Abstract
Cholesterol is essential for the functioning of all human organs, but it is nevertheless the cause of coronary heart disease. Over the course of nearly a century of investigation, scientists have developed several lines of evidence that establish the causal connection between blood cholesterol, atherosclerosis, and coronary heart disease. Building on that knowledge, scientists and the pharmaceutical industry have successfully developed a remarkably effective class of drugs—the statins—that lower cholesterol levels in blood and reduce the frequency of heart attacks.
Keywords: cholesterol, statin, cholesterol-lowering agent, HMG-CoA reductase inhibitor, atherosclerosis, coronary heart disease
Since it was first isolated from gallstones in 1784, cholesterol has fascinated scientists from many areas of science and medicine. Thirteen Nobel Prizes have been awarded to scientists who devoted major parts of their careers to cholesterol research1) French physician-chemist François Poulletier was the first to obtain pure cholesterol from gallstones. Some thirty years later, French chemist Michel E. Chevreul named it cholesterine (solid bile in Greek: chole for bile and stereos for solid). The exact molecular formula of cholesterol was accurately established in 1888 by Austrian botanist Friedrich Reinitzer.2) The cholesterol molecule has four rings, and this tetra-cyclic skeleton made it extremely challenging to elucidate its structure, which occupied scientists for a good part of the first quarter of the twentieth century. Proof of the structure of cholesterol was obtained chiefly through the brilliant work of Heinrich O. Wieland and Adolf Windaus. They won the Nobel Prize in Chemistry in 1927 and 1928, respectively, for their cholesterol work.1)
Milestones on the road to the development of the statins
Animal models in atherosclerosis.
During the 19th century, arteriosclerosis was well recognized, but its etiological and pathological significance had not been established. The hypotheses explaining it ranged from disturbed arterial metabolism to adherent blood clots that gradually changed into arteriosclerotic plaques. The first hint that cholesterol was related to atherosclerosis goes back to 1910, when Windaus reported that atherosclerotic plaques from aortas of human subjects contained over 20-fold higher concentrations of cholesterol than did normal aortas.3) Three years later, the Russian pathologist Nikolai Anitschkow fed pure cholesterol to rabbits, which produced marked hypercholesterolemia and severe atherosclerosis of the aorta.4) This was the first experimental production of atherosclerosis. At that time, however, his findings were largely rejected or at least not followed up. Serious research on the role of cholesterol in human atherosclerosis did not really get underway until the 1940s, due to a prevailing view that the disease was a simple consequence of aging and could not be prevented.
Genetic connection.
The genetic connection between cholesterol and heart attacks was first made in 1939 by Norwegian clinician Carl Müller, who described several large families in which high blood-cholesterol levels and premature heart attacks together were an inherited trait. In the mid-1960s,5) the genetic understanding of this syndrome, which came to be known as familial hypercholesterolemia (FH), was more extensively studied by Avedis K. Khachadurian,6) He delineated two clinically distinct forms of FH in inbred families—the homozygous form, in which affected individuals manifest severe hypercholesterolemia at birth (with plasma cholesterol levels of about 800 mg/dl) and heart attacks that occur as early as 5 years of age, and the heterozygous form, characterized by levels in the 300- to 400-mg/dl range and premature heart attacks that occur typically between 35 and 60 years of age. In addition to studies with animal models, the genetic studies strongly suggested a causal relationship between cholesterol and atherosclerosis and coronary heart disease.
Epidemiologic studies.
In the early 1950s, the epidemiologic study of the cholesterol-coronary connection was unfolded by John Gofman at the University of California at Berkeley, who used the newly developed ultracentrifuge to separate plasma lipoproteins by flotation. Gofman found not only that heart attacks correlated with elevated levels of blood cholesterol but also that the cholesterol was contained in low density lipoprotein (LDL). He also observed that heart attacks were less frequent when the blood contained elevated levels of high density lipoprotein (HDL).7–9)
The epidemiologic connection between blood cholesterol and coronary atherosclerosis was firmly established by a physiologist at the University of Minnesota, Ancel Keys, whose Seven Countries Study (beginning in the mid-1960s) showed that the incidence of heart attacks in 15,000 middle-aged men followed for 10 years was linearly proportional to the blood level of cholesterol.10–12) The Framingham Heart Study was carried out by the National Heart Institute in Framingham, Ma. It provided the first solid and unarguable evidence that individuals with higher blood cholesterol levels at the time of the baseline examination were more likely to experience a myocardial infarction in the subsequent years of follow-up. It also showed that the risk was increased by a number of other factors such as high blood pressure and smoking.13,14)
Cholesterol biosynthetic pathway.
The clinical interest in cholesterol led to an intense effort in the 1950s to determine the pathway by which cholesterol was synthesized in the body. Most of the crucial steps in this complex pathway, involving 30 enzymatic reactions, were worked out by four biochemists—Konrad E. Bloch, Feodor Lynen, John Cornforth, and George Popják.15–19) Cholesterol synthesis takes place in four stages:
(1) condensation of three acetate units to form a six-carbon intermediate, mevalonate; (2) conversion of mevalonate to activated isoprene units; (3) polymerization of six 5-carbon isoprene units to form the 30-carbon linear squalene; (4) cyclization of squalene to form the steroid nucleus, with a further series of changes to produce cholesterol.
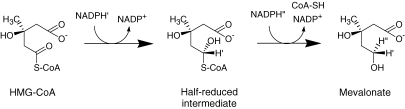
The third reaction in the first stages is the committed and rate-limiting step: reduction of HMG-CoA to mevalonate is the major point of regulation on the pathway to cholesterol (Fig. 1). The major outlines of this pathway were completed by 1960, and Bloch and Lynen were awarded the Nobel Prize in 1964.
