Abstract
Photochromism is defined as a reversible transformation of a chemical species between two isomers upon photoirradiation. Although vast numbers of photochromic molecules have been so far reported, photochromic molecules which exhibit thermally irreversible photochromic reactivity are limited to a few examples. The thermal irreversibility is an indispensable property for the application of photochromic molecules to optical memories and switches. We have developed a new class of photochromic molecules named “diarylethenes”, which show the thermally irreversible photochromic reactivity. The well designed diarylethene derivatives provide outstanding photochromic performance: both isomers are thermally stable for more than 470,000 years, photoinduced coloration/decoloration can be repeated more than 105 cycles, the quantum yield of cyclization reaction is close to 1 (100%), and the response times of both coloration and decoloration are less than 10 ps. This review describes theoretical background of the photochromic reactions, color changes of the derivatives in solution as well as in the single crystalline phase, and application of the crystals to light-driven actuators.
Keywords: photochromism, diarylethene, single crystal, optical memory, optical switch, actuator
Introduction
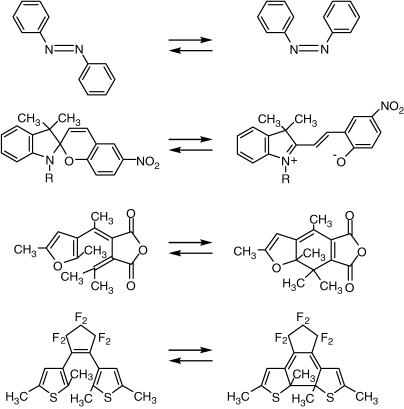
Photochromic molecules are characterized by their reversible color changes upon photoirradiation.1–3) Typical examples of photochromic molecules, which show such color changes, are shown in Fig. 1. The upper two molecules, azobenzene and spiropyran, change the color from colorless to orange and blue, respectively. These traditional molecules are classified into T-type (thermally reversible) photochromic ones. The photogenerated right-side isomers are thermally unstable and the colors slowly disappear in the dark. On the other hand, recently invented lower two molecules, furylfulgide and diarylethene, both of which change the color from colorless to red, are classified into P-type (thermally irreversible, but photochemically reversible) photochromic ones. In these P-type photochromic molecules, photogenerated right-side isomers are thermally stable and scarcely return to the left-side isomers in the dark at room temperature. The thermally irreversible property is indispensable for the application to optical memories and switches. The thermal irreversibility of diarylethene derivatives will be discussed in detail later.
Figure 1.
Typical examples of photochromic molecules.
The color changes are ascribed to photoinduced electronic structural changes of the molecules from the left- to the right-side isomers. During the electronic structural changes the molecules also change the geometrical structures. Azobenzene isomerizes from the trans-isomer to the cis-isomer. Spiropyran converts from the closed-ring form to the open-ring form. Photochromic molecules change both their electronic as well as geometrical structures upon photoirradiation.
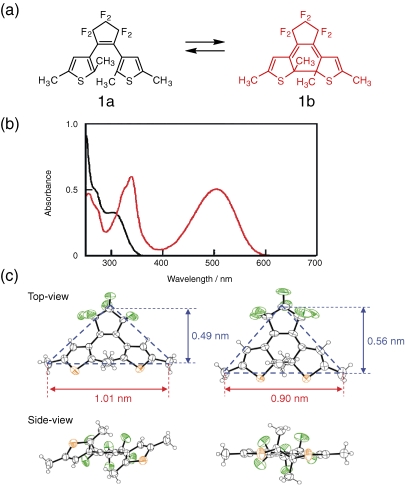
A typical example of the electronic and geometrical structural changes of a diarylethene derivative is shown in Fig. 2. Figure 2a and b show the chemical structures of the open- and the closed-ring isomers of 1,2-bis(2,5-dimethyl-3-theinyl)perfluorocyclopentene (1a) and their absorption spectral changes (color changes).4) In the left-side open-ring isomer two thiophene rings have no interaction and the spectrum is similar to a substituted thiophene. On the other hand, in the right-side closed-ring isomer π-electrons are delocalized throughout the molecule and the HOMO–LUMO gap becomes small. As a result, the spectrum shifts to longer wavelengths. The red-color is ascribed to the delocalization of π-electrons. At the same time, the molecule changes the geometrical structure. The top- and side-views of the molecule are shown in Fig. 2c. As can be seen from the side-view, thickness of the molecule becomes thinner when the molecule undergoes the photocyclization. The top-view of the molecule indicates that the base width of the triangle shape (blue broken line) decreases from 1.01 nm to 0.90 nm and the height increases from 0.49 nm to 0.56 nm. Inferring from the shape change it is concluded that the diarylethene molecule shrinks upon photocyclization and expands upon photocycloreversion.5,6)
Figure 2.
(a) Chemical structures of the open- and the closed-ring isomers of 1,2-bis(2,5-dimethyl-3-theinyl)perfluorocyclopentene (1a) (b) absorption spectra of the open- (solid line) and the closed-ring (red line) isomers and (c) top- and side-views of the geometrical structures of the open- and closed-ring isomers.
