Abstract
Objectives. To examine the all-cause mortality rate and factors associated with mortality in US veteran men with RA.
Methods. Men with RA were enrolled and followed until death or censoring. Vital status was ascertained through systematic record review and standardized mortality ratios (SMRs) were calculated using US life tables for men. Multivariate Cox proportional hazards regression was used to examine the independent associations of patient factors including socio-demographics, comorbidity, measures of RA disease activity/severity and medication use with mortality. Measures of RA disease activity and medications were examined as time-varying factors.
Results. A total of 138 deaths were observed during 2314 patient-years of follow-up (n = 1015 patients), corresponding to a crude morality rate of 5.9 deaths per 100 patient-years (95% CI 5.0, 7.0) and an SMR of 2.1 (95% CI 1.8, 2.5). After multivariate adjustment, factors independently associated with higher mortality risk in men with RA included older age, Caucasian race, low body weight, an increased frequency of rheumatology visits, higher ESR and RF concentrations, increased DAS28, subcutaneous nodules and prednisone use. In contrast, MTX use [hazard ratio (HR) 0.63; 95% CI 0.42, 0.96] was associated with ∼40% lower mortality risk.
Conclusion. Mortality rates among US male veterans with RA are more than twice those of age-matched men in the general population. These results suggest that optimizing disease control, particularly with regimens that include MTX and minimize glucocorticoid exposure, could improve long-term survival in this population.
Keywords: Rheumatoid arthritis, Mortality, US veterans, Men, MTX, Glucocorticoids
Introduction
RA is a chronic inflammatory disease affecting 0.5–1% of the population, a condition that is associated with significant morbidity, work-related disability and mortality. Despite the use of increasingly aggressive therapeutic approaches and the availability of novel and highly effective treatment options, the mortality rate for patients with RA has remained relatively constant over the past several decades [1, 2]. Pooled standardized mortality ratios (SMRs) for RA range from ∼1.6 to 1.8 [1, 2], underscoring the higher mortality for RA patients compared with the general population. Reflecting the demographics of RA, most studies examining disease-related mortality and determinants of mortality have included primarily women. The lack of mortality studies in men with RA represents an important knowledge deficit because available data, albeit limited, suggest that men with RA have disproportionately higher mortality rates than women with RA [3].
Veterans Affairs (VA) hospitals and clinics, which provide comprehensive health care for eligible military veterans, represent the single largest integrated health system in the USA. Although serving a growing number of veteran women, >90% of its beneficiaries are men. The VA Health System includes >150 hospitals and in excess of 800 ambulatory care clinics across the USA, currently providing health care for >5 million US military veterans [4]. Although its prevalence in the VA has not been precisely defined, it has been estimated that the VA currently provides care for >100 000 RA patients based on available claims data and corresponding diagnostic codes (personal communication: J. Curtis, 1 March 2010, Birmingham VAMC). Although the VA represents one of the single largest health providers in the USA for patients with RA, investigations of health outcomes in this population are limited and to date, there have been no studies of RA-related mortality in US veterans. This is particularly relevant since veterans with RA are older and have substantial comorbid illness [5], factors that are associated with greater disease-specific mortality [6].
In addition to male gender, determinants of higher mortality in patients with RA have included several measures of disease activity and severity in addition to poor functional status and lower educational levels [7–15]. Recognizing that most prior studies have been conducted in populations consisting mostly of women, determinants of mortality risk in men with RA, specifically those receiving health care in the VA, remain unknown. With an ageing and predominantly male patient population with high rates of prevalent comorbidity, the VA represents a unique study population for the study of chronic disease outcomes. In this study, we sought to examine the mortality rate as well as factors associated with mortality risk in men participating in the longitudinal Veterans Affairs Rheumatoid Arthritis (VARA) Registry.
Materials and methods
Study subjects
The VARA Registry is a longitudinal observational study of US veterans with RA [16–18]. In addition to serving as a biological repository with banked DNA, serum and plasma collected at the time of study enrolment, VARA includes clinical data that are collected both at baseline and at subsequent rheumatology clinic visits, which are dictated by usual care. Initiated in October of 2002, VARA is a multicentre effort that to date involves collection sites at nine VA Medical Centres across the USA (Brooklyn, NY; Dallas, TX; Denver, CO; Iowa City, IA; Jackson, MS; Omaha, NE; Portland, OR; Salt Lake City, UT; and Washington, DC, USA). All VARA participants satisfy the ACR classification criteria for RA [19] and all have a disease onset after 18 years of age. Eligible patients are systematically enrolled from participating rheumatology clinics with RA patient characteristics that are reflective of the national VA population [5]. The VARA Registry has received Institutional Review Board (IRB) approval at each site, and all study subjects provide written informed consent before enrolment. All ancillary studies require approval from the VARA Scientific Ethics and Advisory Committee. In addition to having IRB approval, this study was also approved by the VARA Scientific Ethics and Advisory Committee.
