probe (F): δ ∼ λ ∼ (1/F). Therefore, the higher the frequency of the probe, the better the resolution and the smaller the measured distance. On the other hand, the wavelength is also dependent on ultrasound velocity (c) in the propagation medium (λ = c/F). Weichenthal et al showed in a small melanoma sample that ultrasound velocities and therefore the measured distances varied from one tumor to another and within the same tumor, but the relative amount of variation seemed to be low.1
Since the early 1980s, several in vivo and in vitro studies2–7 have shown that ultrasound using high-frequency probes (7–20 mHz) produced thickness measurements superimposable on histologic measurements, with differences of less than 0.2 mm8 and correlation coefficients ≥0.95 because of a high resolution rate (precision 0.01 mm).5,9 Therefore, correlation between sonometry and histometry is high but not ideal, in particular for lesions less than 1 mm in thickness, and many authors have suggested that the accuracy of ultrasound measurement would be improved by using probes of very high frequency (>20 mHz). Some authors have proposed use of 100 mHz ultrasound for skin lesions less than 1 mm thickness.10 A recent study by Guitera et al enhanced the use of frequency probes of 75 mHz, and their results showed that in situ melanoma tended to have a poor correlation with histometry in contrast with invasive melanoma.11 Generally, sonometry is reported to be highly correlated with histometry for lesions greater than 0.75 mm in thickness.12
Nevertheless, because of its noninvasiveness and accurate measurement, high frequency ultrasound remains an effective preoperative imaging tool to adjust the operative strategy at the outset according to the index and then avoiding reoperations that had to be performed in 30% of cases when Breslow index was measured subsequently at surgery.13–15
Morphologic analysis in gray scale ultrasound: malignant or benign lesion
Assessment and management of pigmented lesions of the skin (melanoma, basal/squamous cell carcinoma, nevus, keratosis, lentigo, capillary thrombosis, histiocytofibroma) are based on clinical evaluation, but differentiation between benignancy and malignancy is often problematic. In a recent study including 39 blue skin lesions, high frequency ultrasonography (20 mHz) was reported to be more specific than clinical examination and dermoscopy for differentiating melanoma from blue nevi (specificity 94%, 77%, and 74%, respectively) but clinical diagnosis was more sensitive (77.9% versus 70% for dermoscopy and 70.8% for sonography).16
In gray scale mode, the image formation corresponds with a two-dimensional representation of the ultrasound signal arising from several acoustic phenomena, ie, reflection (allowing visualization of an interface between two media with different acoustic properties), diffusion (determining signal amplitude and intensity and then coded in gray scale), and absorption of the ultrasound beam through the tissue.
Depending on the brightness of each pixel on the video screen, an image is constructed, and on ultrasound imaging the majority of skin lesions appear as hypoechoic cutaneous or subcutaneous thickening. Sonographic features that can assist in the differential diagnosis between benign pigmented lesions and melanoma have been widely discussed in literature, ie, location, echogenicity, homogeneity, shape, margins, increased posterior echogenicity, and acoustic shadowing. Melanomas have been generally described as more homogeneous with well defined margins compared with other lesions, with a sensitivity of 100% for these criteria in a series of 114 lesions.7
Harland et al studied quantitative acoustic parameters provided from high resolution ultrasound in melanoma and benign pigmented lesions by using objective measures of acoustic shadowing, intralesional sound reflection, and surface sound reflectance characteristics. They confirmed that significant acoustic differences arise between melanoma and benign pigmented lesions, ie, melanomas were reported to be less attenuating than benign lesions and, furthermore, they showed that melanomas were more homogeneous compared with seborrheic keratoses (specificity of 79% and sensitivity of 100%).6,17
Nevertheless, these morphologic criteria alone are insufficient in lesion characterization and assessment. Some authors have now focused on tumor microvasculature analysis.