The electronic as well as geometrical structural changes at the molecular level can be adopted in various macro-scale applications. The electronic structural changes can be applied to optical memory media and photo-switching devices.7–10) On the other hand, the geometrical structural changes can be potentially applied to light-driven actuators.11–15) For such applications, photochromic molecules should fulfill the following requirements.
-
1.
Thermal stability of both isomers
-
2.
Fatigue-resistant property
-
3.
High sensitivity
-
4.
Rapid response
-
5.
Reactivity in solid state
We have developed a new class of photochromic molecules named “diarylethenes”, which fulfill the above all requirements simultaneously.16–19) Diarylethenes are derivatives of stilbene. When phenyl rings of stilbene are replaced with five-membered heterocyclic rings, such as thiophene or furan rings, both isomers become thermally stable and coloration/decoloration cycles can be repeated many times. Characteristic properties of diarylethene derivatives are summarized as follows.
-
1.
Both isomers are thermally stable: half-life times at room temperature are as long as 470,000 years
-
2.
Coloration/decoloration can be repeated more than 105 cycles
-
3.
The quantum yield of coloration is close to 1 (100%).
-
4.
Response times of both coloration and decoloration are less than 10 ps
-
5.
Many of diarylethene derivatives undergo photochromism even in the single crystalline phase
In this review, theoretical background of the photochromic reactions, color changes of the derivatives in solution as well as in the single crystalline phase, and application of the geometrical structural changes to light-driven actuators will be described.
Theoretical study
Thermal irreversibility is an indispensable property for the application of photochromic molecules to memories and switches. Although tremendous efforts were carried out in the 70s–80s to provide the thermal irreversibility to photochromic systems, all attempts to modify existing photochromic molecules failed.7) We had to wait until fortunate finding of thermally irreversible photochromic molecules. Finally in the beginning and the middle of the 80s furylfulgide and diarylethene were found to undergo thermally irreversible photochromic reactions, respectively.20–23) Although the photogenerated colors of these molecules remained stable at room temperature, the reason why the molecules show the thermal irreversibility was not understood. It was strongly desired to reveal the basic principle of the thermally irreversible photochromic reactivity. We tried to solve the problem based on MO theory.
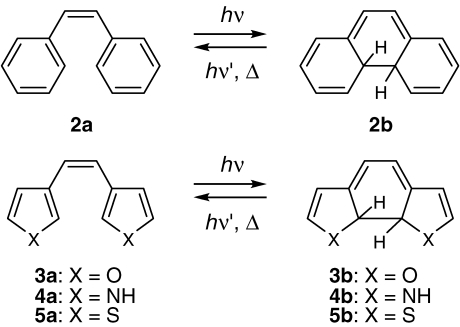
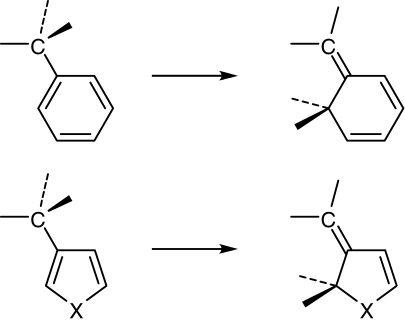
A theoretical study on 1,3,5-hexatriene to cyclohexadiene-type photochromic reactions was carried out to reveal the thermally irreversible reactivity.24) Typical molecules of the hexatriene molecular framework are diarylethene derivatives having phenyl or heterocyclic rings. Semiempirical MNDO calculation was carried out for diarylethene derivatives 2–5 (Scheme 1).
Scheme 1.
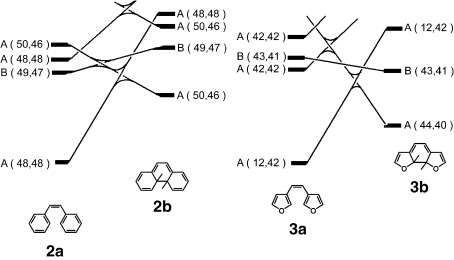
Figure 3 shows state correlation diagrams for the electrocyclic reactions from 2a to 2b and from 3a to 3b in a conrotatory mode. Full lines of the figure show that the interconnecting states belong to the same symmetry groups. According to the Woodward-Hoffmann rule based on π-orbital symmetries for 1,3,5-hexatriene, the cyclization reaction is allowed in the conrotatory mode in the photoexcited state. The cyclization reactions of both molecules can not proceed in the ground state because of large energy barriers. The open-ring isomers excited to B states convert to the closed-ring isomers in the ground state.
Figure 3.
State correlation diagrams in conrotatory mode for the reaction from 2a to 2b and from 3a to 3b.
Although full lines of Fig. 3 suggests that the closed-ring isomers, 2b and 3b, can convert to the open-ring isomers, 2a and 3a, in the ground state, the cycloreversion reactions have to overcome some energy barriers. The barriers correlate with the ground state energy difference between the open- and the closed-ring isomers. The calculated values of the difference for 2, 3, 1,2-di(3-pyrrolyl)ethene (4), and 1,2-di(3-thienyl)ethene (5) are shown in Table 1. When the energy difference is large, as in the case of 2, the energy barrier becomes small and the reaction is expected to take place readily. On the other hand, the reaction barrier becomes large when the energy difference is small as shown for 3. In this case, the cycloreversion reaction hardly takes place. The energy difference between the open- and the closed-ring isomers controls the stability of the photogenerated closed-ring isomers.