For these analyses, patients from three recently added VARA enrolment sites with limited patient follow-up and no recorded deaths were excluded from the analysis. Given the younger age of women compared with men (55 vs 67 years at enrolment) and the relatively small proportion of women enrolled in VARA (<10% of total participants), all analyses were restricted to men.
Clinical measurements
At each clinic visit, VARA participants undergo a standardized examination that includes tender and swollen joint counts (0–28 joints), a provider global assessment [measured using a 100-mm visual analogue scale (VAS)] and an ESR measurement. Patient-reported outcomes collected at each visit include a self-reported pain score (0–10), a patient global well-being score (100-mm VAS) and a 10-item multidimensional HAQ (MD-HAQ; range 0–3) [20]. Medication use, the presence of comorbid health conditions and individual ACR criteria (including RF positivity, the presence of rheumatoid nodules and radiographic damage) are recorded at enrolment and updated at all subsequent clinic visits. Information regarding medication dose (daily or cumulative) is not routinely collected as part of VARA. Additional variables collected at enrolment include socio-demographics (age, gender, self-reported race and education), date of RA diagnosis, smoking status (current, former or never) and BMI. Anti-CCP antibody (immunoglobulin G) is measured on banked serum collected at enrolment using a second generation ELISA (Diastat; Axis-Shield Diagnostics Ltd, Dundee, UK; positivity ≥5 U/ml) [17]. Both RF (positive, ≥15 IU/ml) and high-sensitivity CRP (hsCRP; mg/l) are determined by nephelometry (Siemens Healthcare Diagnostics, Munich, Germany) [17].
Vital status
For the present study, patients were followed longitudinally from time of enrolment to June 2009, and all deaths were identified through systematic review of the VA Computerized Patient Record System (CPRS). Mortality events were captured by yearly CPRS abstraction, next of kin notification or by periodic regional VA surveillance. All VA beneficiaries are tracked for vital status and death benefit eligibility using social security numbers on a quarterly basis by the VA. In the absence of death, patients were censored at the time of their last documented follow-up in the VA health system. Cause of death was not systematically available for decedents and therefore not subject to further study. A crude mortality rate was calculated by dividing the total number of deaths by the cumulative person-years of observation—where the at-risk period included elapsed time from VARA enrolment to the time of death or censoring.
Statistical analysis
Age-adjusted SMRs and corresponding 95% CIs were calculated using US life tables for men from 2002 through 2006 (available at http://mortality.org). All observations occurring after this time were compared with the 2006 national mortality rates as more recent US life tables were not available at the time of this study. Life tables for the US veteran population or VA beneficiaries were not available for this study. For descriptive purposes in post hoc analyses, we also calculated SMRs and corresponding CIs for patient subgroups defined by BMI category (<20, 20 to <25, 25–30 and >30 kg/m2), ESR quartile (≤10, 11–21, 22–38 and ≥39 mm/h), MTX and prednisone use—with these factors measured at the clinical encounter most proximate to death or censoring.
Age-adjusted associations of the aforementioned patient factors (socio-demographics, comorbidity, measures of disease activity and other disease characteristics) with mortality were examined using Cox proportional hazards regression. Additional factors examined included age at RA onset, disease duration, rheumatology clinic visit frequency and 28-joint DAS (DAS-28) [21]. Race was categorized as Caucasian vs non-Caucasian while educational status was examined as high school graduate (≥12 years of education) vs less than high school graduate. Comorbidity was examined using a count of prevalent diabetes mellitus, coronary artery disease, cerebrovascular disease, hypertension, hyperlipidaemia, depression, chronic obstructive and interstitial lung disease and chronic kidney disease (range of scores 0–9). To account for co-linearity in patient-reported outcomes, we examined associations of the Routine Assessment of Patient Index Data (RAPID)-3, a composite disease activity measure that incorporates the MD-HAQ, pain and patient global well-being [22].