Figure 1.
HMG-CoA reductase reaction.
At the Nobel Banquet in Stockholm, December 10, 1964 when Konrad Bloch and Feodor Lynen, were awarded the Nobel Prize, S. Friberg, Rector of the Caroline Institute, made the following remarks: “Your discoveries may provide us with weapons against some of mankind’s gravest maladies, above all in relation to cardiovascular diseases. Achievements like yours make it not unrealistic to look forward to a time, when mankind will not only live under vastly improved conditions, but will itself be better”.20)
Regulation of cholesterol metabolism.
Regulation of cholesterol metabolism was extensively studied by several scientists in the U.S. in the 1960s.21–26) Cholesterol in the body can be derived from what is absorbed from the diet and from what is synthesized in the body, mainly by the liver. The former type is supplemented by the latter if the required levels are not met, but if the former type of “exogenous” cholesterol reaches its required level, the synthesis function of the liver is suppressed to prevent excessive production of cholesterol. Feedback suppression of cholesterol synthesis in the liver by dietary cholesterol is mediated through changes in the activity of HMG-CoA reductase that catalyzes the conversion of HMG-CoA to mevalonate Changes in reductase activity are closely related to changes in the overall rate of cholesterol synthesis. In humans, cholesterol produced in the liver exceeds what is absorbed from the diet, even when a large quantity of cholesterol is ingested. These findings suggest that the inhibition of HMG-CoA reductase would be an effective means of lowering plasma cholesterol in humans.
Searching for cholesterol synthesis inhibitors.
As evidence grew that high blood cholesterol levels were linked to heart disease, scientists in both academia and industry began searching for drugs to lower blood cholesterol. In the 1950s and 1960s, many companies were searching for molecules that would block one of the 30 steps in the synthesis of cholesterol from acetyl-coenzyme A (CoA). Many molecules homologous to intermediates along the pathway were synthesized. Some molecules were effective in animals, but none of those were effective at the clinical level.27) Triparanol(MER/29), which was introduced into clinical use in the U.S. in 1959, was the first cholesterol-lowering agent that inhibited cholesterol synthesis. However, it was withdrawn from the market in the early 1960s because of serious side effects, including cataracts.28–31) Triparanol inhibited the final stage in the cholesterol synthetic pathway, resulting in the accumulation of other sterols.
Cholesterol-lowering agents available in the 1960s.
The cholesterol-lowering properties of nicotinic acid were discovered in 1955 by Canadian pathologist Rudolf Altschul.32) At that time, nicotinic acid was the only drug effective in lowering both cholesterol and triglycerides. Clofibrate was synthesized at Imperial Chemical Industries (ICI) in England and marketed in 1958.33) In the 1960s, many derivatives of clofibrate, called fibrates, that were more potent and safer than clofibrate were developed. In most patients, the cholesterol-lowering effect of fibrates was minimal to moderate. Details of their actions at the biochemical level were not well understood.34) Cholestyramine, an anion-exchange resin, acts by binding bile acids within the intestinal lumen, thus interfering with their reabsorption and enhancing their fecal excretion.35,36) Cholestyramine is highly effective in the treatment of many patients with hypercholesterolemia, but unfortunately, it is not tolerated by all patients.34) Therefore, none of these agents could be considered ideal in terms of efficacy or safety.
Birth of the statins
I (Akira Endo) was born into a rural farming family in northern Japan, in Akita, where I lived for 17 years with my extended family, including my grandparents. My grandfather, who had an interest in medicine and science, was a great home teacher to me. Thanks to his influence, I became fascinated with mushrooms and other molds, and at the age of 10, I dreamt of becoming a scientist, much like renowned Japanese scientist Hideyo Noguchi, who, in 1900 at the age of 24, went to the United States and studied syphilis and yellow fever at the Rockefeller Institute in New York.37,38)
The antibiotic era.
After finishing high school in Akita, I entered Tohoku University’s College of Agriculture in Sendai, where I was inspired by the biography of Alexander Fleming, who discovered penicillin in the blue-green mold belonging to the genus Penicillium in 1928.39) A decade later, penicillin was developed as a systemic therapeutic agent by a research group at Oxford University headed by Florey, Chain and Abraham. In 1940, Selman Waksman isolated streptomycin, an antibiotic active against the tubercle bacillus, from Streptomyces griseus.40) Since then, many antibiotics have been isolated from a variety of microorganisms. At that time, many drug companies and universities in Japan were conducting active research and development in finding effective antibiotics. As a result, I was deeply impressed by the knowledge that antibiotics had saved the lives of many patients with infectious diseases.
Upon graduating in 1957, I joined the pharmaceutical company Sankyo in Tokyo, where I was assigned to one of the applied microbiology groups. I worked toward developing a new pectinase that hydrolyzed viscid pectin contaminated wines and ciders. In 1958, I found a grape-parasitic fungus, Coniothyrium diplodiella, to be a potent producer of such an enzyme. One year later, this new enzyme was commercialized. I then purified it and elucidated its properties.41–44)
Study in the US.