Table 1.
Relative ground-state energy differences between the open- and closed-ring isomers
| Compd | Conrotatory (kcal/mol) |
|
|---|---|---|
| 1,2-diphenylethene | (2) | 27.3 |
| 1,2-di(3-pyrrolyl)ethene | (4) | 15.5 |
| 1,2-di(3-furyl)ethene | (3) | 9.2 |
| 1,2-di(3-thienyl)ethene | (5) | −3.3 |
During the cyclization reaction phenyl and heterocyclic rings change the structures as shown in Scheme 2. The structural changes indicate that aromaticity of the groups change during the reactions. The energy difference between the right and left side groups was calculated, as shown in Table 2. The aromatic stabilization energy of the aryl groups correlates well with the ground state energy difference. The highest energy difference was calculated for the phenyl group and the lowest for the thienyl group. The aromaticity is the key molecular property which controls the energy barrier or the thermal stability of the closed-ring isomers.
Scheme 2.
Table 2.
Aromatic stabilization energy difference
| Group | Energy (kcal/mol) |
|---|---|
| Phenyl | 27.7 |
| Pyrrol | 13.8 |
| Furyl | 9.1 |
| Thienyl | 4.7 |
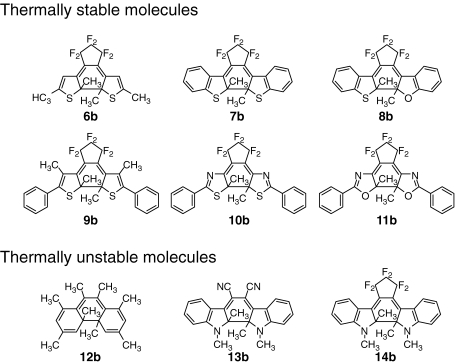
The theoretical prediction was confirmed by the synthesis of diarylethene derivatives with various types of side groups, as shown in Fig. 4.16) When the side groups are furan, thiophene, benzothiophene, benzofuran, thiazole, oxazole rings, which have low aromatic stabilization energy, the closed-ring isomers are thermally stable and hardly return to the open-ring isomers. On the other hands, photogenerated close-ring isomers of diarylethenes with phenyl or indole rings, which have rather high aromatic stabilization energy, are thermally unstable. They return to the open-ring isomers even in the dark. The basic principle that diarylethene derivatives having side groups with low aromatic stabilization energy exhibit thermal irreversibility has been established based on the theoretical consideration as well as the supporting experimental results.
Figure 4.
Thermal stability of photogenerated closed-ring isomers of diarylethene derivatives having various types of side groups.
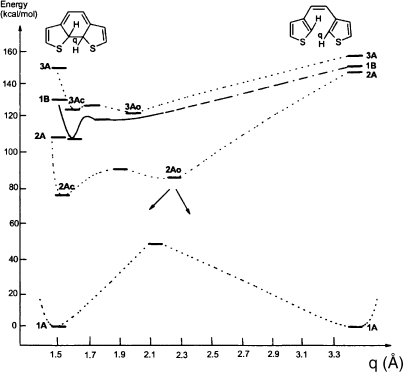
To reveal the details of the photoisomerization reactions ab initio MO study was carried out for a model system.25–27) The energy profile of the potential surface is shown in Fig. 5 as a function of the internuclear distance (q) between two reactive carbons. The photocyclization reaction of the open-ring isomer proceeds as follows. Upon excitation from 1A to 1B state, the 1B state will relax to the minimum having C–C distance of 1.779 Å or crossover into 2A state and then relax to 2Ao. The 2Ao minimum is located between the closed- and the open-ring isomers. From this 2Ao point the system decays to the ground state open- or closed-ring isomers. The photocyclization reaction proceeds without any reaction barrier.
Figure 5.
Potential energy profile of the model system.
The cycloreversion reaction of the closed-ring isomers to the open-ring isomer proceeds as follows. Upon excitation to the 1B state, the system reaches 2A state and then 2Ac state will be populated. Going from the 2Ac closed-ring excited state to 2Ao, there is a barrier caused by C–C bond fission. When the system arrives at the 2Ao point, both cycloreversion as well as cyclization reactions can take place. The rate of population of the 2Ao state by the cycloreversion process is less than the population from the cyclization process, because of the presence of an energy barrier. This is consistent with the experimental results that the cyclization quantum yield is larger than that of cycloreversion in dithienylethene derivatives.
The energy barrier from 2Ac to 2Ao controls the quantum yield of cycloreversion reaction. When the barrier is large, the cycloreversion quantum yield is expected to decrease.28) The experimental quantum yields were plotted against the calculated energy differences between 2Ao and 2Ac states, E(2Ao) − E(2Ac), for five diarylethene derivatives. The quantum yields were found to decrease linearly with increasing energy differences. The good correlation indicates that the energy surface in the excited state well explains the cycloreversion reaction process. The theoretical calculations successfully predict the photochemical quantum yields.