In our primary analyses, we examined the associations of patient factors at the time of enrolment and as time-varying covariates for measures of disease activity (ESR, DAS-28, etc.), disease severity (nodules, radiographic damage) and treatments (e.g. prednisone, MTX, etc.). An s.e. adjustment was applied to all models to account for observation clustering by enrolment site (n = 6 sites). A multivariate Cox regression model was then constructed with age, race and comorbidity count forced into the model based on their anticipated significance. Results were reported as a HR with 95% CI. All variables with P < 0.1 during the initial univariate analysis were entered into a multivariate model with stepwise removal until all remaining variables had a P < 0.05.
To examine the durability of treatment effects in sensitivity analyses, we replaced time-varying use of MTX and prednisone with baseline use of these agents in our multivariate model. To further assess whether our results could be impacted by loss of follow-up and subsequent misclassification of vital status, all individuals lost to follow-up for ≥2 years were reclassified as being deceased with a date of death corresponding to 2 years after their last clinical observation. All analyses were performed in Stata v10.1 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
A total of 1015 men with RA were enrolled in the VARA Registry from 2002 through June 2009. Baseline patient characteristics, including those for decedents and survivors, are summarized in Table 1. The mean (s.d.) age at enrolment in this cohort was 65 (11) years, with a mean disease duration of 12 (12) years. Approximately 80% of patients were Caucasian with 81 and 76% of patients positive for RF and anti-CCP antibody, respectively. Veterans in our cohort exhibited high levels of comorbid illness [including diabetes mellitus (22%), cardiovascular disease (24%), chronic obstructive pulmonary disease (22%) and depression (13%)], and 82% of patients reported either current or former cigarette smoking.
Table 1.
Patient enrolment characteristics among male US veterans with RA (n = 1015)
| Characteristics | Died during follow-up (n = 138) | Alive at end of study (n = 877) | Total |
|---|---|---|---|
| Socio-demographics, smoking history, visit frequency and disease duration | |||
| Age at enrolment, years | 70 (9) | 64 (11) | 65 (11) |
| Caucasian race, % | 90 | 78 | 79 |
| ≥High school education, % | 79 | 83 | 82 |
| Smoking history at enrolment, % | |||
| Never | 16 | 19 | 18 |
| Former | 57 | 53 | 54 |
| Current | 28 | 28 | 28 |
| Follow-up duration, years | 2.0 (1.3) | 2.3 (1.5) | 2.3 (1.5) |
| Mean rheumatology visits per year | 5.4 (3.7) | 5.1 (2.9) | 5.1 (3.0) |
| Disease duration at enrolment, years | 16 (14) | 11 (11) | 12 (12) |
| BMI and comorbidity | |||
| BMI, kg/m2 | 26.1 (5.6) | 28.4 (5.5) | 28.1 (5.5) |
| Comorbidity count (0–9) | 2.6 (1.6) | 2.0 (1.4) | 2.1 (1.4) |
| Hypertension, % | 64 | 61 | 61 |
| Hyperlipidaemia, % | 41 | 47 | 46 |
| Coronary artery disease, % | 41 | 21 | 24 |
| Diabetes mellitus, % | 31 | 21 | 22 |
| Chronic pulmonary disease, % | 32 | 20 | 22 |
| Depression, % | 16 | 12 | 13 |
| Cerebrovascular disease, % | 11 | 6 | 7 |
| Interstitial lung disease, % | 12 | 7 | 7 |
| Chronic kidney disease, % | 14 | 5 | 6 |
| Measures of RA disease activity and severity | |||
| MD-HAQ (0–3) | 1.2 (0.6) | 0.9 (0.6) | 1.0 (0.6) |
| ESR, mm/h | 36.3 (28.4) | 24.8 (21.6) | 26.4 (23.0) |
| hsCRP, mg/l | 22.1 (30.4) | 12.2 (18.7) | 13.6 (21.0) |
| DAS-28 | 4.4 (1.7) | 3.9 (1.6) | 4.0 (1.6) |
| Swollen joint count (0–28) | 5.2 (6.6) | 4.4 (5.6) | 4.5 (5.8) |
| Tender joint count (0–28) | 6.0 (7.4) | 5.4 (6.9) | 5.4 (7.0) |
| Pain (0–10) | 4.6 (2.9) | 4.6 (2.9) | 4.6 (2.9) |
| Patient global well-being (0–100 mm) | 47.5 (27.9) | 41.9 (26.0) | 42.6 (26.3) |
| Provider global well-being (0–100 mm) | 31.5 (23.0) | 38.1 (23.4) | 37.8 (23.4) |
| RAPID-3 score | 3.0 (1.5) | 2.6 (1.4) | 2.6 (1.5) |
| Subcutaneous nodules, % | 54 | 33 | 36 |
| RF factor positivity, % | 88 | 79 | 81 |
| RF concentration, IU/ml | 417.3 (724.1) | 346.3 (743.2) | 356.6 (740.5) |