Vascular analysis: color and power Doppler
Since work by Srisvastava et al18 was published in 1989, several studies have focused on analysis of tumor microvasculature. It has been shown in animal models that Doppler mode (color or power Doppler) allows microvessel detection with a threshold of 100 μm. A high correlation with histologic and immunohistochemical specimens in terms of vascular density has been reported.8,13
Detection of microvessels in a skin lesion increases the conspicuity for malignancy, but reports in the literature are still variable in that microvessel detection has shown a good specificity for malignancy (90%–100%) but sensitivity has been in the 34%–100% range.19 These variations could be related to the natural history of angiogenesis in tumors and also to the advances in ultrasound. Srisvastava et al showed in a small series of 21 patients that neovascularization is detectable by pulsed Doppler until a lesion thickness of 0.8 mm.18 In a most recent series using color Doppler in 67 and 107 patients,7,20 the thickness threshold for vessel detection was 2 mm; in these patients, 87% of skin melanomas more than 2 mm in thickness were perfused and 95% of melanoma less than 2 mm in thickness were not. Thus, the authors concluded that the conspicuity for malignancy was strong when vessels were detected in a hypoechoic lesion more than 2 mm in thickness. Conversely, the absence of vessels in a lesion of less than 2 mm does not enable benignancy to be confirmed.
Moreover, vascular density, as observed by Doppler ultrasound, has been shown to be correlated with metastatic potential.21,22 Hence, in a study involving 107 lesions with a follow-up of five years, Breslow index and vascular density as determined by color Doppler were shown to be independently and significantly correlated with metastatic dissemination.23 Therefore, neovascularization is a prognostic factor for metastasis equivalent to the Breslow index. The assessment of tumor perfusion is therefore becoming an issue in the management of these patients.
Functional assessment
Early assessment of treatment response
The emergence of new targeted therapies in oncology led to reconsideration of assessment and treatment through functional evaluation rather than morphologic evaluation which does not detect early biologic tumor changes.
It is now recognized that morphologic criteria alone cannot determine rapidly if treatment is effective or not.24 It has yet to be established which melanomas may be susceptible to treatments with antiangiogenic agents in combination with chemotherapy.25 In fact, agents that neutralize the biologic activity of human vascular endothelial growth factor may be useful in melanoma management by inducing a decrease in angiogenesis, followed by hypoxia and cell death.26
Animal studies in the late 1990s27,28 and Phase I and II human studies in the early 2000s29 showed that a decrease in tumor vessels, as determined by Doppler ultrasound, reflected the effectiveness of targeted treatments before any reduction in tumor volume was detected. Similarly, in 2003, a study performed in 18 patients with in transit melanoma metastases treated by isolated limb perfusion (using tumor necrosis factor alpha) showed that an early decrease in vascularization, as determined by ultrasound color Doppler, predicted a complete response before any morphologic changes could be detected.30 Furthermore, in skin melanoma, Grünhagen and Eggermont hypothesized that initial vascular density should be predictive of tumor response after isolated limb perfusion because the more vascularized tumors should respond better to treatment due to greater infiltration by chemotherapy.31
Contrast-enhanced ultrasound
Since 1999, the use of ultrasound contrast agents has made great strides in evaluating tumor microvasculature. The contribution of dynamic contrast-enhanced ultrasound in antiangiogenic treatment assessment has been widely discussed by Lassau et al,24,32 underscoring the usefulness of this method as a functional modality for monitoring targeted treatments.