Color changes
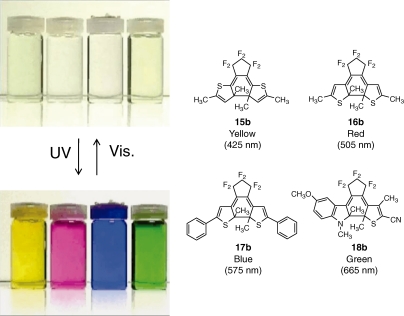
As noted in the introduction, photoinduced color changes are the most pronounced phenomenon observed in photochromic molecules. Figure 6 shows typical color changes of several diarylethene derivatives. When hexane solutions of the derivatives are irradiated with UV light, the colorless solutions turn to yellow, red, blue and green. The chemical structures of the derivatives, 15b–18b, are also shown in the figure. The color change is ascribed to the electronic structure change of the diarylethenes from the open- to the closed-ring isomers. The color is controlled by the length of π-conjugation of the closed-ring isomers. In 15b the π-conjugation is localized in the central part, while in 16b and 17b the π-conjugation delocalizes throughout the molecules and the length of 17b is longer than that of 16b. Therefore, the absorption maximum of 17b (blue) is longer than that of 16b (red). Nowadays, it is possible to estimate the colors using theoretical calculations.
Figure 6.
Typical color changes of several diarylethene derivatives in hexane solution upon photoirradiation.
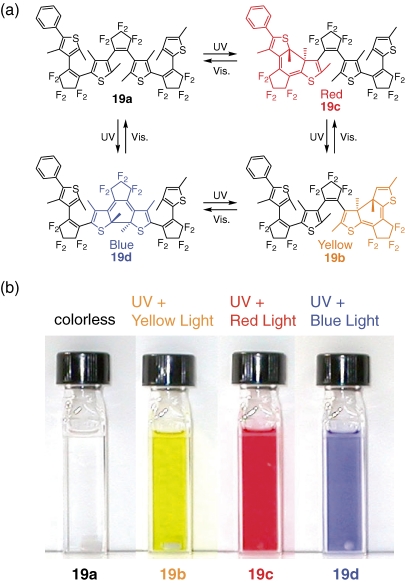
In normal photochromic systems they interconvert between only two states: colorless and colored. If multi-color photochromic molecules are prepared, they are useful for multifrequency optical memories and displays. We took an approach to incorporate three photochromic units in one molecule.29) The advantage of the one-molecule system over mixture systems is high image resolution, constant color balance in a large area and possible application to multifrequency single-molecule memory. Figure 7a shows the fused trimer (19a) along with anticipated photochromic reactions. The fused trimer has three dithienylethene moieties, bis(2-thienyl)ethene, (2-thienyl)(3-thienyl)ethene and bis(3-thienyl)ethene, with two thiophene rings in common. Upon irradiation with UV (313 nm) light the hexane solution containing 19a turned black. When UV (313 nm) and blue (460 nm) light was simultaneously irradiated, the solution turned sky blue. When the solution was irradiated with UV (313 nm) and red (633 nm) light, the solution turned to red. The solution turned to yellow when the solution was irradiated UV (313 nm) and yellow (578 nm) light. The solution containing 19a can develop blue, red, and yellow colors upon irradiation with appropriate wavelength of light, suggesting that all of the three diarylethene moieties can cyclize upon irradiation, as shown in Figure 7b.
Figure 7.
Photochromic reactions of fused trimer 19 and the color changes upon irradiation with the appropriate wavelength of light.
Most of diarylethene derivatives undergo photochromism even in the single crystalline phase, because the geometrical structural change during the photochromic reaction is very small. Figure 8 shows typical color changes of several diarylethene single crystals.30) Upon irradiation with UV light, colorless crystals turn yellow, red, blue or green, depending on the molecular structure of the diarylethenes. The colored forms are stable even at 100 ℃ and never return to the initial colorless form at room temperature in the dark. The color disappeared by irradiation with visible light. The photoinduced coloration/decoloration cycles can be repeated more than 104–105 times without any destruction of the crystals.
Figure 8.
Color changes of several diarylethene single crystals upon photoirradiation.
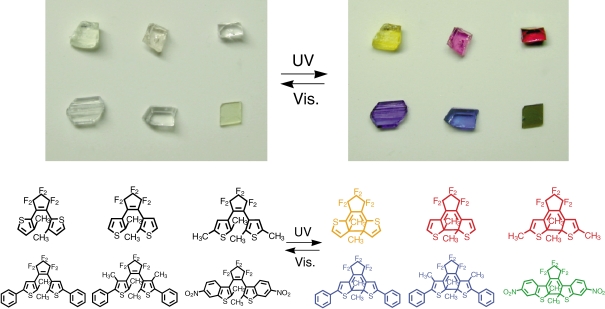
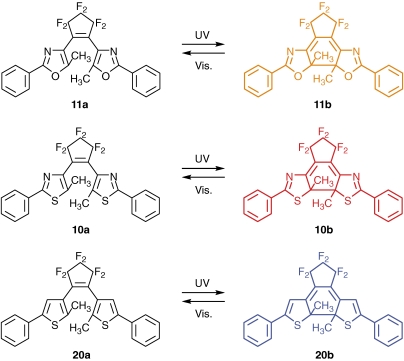
Multi-color single crystals can be prepared by mixing appropriate diarylethene derivatives with similar geometrical structures.31–33) We prepared mixed crystals composed of above derivates, 11, 10 and 20 (Scheme 3).33)
Scheme 3.