At this point, I became interested in cholesterol biosynthesis. Towards the end of 1965, I wrote a letter to Konrad Bloch, who had received the Nobel Prize for his research on cholesterol biosynthesis in 1964,15) expressing my wish to study under him. Unfortunately, the autumn 1966 class for which I was applying was already full. So I eventually worked on the role of phospholipids in an enzyme system involved in the synthesis of bacterial cell wall lipopolysaccharides in the department of a great biochemist, Bernard Horecker, at the Albert Einstein College of Medicine in New York City for 2 years, from 1966 to 1968.45,46)
While living in New York, I was very surprised by the large number of elderly and overweight people, and by the rather rich dietary habits of Americans compared to those of the Japanese. In the residential area of the Bronx where I lived, there were many elderly couples living by themselves and I often saw ambulances going to take an elderly person who had suffered a heart attack to the hospital. At that time, coronary heart disease was the main cause of death in the United States. The number of patients with hypercholesterolemia, a precursor to coronary heart disease, was said to exceed 10 million.
How to search for HMG-CoA reductase inhibitors.
After coming back to Tokyo in 1968, Sankyo Research Laboratories gave me an opportunity to work on a project of my own choosing. Antibiotics were shown to inhibit many different kinds of enzymes, not only in bacterial cells but also in mammalian cells.47) Although no metabolites that inhibited any enzymes involved in cholesterol synthesis had been isolated previously, I speculated that fungi like molds and mushrooms would produce antibiotics that inhibited HMG-CoA reductase. Inhibition of HMG-CoA reductase would thus be lethal to these microbes.
At that time, HMG-CoA reductase was assayed principally by measuring the incorporation of radioactivity from [14C]HMG-CoA into mevalonate.48) As [14C]HMG-CoA was too expensive to use for determining the inhibitory activity of thousands of samples, we first searched for microbial culture broths that inhibited the incorporation of [14C]acetate into nonsaponifiable lipids.49) The active broths were then tested for their ability to inhibit lipid synthesis from [3H]mevalonate. Culture broths that were active in the first assay but not active in the second determination were suspected to contain a compound (or compounds) that inhibited the early stages between acetate and mevalonate in the cholesterol synthetic pathway. The principal active component(s) in these culture broths were then isolated. Rat liver enzymes were used for these assays.50)
Isolation of citrinin.
Inspired by Fleming’s success with molds, we began our project using culture broths of thousands of fungi in April 1971, and one year later, after 3800 strains of fungi, we found that a culture broth of mold showed potent inhibitory activity. The active principle turned out to be a known substance—citrinin.51) Citrinin strongly inhibited HMGCoA reductase and, furthermore, lowered serum cholesterol levels in rats.51,52) However, the research was suspended because of its toxicity to the kidneys. Nevertheless, the experience with citrinin gave us hope and courage that we might be able to discover much better active substances in the near future.
Discovery of compactin (ML-236B).
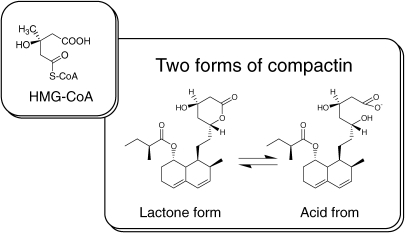
In the mid-summer of 1972, We found a second active culture broth of blue-green mold, Penicillium citrinum Pen-51, which was isolated from a rice sample collected at a grain shop in Kyoto. This mold is similar to the blue-green molds that contaminate fruits, like oranges and melons. It took us one year to isolate the active principles from the culture broth, mainly because of the very low productivity of active compounds. Finally, in July 1973, we were able to isolate three active metabolites from the culture broth by solvent extraction, silica gel chromatography, and crystallization.53–55) These metabolites showed potent activity to inhibit cholesterol synthesis both in vitro and in vivo.56) The most active product of these three, called ML-236B, was used to further developmental study. ML-236B, for historical reasons discussed later, was referred to in the early years as compactin and the name stuck. I shall continue to refer to it that way in this review.
HMG-CoA reductase is the rate-controlling enzyme in cholesterol synthesis. We soon realized the structural similarities between compactin and HMG-CoA, the substrate of the HMG-CoA reductase reaction55,57) (Fig. 2). The structure of compactin was exactly as we had previously envisioned. Compactin was an extremely potent inhibitor of HMG-CoA reductase, and its mechanism of action, as suggested by its structure, was that of a competitive inhibitor.57) I had set my sights on finding a competitive inhibitor of HMG-CoA reductase, and compactin seemed to be a wonderful gift from nature.
Figure 2.
Structual similarities between compactin and HMG-CoA.
Inhibition of sterol synthesis in cultured cells.
In 1973, Michael Brown and Joseph Goldstein demonstrated that in cultured fibroblasts of patients with familial hypercholesterolemia (FH),58,59) regulation of HMG-CoA reductase was partially or completely lost, resulting in high reductase activity even in the presence of low-density lipoprotein (LDL). As expected, compactin strongly inhibited sterol synthesis from acetyl-CoA in a variety of cultured mammalian cells and worked just as well in cells with patients with familial hypercholesterolemia as it did in normal cells. Inhibition was 50% at as low as 0.4 ng/ml (10−9 M) in both types of cell.60)
At very high concentrations, at which sterol synthesis and HMG-CoA reductase were strongly inhibited, cells were not able to grow and died, even in the presence of lipoproteins. But, this inhibition was overcome and cells grew normally by adding a small amount of mevalonate, the product of the HMG-CoA reductase reaction,60) indicating that inhibition by compactin was very specific to HMG-CoA reductase. In 1976 we published two papers reporting the discovery and characterization of compactin, the first statin.53,57)
In 1978, in collaboration with my group at Sankyo, Brown and Goldstein, adding compactin to cultured human cells, homogenized the cells and measured HMG-CoA reductase activity in vitro. They found a large increase in the amount of enzyme activity. Apparently, the cells tried to produce more of the enzyme. The enzyme was inactive in the cell because compactin was present. When the cells were homogenized, the compactin was diluted and the latent enzyme activity became apparent.61,62)
Compactin does not work in rats.