Injection of contrast medium displays functional information of tumor perfusion. Contrast media used in ultrasound are similar to encapsulated gas microbubbles. These microbubbles have a strictly intravascular distribution and the use of specific software displays more accurate and sensitive information with fewer artifacts (decrease of blooming effect) than color or power Doppler. The acoustic properties of the microbubbles increase the signal-to-noise ratio and sensitivity in microvessel detection. Since the arrival of first-generation contrast media (Levovist®), the threshold in microvessel detection decreased from 100 to 40 μm when compared with power Doppler mode.33
Dynamic contrast-enhanced ultrasound has been used in clinical studies to assess tumor response to antiangiogenic or antivascular therapies. In a study involving patients treated with isolated limb perfusion for sarcoma, functional evaluation (dynamic contrast-enhanced ultrasound performed at days 1, 7, and 14, and at one month and two months by Levovist injection) showed that perfusion changes (in terms of vessel count) were able to predict early (from day 1) response in 49 patients.34 Sensitivity, specificity, positive predictive value (PPV), and the negative predictive value (NPV) were 89%, 100%, 100%, and 90%, respectively. Thus, this imaging modality has been proposed by surgeons to possibly adjust the timetable for surgery.35
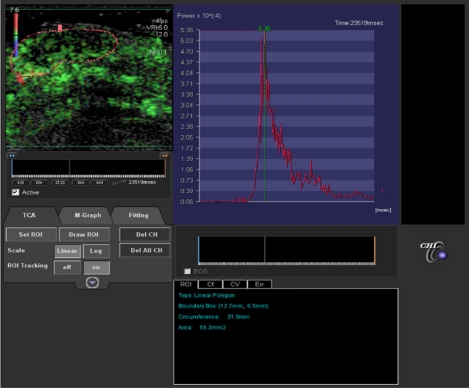
Levovist, which in the early 2000s was the first ultrasound contrast medium used in France, displayed destructive imaging. This method requires significant acoustic power which leads to the destruction of microbubbles. Since 2003, the arrival of second-generation contrast media, eg, SonoVue® enables a new approach to imaging which is no longer a destructive mode but a real-time mode using a low mechanical index while preserving microbubbles. This approach has been made possible as a result of recent technologic developments that combine the advantages of harmonic imaging (in terms of resolution) with those of specific software (specific processing of microbubble signals). This new software can recover the high amplitude signal emitted by microbubbles in spite of nonvascular signals. The duration of the signal depends on the half-life of the microbubbles in the circulation. Real-time imaging allows accurate assessment of tumor enhancement with excellent spatial and temporal resolution, and provides time intensity curves (Figure 1).
Figure 1.
In left window, dynamic contrast-enhanced ultrasound evaluation of superficial skin melanoma after injection of contrast medium (SonoVue®) and using perfusion software (Vascular Recognition Imaging, Toshiba, Japan). Quantitative perfusion software (CHI-Q Laboratory, Toshiba, Japan) provides time intensity curve of enhancement (right window). Several perfusion parameters can be extracted after modelling, ie, maximum intensity, slope, area under wash-out, area under wash-in, time to peak, mean transit time.
Until recently, perfusion evaluation in dynamic contrast-enhanced ultrasound in clinical trials was performed by visual assessment (from Dicom data). It soon became apparent that semiquantitative analysis is an important issue in terms of accuracy and reproducibility to evaluate early vascular changes and therapeutic efficiency. Time intensity curves expressed as native linear raw data provide several perfusion parameters correlated with blood flow and blood volume (maximum intensity, slope, time to peak, mean transit time, area under wash-out, area under wash-in). These parameters could be used for monitoring antiangiogenic therapies.
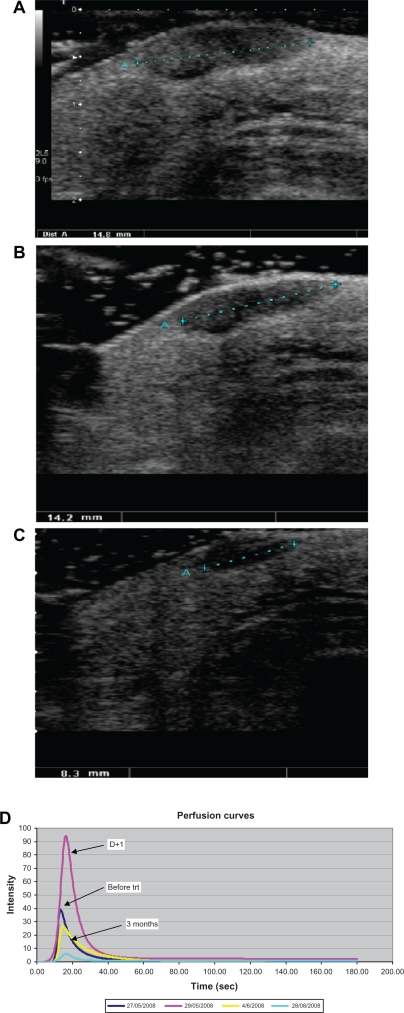
An animal study evaluating the early disruptive effects of AVE 8062 on tumor vasculature showed that perfusion parameters variations extracted from linear raw data were well correlated with microvascular density changes. These were more accurate than power Doppler or visual evaluation from video data.36,37 Likewise, in an initial clinical study involving 50 patients with in transit melanoma metastasis and treated with isolated limb perfusion, quantification showed that initial changes (day 1 after treatment) in some perfusion parameters (maximum intensity, slope, wash-out area, time to peak) were significantly modified in responders38 (Figure 2).