These three diarylethene derivatives have very similar geometrical structures, but the colors of the closed-ring isomers are different. Colorless 11a, 10a and 20a turn yellow, red and blue upon UV irradiation. These three derivatives could be readily mixed in almost any ratios into single crystals. The color change of the three component crystal upon irradiation with the appropriate wavelength of light is shown in Fig. 9. The crystal changes from colorless to yellow, red, and blue upon photoirradiation.
Figure 9.
Color changes of a three-component crystal containing diarylethene 11a, 10a and 20a upon irradiation with the appropriate wavelength of light.
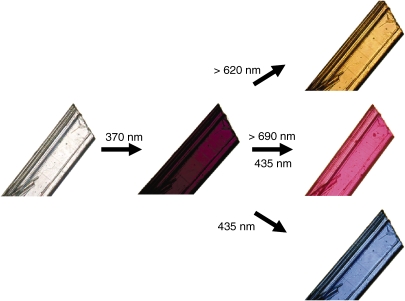
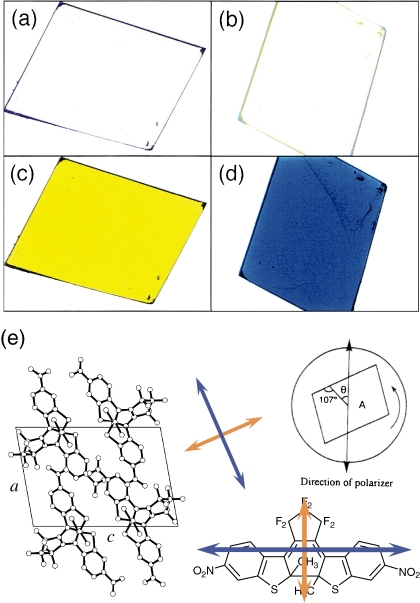
The multicolor change was also observed for a single-component crystal under polarized light.34) Figure 10a–d show the color change of single crystal (010) surface of 1,2-bis(2-methyl-6-nitro-1-benzothiophen-3-yl)perfluorocyclopentene (21a). Before photoirradiation the crystal was colorless (Fig. 10a and b). Upon irradiation with UV light, the crystal turned yellow at a certain angle (θ = 0). When the crystal was rotated as much as 90° (θ = 90), the color turned blue. The yellow color reappeared at 180°. The clear dichroism from yellow to blue is explained by the regular orientation of the diarylethene in the crystals. Figure 10e shows the chemical structure of 21b and the molecular packing of the diarylethenes viewed from (010) surface. The diarylethene has two electronic transitions, long and short axes, and the long and short axes transitions correspond to 600 nm and 465 nm absorption bands. When the polarized light coincides to the long axis, the crystal exhibits blue color (600 nm), while the color changes to yellow (465 nm) when the polarized light coincides to the short axis. Even single component diarylethene crystal can show two colors under polarized light.
Figure 10.
Photographs of single crystal 21a under polarized light before (a, θ = 0°; b, θ = 90°) and after (c, θ = 0°; d, θ = 90°) irradiation with UV light. θ is the rotation angle of the crystal. (e) crystal packing viewed from the (010) surface and the chemical structure of 21a.
Geometrical structural changes
As described in the introductory part, photochromic molecules change electronic as well as geometrical structures upon photoirradiation. Figure 2c shows the typical geometrical structure change of 1 along with the photoisomerization. The diarylethene molecule shrinks when it converts from the open- to the closed-ring isomers, as evidenced by in situ X-ray crystallographic analysis.5,6) If such structural change takes place in a crystal, the crystal bulk shape is anticipated to follow the shape changes of the component diarylethene molecules. The most convenient way to detect the crystal shape change is to measure the surface morphology by using an atomic force microscopy (AFM).
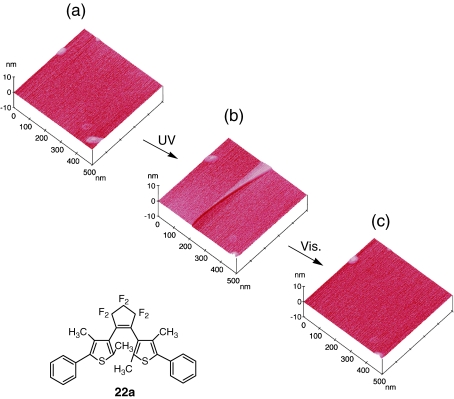
We carried out AFM measurement of the single crystal of 1,2-bis(2,4-dimethyl-5-phenyl-3-thienyl)perfluorocycopentene (22a).35) The surface morphology change was followed before and after UV (365 nm) irradiation and after visible (>500 nm) irradiation. Figure 11 shows the AFM images. Upon irradiation for more than 10 s with UV light, steps appeared on the (100) surface. No step formation was discerned during the irradiation for the initial 10 s but appeared after the induction period. The step height is 1.0 nm. The step disappeared by irradiation with visible light. When the irradiation time was prolonged, the number of steps increased and steps with height of 2.0 and 3.0 nm appeared. The height was always multiples of the minimum step height of 1.0 nm, which corresponds to one molecular layer. The morphological change was reversible and correlated with the color change. This result indicates that the change of molecular shape is linked to macro-scale shape of materials in the densely packed crystal. In densely packed crystalline systems the strain energy generated by the molecular shape change directly influences the shape of the bulk crystal. Based on this view we prepared small-size single crystals (10–100 µm) of photochromic diarylethenes and demonstrated that the bulk shape of crystals changes upon photoirradiation.11)
Figure 11.