The next chapter in the statin story involved a series of animal experiments to determine the efficacy and toxicity of compactin. In 1974, biologists at Sankyo evaluated the efficacy of compactin by feeding rats a diet supplemented with compactin for 7 days. Unfortunately, there was no reduction in serum cholesterol.55)
With these results, there was no hope of convincing the biologists of evaluating compactin, which did not work in rats, in other animal species like dogs and monkeys. It looked as if 2 years of work and over 6,000 tests had led nowhere. Fortunately, although Sankyo Co. was not keenly interested in developing cholesterol lowering drugs, they permitted the project to continue.
We spent the next two years trying to elucidate the mechanism of action of compactin and the reason why it was not effective in rats. These experiments showed that while compactin did not work in rats when given repeatedly for 7 days, it was effective in lowering serum cholesterol levels in the animals between 3 to 8 hours after a single dose. However, after 8 hours, hepatic HMG-CoA reductase activity of rats was induced. When given repeatedly, compactin increased the amount of hepatic HMG-CoA reductase 8- to 10-fold and thereby cancelled out the inhibition of sterol synthesis by compactin.63) This induction was found to be the main reason why compactin did not work in rats. These findings suggested that compactin would be effective in other animal species and patients with elevated blood cholesterol levels.
Compactin was highly effective in hens, dogs and monkeys.
To move the compactin project on, it was essential to show that compactin was active in lowering plasma cholesterol in experimental animals. In the early spring of 1976, Noritoshi Kitano, a pathologist at Sankyo who was keeping laying hens for research purposes, kindly agreed to a joint research project to evaluate compactin using his hens. The experiments were a great success. The plasma cholesterol of laying hens that received compactin decreased by 50% after one month (unpublished results). We were also able to confirm the profound cholesterol-lowering effects of compactin in dogs64) and monkeys.65)
These results defined compactin as a candidate for a new type of drug. So, the ‘Compactin Development Project’—headed by myself and including pharmacologists, pathologists, toxicologists, organic chemists and microbiologists—was launched in August 1976.
Interestingly, at around the same period, researchers from England’s Beecham Pharmaceuticals, now known as GlaxoSmithKline, had also discovered compactin as an antifungal agent from another blue-green mold (Penicillium brevicompactum).66) However, they were unable to develop compactin as a cholesterol-lowering agent due to its inability to lower the blood cholesterol of rats as consistent with our results.67)
Second challenge.
We encountered a second challenge in April 1977, just 8 months after the launch of the Compactin Development Project. The issue was the detection of microcrystalline structures in the liver cells of rats that had been fed extremely large amounts of compactin (more than 500 milligrams per kilogram body weight per day (mg/kg/d) for 5 weeks. The toxicologists insisted that these structures were toxic substances. It took us 9 months to identify these microcrystalline structures as nontoxic cholesterol.
Treatment of patients with severe hypercholesterolemia.
The success of our studies led us to a clinical study in patients that was not sanctioned by Sankyo. Since I am not a physician, I performed this study in collaboration with Akira Yamamoto, a physician at the Osaka University Hospital in Osaka. In February 1978, Yamamoto started treating an 18-year old woman with severe familial hypercholesterolemia. Her serum cholesterol dropped from 1,000 mg per deciliter to ∼700 mg per deciliter during treatment with compactin at a daily dose of 500 mg.68) But after treatment for 2 weeks, trans-aminase levels of blood were elevated, and muscular dystrophy was observed. Both adverse effects were reversible on discontinuation of treatment. While cholesterol levels of the patient were not reduced as expected, tuberous and Achilles tendon xanthomatosis was markedly reduced after treatment at 200 mg of compactin for 5 months.
In the following 6 months, Yamamoto treated five heterozygous patients with familial hypercholesterolemia and three patients with combined hyperlipidemia with compactin, and their cholesterol declined by roughly 30% on average; no severe side effects were noted. We published these studies in a medical journal in 1980.68)
These treatments led to the clinical development of compactin at Sankyo. In November 1978, Sankyo started a phase 1 clinical trial for compactin. In phase 2 of the trial, started in the summer of 1979, compactin was administered to subjects with serious cases of hypercholesterolemia at twelve hospitals. All of the participating hospitals reported positively on the remarkable efficacy and excellent safety profile of compactin.69)
Discontinuation of compactin development.
In August 1980, however, Sankyo discontinued the clinical development of compactin, which had been progressing smoothly until that time. Since then, compactin has never come back. It was said that the drug caused lymphoma in dogs that received astonishingly high doses, 100 or 200 mg/kg/day for 2 years. No abnormalities were noted in the group receiving 20 mg/kg/day (personal communications). Yamamoto had already shown that compactin was fairly effective even in patients with severe hypercholesterolemia at less than 1 mg/kg/day.69) In other words, the dogs were getting about 200 times the dosage that would be used in patients. In the development of the second statin, called pravastatin. Sankyo avoided the same trouble by limiting its maximum dose to 25 mg/kg/day.70) So, regardless of whether lymphomas were actually detected or not, Sankyo could have continued the development of compactin by limiting its maximum dose to 25 mg/kg/day.
Development of lovastatin
Discovery of lovastatin.