Figure 2.
In transit melanoma metastasis treated by isolated limb perfusion in a good responder. Ultrasound B mode (gray scale) scanning of superficial subcutaneous hypoechoic nodule of the right limb. A) Before treatment, the lesion was 14.8 mm in size. B) At day 1 after treatment no significant morphologic change was observed (14.2 mm). C) At day 7, the lesion shows a significant decrease in size (8.3 mm). D) In the same patient, after modelling, perfusion curves expressed as linear raw data show an important increase in perfusion parameters at day 1 after treatment compared with baseline.
Conclusion
Ultrasound is a low-cost and powerful technique for pre-operative melanoma evaluation in terms of detection, characterization, metastatic risk, and monitoring of targeted treatments. Technologic advances in high frequency probes allow accurate morphologic and functional assessments. In particular, melanoma thickness, as measured with high frequency ultrasound, is currently considered as an effective and reliable criterion for preoperative planning. On the other hand, vascular assessment could be optimized by real-time contrast-enhanced ultrasound which is able to provide quantitative perfusion parameters, such as biomarkers for new antiangiogenic therapy monitoring.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weichenthal M, Mohr P, Breitbart EW. The velocity of ultrasound in human primary melanoma tissue – implications for the clinical use of high resolution sonography. BMC Dermatol. 2001;1:1. doi: 10.1186/1471-5945-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus W, Schramm P, Hoede N. First experiences with a high resolution ultrasonic scanner in the diagnosis of malignant melanomas. Arch Dermato Res. 1983;275:235–238. doi: 10.1007/BF00416667. [DOI] [PubMed] [Google Scholar]
- 3.Dines K, Sheets P, Bink J, et al. High-frequency ultrasonic imaging of skin: Experimental results. Ultrason Imaging. 1984;6:408–434. doi: 10.1177/016173468400600403. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman K, Jung J, El Gammal S, Altmeyer P. Malignant melanoma in 20-MHz B scan US. Dermatology. 1992;185:49–55. doi: 10.1159/000247403. [DOI] [PubMed] [Google Scholar]
- 5.Fornage B, McGavran M, Duvic M, Waldron C. Imaging of the skin with 20 MHz sonography. Radiology. 1993;189:69–79. doi: 10.1148/radiology.189.1.8372222. [DOI] [PubMed] [Google Scholar]
- 6.Harland C, Kale S, Jackson P, Mortimer P, Bamber J. Differentiation of common benign pigmented skin lesions from melanoma by high-resolution ultrasound. Br J Dermatol. 2000;143:281–289. doi: 10.1046/j.1365-2133.2000.03652.x. [DOI] [PubMed] [Google Scholar]
- 7.Bessoud B, Lassau N, Koscielny S, et al. High-frequency sonography and color Doppler in the management of pigmented skin lesions. Ultrasound Med Biol. 2003;29:875–879. doi: 10.1016/s0301-5629(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 8.Lassau N, Spatz A, Avril MF, et al. Value of high-frequency US for preoperative assessment of skin tumors. Radiographics. 1997;17:1559–1565. doi: 10.1148/radiographics.17.6.9397463. [DOI] [PubMed] [Google Scholar]
- 9.Harland C, Bamber JC, Gusterson B, Mortimer P. High-frequency, high resolution B-scan ultrasound in the assessment of skin tumours. Br J Dermatol. 1993;128:525–532. doi: 10.1111/j.1365-2133.1993.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Gamblicher T, Moussa G, Bahrenberg K, et al. Preoperative ultrasonic assessment of thin melanocytic skin lesions using a 100-MHz ultrasound transducer: A comparative study. Dermatol Surg. 2007;33:818–824. doi: 10.1111/j.1524-4725.2007.33175.x. [DOI] [PubMed] [Google Scholar]