AFM images of the (100) surface of single crystal 22a (a) before and (b) after irradiation with 365 nm light and (c) after irradiation with visible (λ > 500 nm) light.
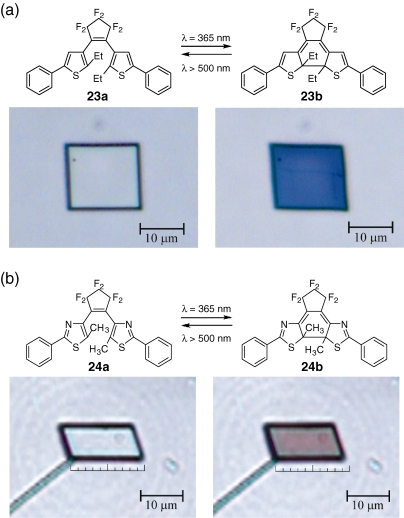
Figure 12 shows the color and shape changes of 1,2-bis(2-ethyl-5-phenyl-3-thienyl)perfluorocyclopentene (23a) and 1,2-bis(5-methyl-2-phenyl-4-thiazolyl)perfluorocyclopentene (24a) crystals upon irradiation with UV (365 nm) and visible (>500 nm) light. The color of the two crystals turned blue and violet, respectively. The colors are stable in the dark, but disappeared on irradiation with visible light. UV irradiation changed the corner angle of the single crystal 23 from 88° and 92° to 82° and 98° and hence the shape from a square to a lozenge. On the other hand, the crystal 24 exhibited reversible contraction/expansion in length as much as 5–7% upon alternate irradiation with UV and visible light.
Figure 12.
Photoinduced color and shape changes of single crystals of (a) 23a and (b) 24a upon irradiation with UV and visible light.
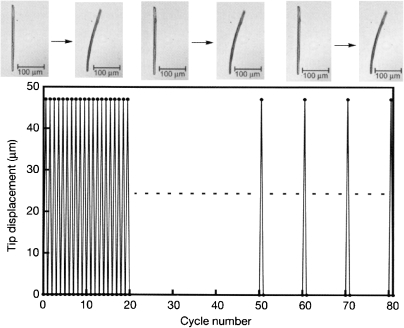
A rod-like crystal was also prepared from 24a by sublimation. The rod-like crystal mounted at one end on a glass surface bent upon irradiation with UV light. The rod bent towards the direction of the incident light. This effect is due to the gradient in the extent of photoisomerization caused by the high absorbance of the crystal, so that the shrinkage of the irradiated part of the crystal causes bending just as in a bimetal. The bent rod-like crystal became straight upon irradiation with visible light. Such reversible bending could be repeated over 80 cycles, as shown in Fig. 13.
Figure 13.
Reversible bending a crystal-rod of 24a upon irradiation with UV and visible light.
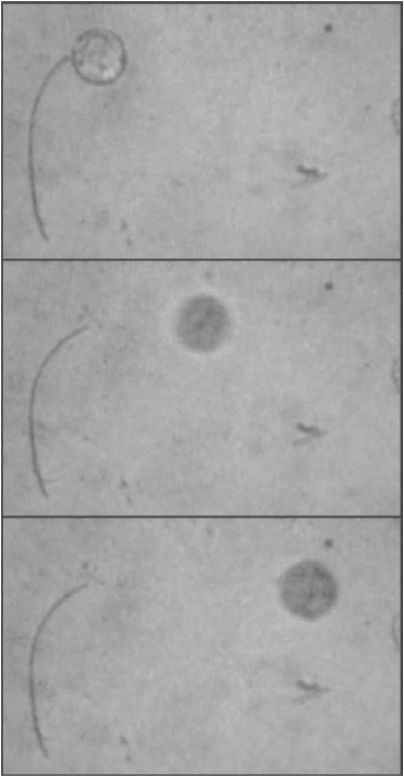
The crystal rod (about 200 micrometers long and 5 micrometers diameter) was used to launch a tiny silica-particle (diameter 80 micrometers), as shown in Fig. 14. The rapid response and relatively large elastic modulus of the crystal rod enabled to hit the silica-particle as if it were a tennis ball.
Figure 14.
A shot of a silica micro-particle by crystal-rod of 24a upon irradiation with 365 nm light pulse.
It is a long-standing ambition for chemists to construct molecular systems which exhibit mechanical movement based on geometrical structural changes of individual molecules and have the systems perform mechanical work.36,37) The present result indicates that densely packed crystals can be used to link the molecular scale events to macro-scale phenomena. The shape change of component molecules in crystal upon photoirradiation is directly linked to the macro-scale shape change of the crystal. The photoinduced change of molecular shape at the molecular level is successfully linked to the macro-scale mechanical motion of the crystal and resulted in a net task to launch a tiny silica-particle.