At the end of the 1970s, the findings showing the dramatic effects of compactin in dogs and monkeys inspired many pharmaceutical companies to begin searching for another statin. Merck was first out of the gate. In July 1976, Roy Vagelos, President of Merck Research Laboratories, signed a confidentiality agreement with Sankyo and obtained samples of compactin and our confidential experimental data. Merck researchers confirmed our findings and were astonished at the potency of the drug. Under the direction of Alfred Albert, Merck set out to find its own statins and in February 1979 isolated a statin very similar to compactin in chemical structure, called mevinolin, from the fungus Aspergillus terreus.71–73)
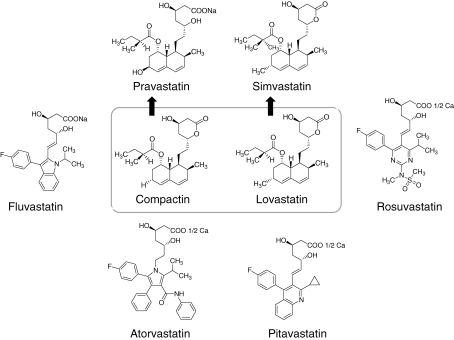
At the end of 1978, I left Sankyo and moved to Tokyo Noko University (Tokyo University of Agriculture and Technology), where I continued my work on reductase inhibitors, and in February 1979 isolated another statin (named monacolin K) from cultures of Monascus ruber.74) In the fall of the same year, it was confirmed that monacolin K and mevinolin were the same compound (later both changed to lovastatin) (Fig. 3).
Figure 3.
Compactin and other commercialized statins (compactin analogs).
Suspension of lovastatin development.
Merck began preliminary clinical studies of lovastatin in April 1980 but after 5 months, in September 1980, they discontinued the clinical trials because of rumors that the closely related compound, compactin, caused certain cancers in dogs. They could not ignore the rumors and it appeared that the lovastatin project was dead.72,73)
In early 1981, Brown and Goldstein reported that lovastatin could raise liver LDL receptors in dogs, and this led to a profound fall in plasma LDL levels.75) Lovastatin was then tested on people, and it was shown to produce a profound fall in plasma LDL levels.76)
Seven months later, Hiroshi Mabuchi’s group at Kanazawa University reported the impressive results of their highly successful compactin treatment of severe heterozygous patients with familial hypercholesterolemia.77) The LDL cholesterol, or bad cholesterol, of these patients declined by an average of 30% with no fall in HDL cholesterol, or good cholesterol; rather, a slight increase was noted. Subsequently, Mabuchi used a combination of compactin and cholestyramine in patients with heterozygous familial hypercholesterolemia. LDL cholesterol was reduced by 50–60% by the combination.78)
Birth of the first commercial statin.
In July 1982, several clinicians stimulated by Mabuchi’s report,77) including Roger Illingworth of Oregon Health Sciences University and Scott Grundy and David Bilheimer of the University of Texas, treated patients with severe hypercholesterolemia unresponsive to available agents.72,73) The drug showed dramatic activity in lowering LDL cholesterol, with very few side effects. This led Merck to begin large-scale clinical trials of lovastatin in patients at high risk and long-term toxicity studies in dogs in 1984. The drug dramatically reduced cholesterol levels and was well tolerated. No tumors were detected. In November 1986, Merck sent the New Drug Application (NDA) to the U.S. FDA and lovastatin was given FDA approval to become the first commercial statin in September 1987.72,73) Two years earlier, in 1985, Brown and Goldstein were awarded the Nobel Prize in Physiology or Medicine for their discoveries concerning the regulation of cholesterol metabolism.1)
Conclusion
Since lovastatin had been commercialized, 6 statins, including 2 semi-synthetic statins (simvastatin and pravastatin) and 4 synthetic statins (fluvastatin, atorvastatin, rosuvastatin and pitavastatin) have been introduced to the market55,79) (Fig. 3). Lovastatin was followed by a new statin, simvastatin. Sankyo, in turn, developed pravastatin and launched it in 1989. Four synthetic statins, shown in Fig. 3, were subsequently developed. Today the most popular statin is Pfizer’s atorvastatin (Lipitor®).
Statins have now been tested in many large-scale clinical trials, involving 90,000 subjects who were followed for 5 years.27,80,81) The results in all these studies have been consistent: treatment with statins lowers plasma LDL levels by 25–35% and reduces the frequency of heart attacks by 25–30%. It is said that the percentage reduction in coronary events would be even more dramatic if the treatment were longer and if statin therapy were started earlier. No major adverse effects of lowering cholesterol were noted in any of the studies. The remarkable safety of statins derives from their unique mechanism of action. The statins are the largest selling class of drugs currently taken by patients throughout the world. Sales for this one class of drugs in 2005 were $25 billion.
Today, an estimated 30 million people worldwide are taking statins. It is said that the lives of millions of people have been extended through statin therapy. I believe that the words Rector Friberg spoke at the 1964 Nobel Prize Banquet—that we may “look forward to a time when mankind will not only live under vastly improved conditions, but will itself be better”—have truly been realized.82)
Profile
Akira Endo, born in 1933, obtained a BA at Tohoku University (Faculty of Agriculture) in Sendai in 1957 and a PhD in biochemistry at the same university in 1966. From 1957 to 1978 he worked as a biochemist at Sankyo Co. He spent two years (1966–1968) at the Albert Einstein College of Medicine in New York as a research associate. From 1979 to 1997 he worked as an associate professor (1979–1986) and later a full professor (1986–1997) at the Tokyo University of Agriculture and Technology (TUAT), and after official retirement, besides becoming the director of Biopharm Research Laboratories Inc. (Tokyo), he serves as a professor on Special Mission at Tohoku University and Waseda University and a Visiting Professor at Kanazawa University and Hitotsubashi University. Prizes and honors received include the 1987 Heinrich Wieland Prize (Germany), the 1987 Toray Science-Technology Prize (Japan), the 2000 Warren Alpert Foundation Prize (U.S.A.), the 2006 Japan Prize, the 2006 Massry Prize (U.S.A.), the 2008 Albert Lasker∼DeBakey Clinical Medical Research Award (U.S.A.), Distinguished Professor Emeritus at TUAT (2008) Honorary Citizen of Akita Prefecture, Japan (2008), Outstanding Achievement Award (2009, International Society of Atherosclerosis) and the 2008 Lasker Awardee Special Lecture (American Heart Association).