| Anti-CCP antibody positivity, % | 81 | 75 | 76 |
| Anti-CCP antibody concentration, U/ml | 358.5 (496.3) | 273.7 (473.0) | 285.6 (477.0) |
| Radiographic changes satisfying ACR classification criteria,a % | 65 | 53 | 55 |
| Medication use | |||
| Prednisone, % | 59 | 42 | 45 |
| MTX, % | 50 | 57 | 56 |
| SSZ, % | 10 | 16 | 15 |
| HCQ, % | 32 | 32 | 32 |
| LEF, % | 17 | 13 | 14 |
| Anti-TNF agent, % | 27 | 29 | 29 |
| Any biologic agent, % | 29 | 29 | 29 |
Values are expressed as mean (s.d.) unless otherwise noted. ahsCRP median (range): 5.8 (0.2–163 mg/l); RF median (range): 115 (0–5427 IU/ml); anti-CCP antibody median (range): 132 (0–5430 U/ml).
Overall mortality and SMR
A total of 138 patients died during 2314 patient-years of follow-up [mean follow-up period of 2.3 (1.5) years]. This corresponds to a crude mortality rate of 5.9 deaths (95% CI 5.0, 7.0) per 100 person-years of follow-up. When compared with men in the general population, we noted a substantially higher age-adjusted SMR of 2.1 (95% CI 1.8, 2.5) among male veterans with RA.
Age-adjusted associations with mortality
Age-adjusted associations of patient factors with mortality risk in men with RA are shown in Table 2. There were significant associations of increased mortality risk in RA patients with Caucasian race (vs non-Caucasian race), current smoking history (vs never smoking), rheumatology clinic visit frequency, disease duration, increased disease activity (as defined by DAS-28, ESR, hsCRP and RAPID-3), autoantibody seropositivity, higher MD-HAQ scores reflecting lower levels of physical functioning and ongoing prednisone use. Higher BMI values and MTX use (the latter with and without combination anti-TNF therapy) were associated with a significantly lower mortality risk. Associations of mortality with other DMARDs commonly used in the treatment of RA (SSZ, HCQ, LEF, anti-TNF or any biologic therapy) did not reach statistical significance in age-adjusted analyses.
Table 2.
Age-adjusted associations of patient characteristics with all-cause mortality among US veterans with RAa
| Characteristics | HR (95% CI) |
|---|---|
| Socio-demographics, smoking history, visit frequency and disease duration | |
| Caucasian race (vs non-Caucasian) | 1.77 (1.10, 2.84) |
| ≥High school graduate | 1.08 (0.78, 1.50) |
| Smoking history at enrolment | |
| Never | – |
| Former | 1.20 (0.87, 1.67) |
| Current | 2.26 (1.27, 4.00) |
| Mean rheumatology visits per year | 1.22 (1.18, 1.26) |
| Disease duration at enrolment, years | 1.02 (1.00, 1.02) |
| BMI and comorbidity | |
| BMI, kg/m2 | 0.95 (0.92, 0.98) |
| Comorbidity count (0–9) | 1.23 (0.98, 1.54) |
| Measures of RA disease activity and severityb | |
| MD-HAQ | 2.25 (1.95, 2.60) |
| ESR per 10 mm/h | 1.26 (1.22, 1.30) |
| hsCRP per 10 mg/l | 1.12 (1.10, 1.13) |
| DAS-28 | 1.34 (1.16, 1.54) |
| Swollen joint count | 1.02 (0.98, 1.05) |
| Tender joint count | 1.03 (1.01, 1.05) |
| Pain | 1.11 (1.07, 1.14) |
| Patient global well-being per 10 mm | 1.24 (1.13, 1.36) |
| Provider global well-being per 10 mm | 1.31 (1.18, 1.46) |
| RAPID-3 score | 1.53 (1.37, 1.70) |
| Subcutaneous nodules | 1.89 (1.50, 2.39) |
| RF positivity | 1.86 (1.07, 3.23) |
| RF concentration per 100 IU/ml | 1.02 (1.00, 1.03) |
| Anti-CCP antibody positivity | 1.45 (1.04, 2.02) |
| Anti-CCP antibody concentration per 100 U/ml | 1.03 (1.00, 1.06) |
| Radiographic changesa | 1.17 (0.98, 1.40) |
| Medication useb | |
| Prednisone | 1.88 (1.44, 2.46) |
| MTX | 0.51 (0.35, 0.73) |
| SSZ | 0.59 (0.34, 1.03) |
| HCQ | 1.06 (0.83, 1.34) |
| LEF | 0.72 (0.39, 1.33) |
| Anti-TNF agent | 0.78 (0.46, 1.32) |
| Anti-TNF/MTX combination | 0.64 (0.44, 0.91) |
aHRs and 95% CIs are calculated using Cox proportional hazards regression; radiographic changes based on documentation of corresponding ACR classification criterion for RA. bMeasures of disease activity/severity and medications examined as time-varying covariates with exception of hsCRP, RF (status and concentration) and anti-CCP antibody (status and concentration), which were measured using banked serum collected at enrolment.