Acknowledgments
The author is grateful for the support from the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research in Priority Area “New Frontiers in Photochromism (471)” No. 19050008). The author also wishes to express his sincere thanks to colleagues and students for their extraordinary efforts to accomplish the research.
Profile
Masahiro Irie received his BS (1966) and MS (1968) degrees from Kyoto University and his PhD (1973) degree in radiation chemistry from Osaka University. In 1968, he joined the Faculty of Engineering, Hokkaido University, as a research associate and started his research on photochemistry. In 1973, he moved to Osaka University and was promoted to associate professor at the Institute of Scientific and Industrial Research in 1978. In 1988, he was appointed professor at the Institute of Advanced Material Study, Kyushu University; in 1996, he was reappointed professor of chemistry at the Faculty of Engineering. In 2007, he moved to Tokyo and is now professor at Rikkyo University. He has been conducting research on photochromic molecular systems for the last 30 years. In the middle of the eighties he invented a new class of photochromic molecules, the diarylethenes, which undergo thermally irreversible and fatigue resistant photochromic reactions. At present, these derivatives are widely used in functional molecular materials as key elements of optical switches. He has received the following awards: Award of the Society of Polymer Science, Japan (1988), Award of the Japanese Photochemical Association (1993), Vinci d’Excellence (1995), Docteur Honoris Causa of University of Bordeaux I (2003), the Chemical Society of Japan Award (2004), Mukai Award (2007), The Purple Ribbon Medal (2007) and Theodor-Förster-Preis (2008).
References
- 1).Brown, G.H. (1971) Photochromism. Wiley-Interscience, New York. [Google Scholar]
- 2).Crano, J.C. and Guglielmetti, R.J. (1998) Organic Photochromic and Thermochromic Compounds. Plenum Press, New York and London. [Google Scholar]
- 3).Dürr, H. and Bouas-Laurent, H. (2003) Photochromism: Molecules and Systems. Elsevier, Amsterdam. [Google Scholar]
- 4).Kobatake S., Yamada T., Uchida K., Kato N., Irie M. (1999) Photochromism of 1,2-bis(2,5-dimethyl-3-thienyl)perfluorocyclopentene in single crystalline phase. J. Am. Chem. Soc. 121, 2380–2386 [Google Scholar]
- 5).Yamada T., Kobatake S., Muto K., Irie M. (2000) X-ray crystallographic study on single-crystalline photochromism of bis(2,5-dimethyl-3-thienyl)perfluorocyclopentene. J. Am. Chem. Soc. 122, 1589–1592 [Google Scholar]
- 6).Yamada T., Kobatake S., Irie M. (2000) X-Ray Crystallographic Study on Single-Crystalline Photochromism of 1,2-Bis(2,5-dimethyl-3-thienyl)perfluorocyclopentene. Bull. Chem. Soc. Jpn. 73, 2179–2184 [Google Scholar]
- 7).Irie, M. (1994) Photo-reactive Materials for Ultrahigh Density Optical Memory. Elsevier, Amsterdam. [Google Scholar]
- 8).Irie M. (2000) Photochromism: memories and switches-introduction. Chem. Rev. 100, 1683–1890 [DOI] [PubMed] [Google Scholar]
- 9).Irie, M. and Matsuda, K. (2001) Memories. In Electron Transfer in Chemistry (ed. Balzani, V.). Springer, Berlin. pp. 215–242. [Google Scholar]
- 10).Irie, M. (2001) Photoswitchable molecular systems based on diarylethenes. In Molecular Switches (ed. Feringa, B. L.). Wiley, Weinheim, pp. 37–62. [Google Scholar]
- 11).Kobatake S., Takami S., Muto H., Ishikawa T., Irie M. (2007) Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 466, 778–781 [DOI] [PubMed] [Google Scholar]
- 12).Al-Kaysi R.O., Müller A.M., Bardeen C.J. (2006) Photochemically driven shape changes of crystalline organic nanorods. J. Am. Chem. Soc. 128, 15938–15939 [DOI] [PubMed] [Google Scholar]
- 13).Al-Kaysi R.O., Bardeen C.J. (2007) Reversible photoinduced shape changes of crystalline organic nanorods. Adv. Mater. 19, 1276–1280 [Google Scholar]
- 14).Uchida K., Sukata S., Matsuzawa Y., Akazawa M., de Jong J.J.D., Katsonis N., et al. (2008) Photoresponsive rolling and bending of thin crystals of chiral diarylethenes. Chem. Commun. (Camb.), 326–328 [DOI] [PubMed] [Google Scholar]
- 15).Koshima H., Ojima N., Uchimoto H. (2009) Mechanical motion of azobenzene crystals upon photoirradiation. J. Am. Chem. Soc. 131, 6890–6891 [DOI] [PubMed] [Google Scholar]