References
- 1).Brown M.S., Goldstein J.L. (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 [DOI] [PubMed] [Google Scholar]
- 2).Reinitzer F. (1888) Contributions to the knowledge of cholesterol. Mon. Chem. 9, 421–441 [Google Scholar]
- 3).Goldstein J.L., Brown H.S. (2003) Cholesterol: a century of research. HHMI Bull. 16(3), 10–19 [Google Scholar]
- 4).Anitschkow N. (1913) Über die Veränderungen der Kaninchenaorta bei experimenteller Cholesterinsteatose. Beitr. Pathol. Anat. 56, 379–404 [Google Scholar]
- 5).Müller C. (1939) Angina pectoris in hereditary xanthomatosis. Arch. Intern. Med. 64, 675–700 [Google Scholar]
- 6).Khachadurian A.K. (1964) The inheritance of essential familial hypercholesterolemia. Am. J. Med. 37, 402–407 [DOI] [PubMed] [Google Scholar]
- 7).Gofman J.W., Lindgren F.T., Elliott H. (1949) Ultracentrifugal studies of lipoproteins of human serum. J. Biol. Chem. 179, 973–979 [PubMed] [Google Scholar]
- 8).Gofman J.W., Lindgren F.T., Elliott H., Manz W., Hewitt J., Herring V. (1950) The role of lipids and lipoproteins in atherosclerosis. Science 111, 166–171 [DOI] [PubMed] [Google Scholar]
- 9).Gofman J.W. (1956) Serum lipoproteins and the evaluation of atherosclerosis. Ann. N.Y. Acad. Sci. 64, 590–595 [DOI] [PubMed] [Google Scholar]
- 10).Keys A., Anderson J.T., Fidanza F., Keys M.H., Swahn B. (1955) Effects of diet on blood lipids in man, particularly cholesterol and lipoproteins. Clin. Chem. 1, 34–52 [PubMed] [Google Scholar]
- 11).Keys A., Aravanis C., Blackburn H.W., Van Buchem F.S., Buzina R., Djordjević B.D., et al. (1966) Epidemiological studies related to coronary heart disease: characteristics of men aged 40–59 in seven countries. Acta Med. Scand. Suppl. 460, 1–392 [PubMed] [Google Scholar]
- 12).Keys A. (1970) Coronary heart disease in seven countries. Circulation 41 (Suppl. 1), 1–211 [PubMed] [Google Scholar]
- 13).Kannel W.B., Dawber T.R., Kagan A., Revotskie N., Stokes J., III (1961) Factors of risk in the development of coronary heart disease—six year follow-up experience. The Framingham Study. Ann. Intern. Med. 55, 33–50 [DOI] [PubMed] [Google Scholar]
- 14).Wilson P.W., Garrison R.J., Castelli W.P., Feinleib M., McNamara P.M., Kannel W.B. (1980) Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am. J. Cardiol. 46, 649–654 [DOI] [PubMed] [Google Scholar]
- 15).Bloch K. (1965) The biological synthesis of cholesterol. Science 150, 19–28 [DOI] [PubMed] [Google Scholar]
- 16).Bucher N.L., Overath P., Lynen F. (1960) β-Hydroxy-β-methylglutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim. Biophys. Acta 40, 491–501 [DOI] [PubMed] [Google Scholar]
- 17).Lynen F. (1966) The biochemical basis of the biosynthesis of cholesterol and fatty acids. Wien. Klin. Wochenschr. 78, 489–497 [PubMed] [Google Scholar]
- 18).Cornforth J.W., Popják G. (1958) Biosynthesis of cholesterol. Br. Med. Bull. 14, 221–226 [DOI] [PubMed] [Google Scholar]
- 19).Popják G., Cornforth J.W. (1960) The biosynthesis of cholesterol. Adv. Enzyme Regul. 22, 281–335 [DOI] [PubMed] [Google Scholar]
- 20).Bloch, K. (1964) Speech at the Nobel Banquet in Stockholm, December 10, 1964. [Google Scholar]
- 21).Dietschy J.M., Wilson J.D. (1970) Regulation of cholesterol metabolism. N. Engl. J. Med. 282, 1128–1138 [DOI] [PubMed] [Google Scholar]
- 22).Dietschy J.M., Wilson J.D. (1970) Regulation of cholesterol metabolism. N. Engl. J. Med. 282, 1179–1183 [DOI] [PubMed] [Google Scholar]
- 23).Dietschy J.M., Wilson J.D. (1970) Regulation of cholesterol metabolism. N. Engl. J. Med. 282, 1241–1249 [DOI] [PubMed] [Google Scholar]
- 24).Dietschy D.M., Siperstein M.D. (1967) Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J. Lipid Res. 8, 97–104 [PubMed] [Google Scholar]
- 25).Dietschy D.M., Wilson J.D. (1968) Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. Clin. Investig. 47, 166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Siperstein M.D., Fagan V.M. (1966) Feedback control of mevalonate synthesis by dietary cholesterol. J. Biol. Chem. 241, 602–609 [PubMed] [Google Scholar]
- 27).Steinberg D. (2006) An interpretive history of the cholesterol controversy, part V. The discovery of the statins and the end of the controversy. J. Lipid Res. 47, 1339–1351 [DOI] [PubMed] [Google Scholar]
- 28).Blohm T.R., MacKenzie R.D. (1959) Specific inhibition of cholesterol biosynthesis by a synthetic compound (MER-29). Arch. Biochem. Biophys. 85, 245–249 [DOI] [PubMed] [Google Scholar]
- 29).Avigan J., Steinberg D., Thompson M.J., Mosettig E. (1960) Mechanism of action of MER-29, an inhibitor of cholesterol biosynthesis. Biochem. Biophys. Res. Commun. 2, 63–65 [DOI] [PubMed] [Google Scholar]