Multivariate associations with mortality
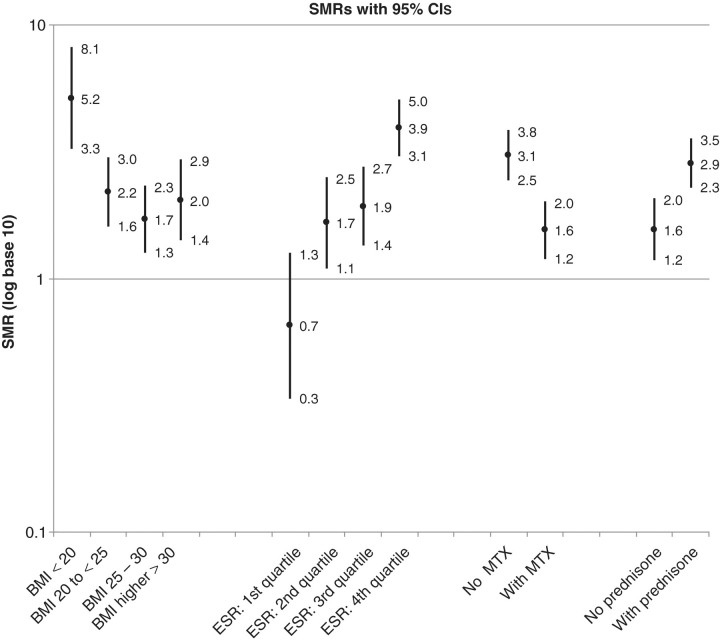
Multivariate associations of patient factors (modelling medications and measures of disease activity and severity as time varying) with mortality risk are shown in Table 3. After multivariate adjustment, patient characteristics significantly associated with increased mortality included older age, visit frequency, Caucasian race (vs non-Caucasian; HR 2.10; 95% CI 1.33, 3.31), prednisone use (HR 1.40; 95% CI 1.15, 1.71), higher RAPID-3 score (HR 1.35; 95% CI 1.17, 1.56), higher ESR and RF concentrations, and the presence of subcutaneous nodules (HR 1.35; 95% CI 1.09, 1.68). The RAPID-3 was more strongly associated with mortality than the DAS-28 with ESR representing the only component value of the DAS-28 independently associated with survival (data not shown). MTX use (adjusted HR 0.63; 95% CI 0.42, 0.96) was associated with a lower mortality risk (Table 3). Likewise, BMI showed a significant inverse association with mortality risk (HR 0.95; 95% CI 0.93, 0.97), an association that appeared to be primarily related to the higher mortality rates observed in underweight individuals (BMI <20 kg/m2). SMRs and corresponding CIs based on patient subgroups defined by BMI category, ESR quartiles, MTX and prednisone use are shown in Fig. 1. SMRs were highest among male RA patients who were underweight with a BMI of <20 kg/m2 (SMR 5.15; 95% CI 3.29, 8.08) and for those with ESR values falling in the highest quartile (SMR 3.93; 95% CI 3.10, 5.04).
Table 3.