- 11.Guitera P, Li LX, Crotty K, et al. Melanoma histological Breslow thickness predicted by 75-MHz ultrasonography. Br J Dermatol. 2008;159:364–369. doi: 10.1111/j.1365-2133.2008.08681.x. [DOI] [PubMed] [Google Scholar]
- 12.Serrone L, Solivetti F, Thorel M, Eibenschuntz L, Donati P, Catricalà C. High frequency ultyrasound in the preoperative staging of primary melanoma: A statistical analysis. Melanoma Res. 2002;12:287–290. doi: 10.1097/00008390-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Lassau N, Mercier S, Koscielny S, et al. Prognostic value of high-frequency sonography for the preoperative assessment of melanomas. Am J Roentgenol. 1999;172:457–461. doi: 10.2214/ajr.172.2.9930803. [DOI] [PubMed] [Google Scholar]
- 14.Lassau N, Paturel-Asselin C, Guinebretiere JM, et al. New hemodynamic approach to angiogenesis: Color and pulsed Doppler ultrasonography. Invest Radiol. 1999;34:194–198. doi: 10.1097/00004424-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Machet L, Belot V, Naouri M, et al. Preoperative measurement of thickness of cutaneous melanoma using high-resolution 20 MHz ultrasound imaging: A monocenter prospective study and systematic review of the literature. Ultrasound Med Biol. 2009;35:1411–1420. doi: 10.1016/j.ultrasmedbio.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Samimi M, Perrinaud A, Naouri M, Maruani E, Vaillant L, Machet L. High-resolution ultrasonography assists the differential diagnosis of blue naevi and cutaneous metastases of melanoma. Br J Dermatol. 2010 Jun 10; doi: 10.1111/j.1365-2133.2010.09903.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Rallan D, Bush N, Bamber J, Harland C. Quantitative discrimination of pigmented lesions using three-dimensional high-resolution ultrasound reflex transmission imaging. J Invest Dermatol. 2007;127:189–195. doi: 10.1038/sj.jid.5700554. [DOI] [PubMed] [Google Scholar]
- 18.Srisvastava A, Hughes L, Woodcock J, Laidler P. Vascularity in cutaneous melanoma detected by Doppler sonography and histology: Correlation with tumor behaviour. Br J Cancer. 1989;59:89–91. doi: 10.1038/bjc.1989.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovagnorio F, Andreoli C, de Cicco ML. Color Doppler sonography of focal lesions of the skin and subcutaneous tissue. J Ultrasound Med. 1999;18:89–93. doi: 10.7863/jum.1999.18.2.89. [DOI] [PubMed] [Google Scholar]
- 20.Lassau N, Koscielny S, Avril MF, et al. Prognostic value of angiogenesis evaluated with high-frequency and color Doppler sonography for preoperative assessment of melanomas. Am J Roentgenol. 2002;178:1547–1551. doi: 10.2214/ajr.178.6.1781547. [DOI] [PubMed] [Google Scholar]
- 21.Barnhill RL, Levy MA. Regressing thin cutaneous malignant melanomas (< or = 1.0 mm) are associated with angiogenesis. Am J Pathol. 1993;143:99–104. [PMC free article] [PubMed] [Google Scholar]