- 16).Irie M., Uchida K. (1998) Synthesis and properties of photochromic diarylethenes with heterocyclic aryl groups. Bull. Chem. Soc. Jpn. 71, 985–996 [Google Scholar]
- 17).Irie M. (2000) Diarylethenes for memories and switches. Chem. Rev. 100, 1685–1716 [DOI] [PubMed] [Google Scholar]
- 18).Kobatake S., Irie M. (2004) Single-crystalline photochromism of diarylethenes. Bull. Chem. Soc. Jpn. 77, 195–210 [DOI] [PubMed] [Google Scholar]
- 19).Morimoto M., Irie M. (2005) Photochromism of diarylethene single crystals: crystal structures and photochromic performance. Chem. Commun. (Camb.), 3895–3905 [DOI] [PubMed] [Google Scholar]
- 20).Darcy P.J., Heller H.G., Strydom P.J., Whittall J. (1981) Photochromic heterocyclic fulgides. Part 2. Electrocyclic reactions of (E)-α-2,5-dimethyl-3-furylethylidene (alkyl-substituted methylene) succinic anhydrides. J. Chem. Soc., Perkin Trans. 1, 202–205 [Google Scholar]
- 21).Heller H.G., Langan J.R. (1981) Photochromic heterocyclic fulgides. Part 3. The use of (E)-α-(2,5-dimethyl-3-furylethylidene)(isopropylidene) succinic anhydride as a simple convenient chemical actinometer. J. Chem. Soc., Perkin Trans. 2, 341–343 [Google Scholar]
- 22).Irie M., Mohri M. (1988) Thermally irreversible photochromic systems. Reversible photocyclization of diarylethene derivatives. J. Org. Chem. 53, 803–805 [Google Scholar]
- 23).Hanazawa M., Sumiya R., Horikawa Y., Irie M. (1992) Thermally irreversible photochromic systems. Reversible photocyclization of 1,2-bis(2-methylbenzo[b]thiophen-3-yl)perfluorocycloalkene derivatives. Chem. Commun. (Camb.), 206–207 [Google Scholar]
- 24).Nakamura S., Irie M. (1988) Thermally irreversible photochromic systems. A theoretical study. J. Org. Chem. 53, 6136–6138 [Google Scholar]
- 25).Guillaumont D., Kobayashi T., Kanda K., Miyasaka H., Uchida K., Kobatake S., et al. (2002) An ab initio MO study of the photochromic reaction of dithienylethenes. J. Phys. Chem. A 106, 7222–7227 [Google Scholar]
- 26).Asano Y., Murakami A., Kobayashi T., Goldberg A., Guillaumont D., Yabushita S., et al. (2004) Theoretical study on the photochromic cycloreversion reactions of dithienylethenes; on the Role of the Conical Intersections. J. Am. Chem. Soc. 126, 12112–12120 [DOI] [PubMed] [Google Scholar]
- 27).Nakamura S., Kobayashi T., Takata A., Uchida K., Asano A., Murakami A., et al. (2007) Quantum yields and potential energy surfaces: a theoretical study. J. Phys. Org. Chem. 20, 821–829 [Google Scholar]
- 28).Shibata K., Kobatake S., Irie M. (2001) Extraordinarily low cycloreversion quantum yields of photochromic diarylethenes with methoxy substituents. Chem. Lett., 618–619 [Google Scholar]
- 29).Higashiguchi K., Matsuda K., Tanifuji N., Irie M. (2005) Full-color photochromism of a fused dithienylethene trimer. J. Am. Chem. Soc. 127, 8922–8923 [DOI] [PubMed] [Google Scholar]
- 30).Fukaminato T., Kobatake S., Kawai T., Irie M. (2001) Three-dimensional erasable optical memory using a photochromic diarylethene single crystal as the recording medium. Proc. Jpn. Acad., Ser. B 77, 30–35 [Google Scholar]
- 31).Morimoto M., Kobatake S., Irie M. (2003) Multicolor photochromism of two- and three-component diarylethene crystals. J. Am. Chem. Soc. 125, 11080–11087 [DOI] [PubMed] [Google Scholar]
- 32).Kuroki L., Takami S., Shibata K., Irie M. (2005) Photochromism of single crystals composed of dioxazolylethene and dithiazolylethene. Chem. Commun. (Camb.), 6005–6007 [DOI] [PubMed] [Google Scholar]
- 33).Takami S., Kuroki L., Irie M. (2007) Photochromism of mixed crystals containing bisthienyl-, bisthiazolyl-, and bisoxazolylethene derivatives. J. Am. Chem. Soc. 129, 7319–7326 [DOI] [PubMed] [Google Scholar]
- 34).Kobatake S., Yamada M., Yamada T., Irie M. (1999) Photochromism of 1,2-bis(2-methyl-6-nitro-1-benzothiophen-3-yl)-perfluorocyclopentene in a single-crystalline phase: dichroism of the closed-ring form isomer. J. Am. Chem. Soc. 121, 8450–8456 [Google Scholar]
- 35).Irie M., Kobatake S., Horichi M. (2001) Reversible surface morphology changes of a photochromic diarylethene single crystal by photoirradiation. Science 291, 1769–1772 [DOI] [PubMed] [Google Scholar]
- 36).Merian E. (1966) Steric factors influencing the dyeing of hydrophobic fibers. Text. Res. J. 36, 612–618 [Google Scholar]
- 37).Irie M. (2008) Photochromism and molecular mechanical devices. Bull. Chem. Soc. Jpn. 81, 917–926 [Google Scholar]