Multivariate associations of patient factors with all-cause mortality among US veterans with RAa
| Characteristic | HR (95% CI) |
|---|---|
| Socio-demographics and visit frequency | |
| Age, years | 1.06 (1.04, 1.08) |
| Caucasian race (vs non-Caucasian) | 2.10 (1.33, 3.31) |
| Clinic visit frequency | 1.21 (1.14, 1.28) |
| BMI and comorbidity | |
| BMI | 0.95 (0.93, 0.97) |
| Comorbidity count | 1.18 (0.99, 1.41) |
| Measures of RA disease activity and severityb | |
| ESR per 10 mm/hb | 1.13 (1.09, 1.18) |
| Subcutaneous nodulesb | 1.35 (1.09, 1.68) |
| RF concentration per 100 IU/mlc | 1.01 (1.00, 1.02) |
| RAPID-3 scoreb | 1.35 (1.17, 1.56) |
| Medication useb | |
| Prednisone useb | 1.40 (1.15, 1.71) |
| MTX useb | 0.63 (0.42, 0.96) |
aHRs and 95% CIs calculated using Cox proportional hazards regression; age, race, BMI and comorbidity forced into model; model developed using backwards regression, entering all variables with P < 0.1 into model; all remaining P-variables with P < 0.05. bModelled as time-varying covariates. cMeasured by nephelometry on banked serum collected at the time of study enrolment.
Fig. 1.
SMRs and 95% CIs among RA subgroups based on BMI, ESR, MTX use and prednisone use categories; factors assessed at clinical visit most proximate to time of censoring or death. ESR quartiles: ≤10, 11–21, 22–38 and ≥39 mm/h. BMI categories: <20, 20–< 25, 25–30 and >30 kg/m2.
Sensitivity analyses
Substituting baseline treatments for time-varying treatments in our multivariate model, the associations of both MTX (HR 0.79; 95% CI 0.58, 1.07) and prednisone use (HR 1.10; 95% CI 0.70, 1.71) were attenuated and not statistically significant. In additional analyses, survivors lost to follow-up for >2 years preceding the censoring date were reclassified as being deceased. This resulted in a change in vital status of 6% of censored subjects. This reclassification attenuated the associations of Caucasian race (HR 1.54; 95% CI 0.82, 2.88), prednisone use (HR 1.14; 95% CI 0.92, 1.43) and nodules (HR 1.04; 95% CI 0.84, 1.28) with mortality but did not impact results referent to other factors in the multivariate model.
Discussion
We have shown that men with RA receiving health care in the VA Health System experience more than twice the mortality of men in the general population, confirming previous observations of a marked increase in the death rate among subjects with RA. Recognizing limitations of comparing results across studies, excess mortality among male veterans with RA (with an observed SMR of 2.1) exceeds previous pooled estimates from other RA populations [1, 2]. In a recent systematic review, Sokka et al. [1] reported a median SMR of 1.63 after pooling data from 31 non-inception cohort studies of RA conducted between 1953 and 2008. To our knowledge, ours is the first study to focus entirely on a large, well-characterized population of men. This unique aspect of our study is significant as men appear to experience greater disease-related reductions in survival than women [3, 9]. Moreover, at least one previous population-based study found that the mortality gap in RA, defined as the difference in age-adjusted mortality in RA vs the general population, continues to increase in men while this gap has stabilized over the previous decades in women [23].
In addition to defining the impact of prevalent RA on survival, we examined a number of possible determinants of mortality and found that select measures including socio-demographics, BMI, measures of disease activity/severity and medication use are independently associated with mortality in this unique patient population. Factors associated with mortality included not only non-modifiable factors such as older age and race, but also included a number of potentially modifiable factors that could ultimately serve as targets in efforts to reduce the widening mortality gap in men with RA. Potentially modifiable factors associated with RA mortality risk in this study included measures of disease activity (ESR and RAPID-3), RA treatments, BMI and the presence of subcutaneous nodules. Along with prior reports, our results suggest that optimizing disease control, particularly with regimens that incorporate MTX [24–26] but minimize glucocorticoid exposure [27–29], could have an important benefit on long-term disease-related survival. Our findings are consistent with other RA studies showing associations of increased disease activity with mortality risk, studies that have examined the predictive value of numerous measures including the presence of subcutaneous nodules and other extra-articular disease features, autoantibody status, measures of acute-phase response, composite measures of disease activity and several other patient-reported outcome measures [7–15]. Perhaps counter-intuitively, we found that BMI was inversely associated with mortality risk, an association that was driven primarily by the higher mortality rates in underweight men with RA (those with BMI <20 kg/m2). This could reflect the presence of rheumatoid cachexia, weight loss and anorexia reflecting long-standing and suboptimally controlled inflammation in RA [30, 31]. It is also possible that other factors mediating weight loss in RA, such as the increased expression of select pro-inflammatory cytokines, could more directly explain this association. Kremers et al. [32] have previously shown that RA patients with low BMI are more than three times as likely as non-RA subjects with normal body weight to experience a cardiovascular-related death.