- 22.Straume O, Akslen LA. Expression of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223–235. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassau N, Lamuraglia M, Koscielny S, et al. Prognostic value of angiogenesis evaluated with high-frequency and colour Doppler sonography for preoperative assessment of primary cutaneous melanomas: Correlation with recurrence after a 5-year follow-up period. Cancer Imaging. 2006;25:24–29. doi: 10.1102/1470-7330.2006.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassau N, Chami L, Péronneau P. Current events about echography in 2006: Position of the ultrasound functional imaging for the early evaluation of targeted therapeutics. Bull Cancer. 2006;93:1207–1211. [PubMed] [Google Scholar]
- 25.Marneros AG. Tumor angiogenesis in melanoma. Hematol Oncol Clin North Am. 2009;23:431–446. doi: 10.1016/j.hoc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: A North Central Cancer Treatment Group study, N047A. Cancer. 2009;115:119–127. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asselin-Paturel C, Lassau N, Guinebretière JM, et al. Transfer of the murine interleukin-12 gene in vivo by the Semliki forest virus induces B16 tumour regression through inhibition of tumour blood vessel formation monitored by Doppler ultrasonography. Gene Ther. 1999;4:606–615. doi: 10.1038/sj.gt.3300841. [DOI] [PubMed] [Google Scholar]
- 28.Kaliski A, Maggiorella L, Rouffiac V, et al. Angiogenic and tumor growth inhibition by an MMP-inhibitor targeting radio-induced MMP-2 and VEGF. Mol Cancer Ther. 2005;11:1717–1728. doi: 10.1158/1535-7163.MCT-05-0179. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Lassau N, Couanet D, et al. Phase II trial of thalidomide in renal-cell carcinoma. Ann Oncol. 2002;13:1029–1035. doi: 10.1093/annonc/mdf213. [DOI] [PubMed] [Google Scholar]
- 30.Hochedez P, Lassau N, Bonvalet S, Bidault S, Leclere J, Avril MF. Treatment of local recurrent melanomas by isolated limb perfusion: Value of Doppler ultrasonography. J Radiol. 2003;84:597–603. [PubMed] [Google Scholar]
- 31.Grünhagen DJ, van Etten B, Brunstein F, et al. Efficacy of repeat isolated limb perfusion with tumor necrosis factor and mephalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol. 2005;12:609–615. doi: 10.1245/ASO.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): A new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53–58. doi: 10.1007/s11523-010-0136-7. [DOI] [PubMed] [Google Scholar]
- 33.Lassau N, Koscielny S, Opolon P, et al. Evaluation of contrast-enhanced color Doppler ultrasound for the quantification of angiogenesis in vivo. Invest Radiol. 2001;36:50–55. doi: 10.1097/00004424-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Lassau N, Lamuraglia M, Vanel D, et al. Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: Prospective study of 49 cases. Ann Oncol. 2005;16:1054–1060. doi: 10.1093/annonc/mdi214. [DOI] [PubMed] [Google Scholar]
- 35.Eggermont AM. Evolving imaging technology: Contrast-enhanced Doppler ultrasound is early and rapid predictor of tumour response. Ann Oncol. 2005;16:995–996. doi: 10.1093/annonc/mdi230. [DOI] [PubMed] [Google Scholar]
- 36.Brule A, Lassau N, Perronneau P. Intérêt des données linéaires ultra-sonores comparées aux données vidéo dans l’étude de la perfusion tumorale. JFR. 2006;87:1381. [Google Scholar]
- 37.Lavisse S, Lejeune P, Rouffiac V, et al. Early quantitative evaluation of tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100–111. doi: 10.1097/RLI.0b013e3181577cfc. [DOI] [PubMed] [Google Scholar]
- 38.Chami L, Cavalcanti A, Benatsou B, Koscielny S, Chebil M, Lassau N. Dynamic contrast-enhanced ultrasound (DCE-US) and melanoma: Early evaluation of response after isolated limb perfusion (ILP). [Abstract]. RSNA SSK11-08. 2008:533.