- 30).Steinberg D., Avigan J., Feigelson E.B. (1960) Identification of 24-dehydrocholesterol in the serum of patients treated with MER-29. Prog. Cardiovasc. Dis. 2, 586–592 [DOI] [PubMed] [Google Scholar]
- 31).Avigan J., Steinberg D. (1962) Deposition of desmosterol in the lesions of experimental atherosclerosis. Lancet 1, 527. [DOI] [PubMed] [Google Scholar]
- 32).Altschul R., Hoffer A., Stephen J.D. (1955) Influence of nicotinic acid on serum cholesterol in man. Arch. Biochem. 54, 558–559 [DOI] [PubMed] [Google Scholar]
- 33).Thorp J.M., Warig W.S. (1962) Modification of Metabolism and distribution of lipids by ethyl chlorophenoxyisobutyrate. Nature 194, 948–949 [DOI] [PubMed] [Google Scholar]
- 34).Thompson, G.R. (1989) A Handbook of Hyperlipidemia. Current Science Ltd., London. [Google Scholar]
- 35).Bergen S.S., Jr., Van Itallie T.B., Tennent D.M., Sebrell W.H. (1959) Effect of an anion exchange resin on serum cholesterol in man. Proc. Soc. Exp. Biol. Med. 102, 676–679 [DOI] [PubMed] [Google Scholar]
- 36).Tennent D.M., Siegel H., Zanetti M.E., Curon G.W., Ott W.H., Wolf E.J. (1960) Plasma cholesterol lowering action of bile acid binding polymers in experimental animals. J. Lipid Res. 1, 469–473 [PubMed] [Google Scholar]
- 37).Koizumi, T. (1939) Hideyo Noguchi (revised). Iwanami Shoten, Tokyo (in Japanese). [Google Scholar]
- 38).Okumura, T. (1933) Hideyo Noguchi. Iwanami Shoten, Tokyo (in Japanese). [Google Scholar]
- 39).Ludovici, L.J. (1952) Fleming. Discoverer of Penicillin. Andrew Dankers Limited, London. [Google Scholar]
- 40).Waksman, S.A. (1954) Living with the Microbes. Simon & Schuster, Inc., New York. [Google Scholar]
- 41).Endo A. (1961) Studies on pectolytic enzymes of molds. I. Survery of enzyme- producing microorganisms by fruit juice clarification. Agr. Biol. Chem. (Japan) 25, 382–388 [Google Scholar]
- 42).Endo A. (1964) Studies on pectolytic enzymes of molds. VIII. Purification and properties of endopolygalacturonase I. Agr. Biol. Chem. (Japan) 28, 535–542 [Google Scholar]
- 43).Endo A. (1964) Studies on pectolytic enzymes of molds. XIII. Clarification of apple juice by the joint action of purified pectolytic enzymes. Agr. Biol. Chem. (Japan) 29, 129–136 [Google Scholar]
- 44).Endo A. (1964) Studies on pectolytic enzymes of molds. XVI. Mechanism of clarfication of apple juice. Agr. Biol. Chem. (Japan) 29, 229–233 [Google Scholar]
- 45).Endo A., Rothfield L. (1969) Studies of a phospholipid-requiring bacterial enzyme. I. Purification and properties of UDP-galactose lipopolysaccharide-α3-galactosyl-transferase. Biochemistry 8, 3500–3507 [DOI] [PubMed] [Google Scholar]
- 46).Endo A., Rothfield L. (1969) Studies of a phospholipid-requiring bacterial enzyme. II. The role of phospholipid in the UDP-galactose: lipopolysaccharide-α3-galactosyl-transferase. Biochemistry 8, 3508–3515 [DOI] [PubMed] [Google Scholar]
- 47).Gale, E.F., Cundliffe, E., Reynold, P.E., Richmond, M.H. and Waring, M.J. (1972) The Molecular Basis of Antibiotic Action. John Wiley & Sons, New York. [Google Scholar]
- 48).Goldfarb S., Pitot H.C. (1971) Improved assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Lipid Res. 12, 512–515 [PubMed] [Google Scholar]
- 49).Bucher N.L.R. (1953) The formation of radioactive cholesterol and fatty acids from C14-acetate by rat liver homogenates. J. Am. Chem. Soc. 75, 498 [Google Scholar]
- 50).Knauss H.J., Porter J.W., Wasson G. (1959) The biosynthesis of mevalonic acid from 1-C14-acetate by a rat liver enzyme system. J. Biol. Chem. 234, 2835–2840 [PubMed] [Google Scholar]
- 51).Endo A., Kuroda M. (1976) Citrinin, an inhibitor of cholesterol synthesis. J. Antibiot. (Japan) 29, 841–843 [DOI] [PubMed] [Google Scholar]
- 52).Tanzawa K., Kuroda M., Endo A. (1977) Time-dependent, irreversible inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by the antibiotic citrinin. Biochim. Biophys. Acta 488, 97–101 [PubMed] [Google Scholar]
- 53).Endo A., Kuroda M., Tsujita Y. (1976) ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J. Antibiot. (Japan) 29, 1346–1348 [DOI] [PubMed] [Google Scholar]
- 54).Endo A. (1988) Chemistry, biochemistry, and pharmacology of HMG-CoA reductase inhibitors. Klin. Wochenschr. 66, 421–427 [DOI] [PubMed] [Google Scholar]
- 55).Endo A. (1992) The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 33, 1569–1582 [PubMed] [Google Scholar]
- 56).Endo A., Tsujita T., Kuroda M., Tanzawa K. (1977) Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur. J. Biochem. 77, 31–36 [DOI] [PubMed] [Google Scholar]
- 57).Endo A., Kuroda M., Tanzawa K. (1976) Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML 236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 72, 323–326 [DOI] [PubMed] [Google Scholar]
- 58).Brown M.S., Dana S.E., Goldstein J.L. (1973) Regulation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 70, 2162–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Brown M.S., Goldstein J.L. (1974) Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc. Natl. Acad. Sci. U.S.A. 71, 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Kaneko I., Hazama-Shimada Y., Endo A. (1978) Inhibitory effects on lipid metabolism incultured cells of ML-236B, a potent inhibitor of 3-hydroky-3-methylgutaryl-coenzyme-A reductase. Eur. J. Biochem. 87, 313–321 [DOI] [PubMed] [Google Scholar]
- 61).Brown M.S., Faust J.R., Goldstein J.L., Kaneko I., Endo A. (1978) Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem. 253, 1121–1128 [PubMed] [Google Scholar]
- 62).Brown M.S., Goldstein J.L. (2004) A tribute to Akira Endo, discoverer of a “penicillin” for cholesterol. Atherosclerosis Suppl. 5, 13–16 [Google Scholar]
- 63).Endo A., Tsujita Y., Kuroda M., Tanzawa K. (1979) Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim. Biophys. Acta 575, 266–276 [PubMed] [Google Scholar]
- 64).Tsujita Y., Kuroda M., Tanzawa K., Kitano N., Endo A. (1979) Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Atherosclerosis 32, 307–313 [DOI] [PubMed] [Google Scholar]
- 65).Kuroda M., Tsujita Y., Tanzawa K., Endo A. (1979) Hypolipidemic effects in monkeys of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Lipids 14, 585–589 [DOI] [PubMed] [Google Scholar]
- 66).Brown A.G., Smale T.C., King T.J., Hasenkamp R., Thompson R.H. (1976) Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J. Chem. Soc., Perkin Trans. 1 1165–1170 [PubMed] [Google Scholar]
- 67).Fears R.B, Richards D.H, Ferres H. (1980) The effect of compactin, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity, on cholesterogenesis and serum cholesterol levels in rats and chicks. Atherosclerosis 35, 439–449 [DOI] [PubMed] [Google Scholar]
- 68).Yamamoto A., Sudo H., Endo A. (1980) Therapeutic effects of ML-236B inprimary hypercholesterolemia. Atherosclerosis 35, 259–266 [DOI] [PubMed] [Google Scholar]
- 69).Abstract Book of the VII International Symposium on Drugs Affecting Lipid Metabolism, held May 28–31, 1980 in Milan, Italy. pp. 224–234. [Google Scholar]
- 70).Interview Format in mevastatin (in Japanese). p. 23, Sankyo Co. 1989. [Google Scholar]
- 71).Alberts A.W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., et al. (1980) Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 77, 3957–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Vagelos P.R. (1991) Are prescribing drug prices high? Science 252, 1080–1084 [DOI] [PubMed] [Google Scholar]
- 73).Vagelos, P.R. and Galambos, L. (2004) Medicine, Science and Merck. Cambridge University Press, Cambridge, United Kingdom. pp. 1–301. [Google Scholar]
- 74).Endo A. (1979) Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. 32, 852–854 [DOI] [PubMed] [Google Scholar]
- 75).Brown M.S., Dana S.E., Goldstein J.L. (1973) Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc. Natl. Acad. Sci. USA 70, 2162–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Brown M.S., Dana S.E., Goldstein J.L. (1974) Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J. Biol. Chem. 249, 789–796 [PubMed] [Google Scholar]
- 77).Mabuchi H., Haba T., Tatami R., Miyamoto S., Sakai Y., Wakasugi T., et al. (1981) Effects of an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase on serum lipoprotein and ubiquinone-10-levels in patients with familial hypercholesterolemia. N. Engl. J. Med. 305, 478–482 [DOI] [PubMed] [Google Scholar]
- 78).Mabuchi H., Sakai T., Yoshimura A., Watanabe A., Wakasugi T., Watanabe A., et al. (1983) Reduction of serum cholesterol in heterozygous patients with familial hypercholesterolemia. Additive effects of compactin and cholestyramine. N. Engl. J. Med. 308, 609–613 [DOI] [PubMed] [Google Scholar]
- 79).Endo A. (2008) A gift from nature: the birth of the statins. Nat. Med. 14, 1050–1052 [DOI] [PubMed] [Google Scholar]
- 80).Scandinavian Simviastatin Survival Study Group (1994) Randomised trial of cholesterollowering in 4444 patients with coronary heart disease: the Scandinavian Simvistatin Survival Study (4S). Lancet 344, 1383–1389 [PubMed] [Google Scholar]
- 81).Shepherd J., Cobbe S.M., Ford I., Isles C.G., Lorimer A.R., Macfarlane P.W., et al. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333, 1301–1307 [DOI] [PubMed] [Google Scholar]
- 82).Goldfine H., Vance D.H. (2001) Obituary: Konrad E. Bloch (1912–2000). Nature 409, 779. [DOI] [PubMed] [Google Scholar]