The impact of therapy on RA mortality has been evaluated, with substantial attention given to the survival benefit associated with MTX use. Our findings indicate an ∼40% significant reduction in mortality risk associated with MTX use, a benefit that is independent of other confounders including measures of disease activity. This corroborates previous work in which MTX was shown to decrease overall mortality by as much as 60–80% [24, 25], a benefit that appears to operate primarily through a significant reduction in cardiovascular-related deaths [26]. In contrast to the protective association with MTX, glucocorticoid use was associated with a ∼40% increased mortality risk in this population, a detrimental effect reported in other RA cohort studies [27–29]. Whether glucocorticoid use simply serves as a surrogate for more severe and inadequately controlled inflammation or has other direct detrimental physiological effects (i.e. increased dyslipidaemia, hypertension, etc.) that impact mortality risk remains to be defined. These results should be interpreted with caution. Although we adjusted for factors likely influencing treatment patterns (age, comorbidity and disease activity), it is possible that the observed associations reflect changes in treatment prescribing patterns towards the end of life, a time in which health care providers may opt away from DMARDs and more readily use prednisone as a salvage therapy. The differences in risk observed between baseline and time-varying treatments show that the associations are most robust with proximate use.
There are methodological issues that must be considered in interpreting results from investigations examining RA-related mortality [1, 2]. In a pooled analysis of 18 cohort investigations, Ward reported a mean SMR of 1.70 across studies [2]. In this analysis, there were important study-related differences in SMRs based on the populations studied and the year of publication with lower mortality burdens in more recently completed studies, findings that were recently corroborated in a review from Sokka et al. [1]. After adjusting for study year, Ward [2] observed the highest mean SMRs in studies of non-inception cohorts (mean SMR 1.69) and studies that included clinic-based samples (mean SMR of 1.60). It is important to recognize that the RA-related SMR observed in our study, using a non-inception VA cohort, exceeded the median SMR for other clinic-based studies reported by Sokka et al. [1] (median SMR of 1.65) and even exceeded the median SMR for earlier studies reported from the period of 1953–1994 (median SMR of 1.86). It is possible that the higher SMR observed in our study reflects systematic differences in the veteran population examined, characteristics that include relatively high rates of cigarette smoking, obesity and comorbid illnesses including hypertension, cardiovascular disease, diabetes and chronic lung disease. Other factors unique to this population could also impact our results including differences in medication and treatment adherence and relatively high rates of post-traumatic stress disorder reported in the VA [33, 34], attributes that could adversely affect mortality risk. Although it would be informative to generate SMRs using veterans without RA as the referent population, the life tables required for these calculations are not currently available.
There are limitations to this study. These findings are not generalizable to other populations including women (veterans and non-veterans alike) and non-veteran men with RA. The ascertainment of vital status in this study involved systematic review of the electronic medical record, which in turn relies on next-of-kin notifications and periodic regional surveillance in the VA. This method may lack sensitivity [35, 36], suggesting that our results are likely to underestimate the true mortality burden in this patient group. This method of classifying vital status also did not provide the opportunity to examine specific cause of death, efforts that would greatly inform future mortality analyses in this population. Based on results from other RA populations [37–39] and studies of older US veterans [40, 41], we anticipate that cardiovascular disease likely accounted for a significant proportion of the excess mortality observed. In addition to resulting in a possible underestimation of overall RA-related mortality, reduced sensitivity in our method of measuring vital status could have contributed to the racial differences observed, findings that were not expected, with Caucasians experiencing twice the mortality risk of non-Caucasians. Indeed, the possibility that misclassification played a role in this finding is supported by results of our sensitivity analysis where the effect of race was substantially attenuated and rendered non-significant when individuals lost to follow-up were reclassified as being deceased. Recognizing these limitations, there are substantial strengths to this study. To our knowledge, this is the largest investigation to date evaluating the mortality burden and factors associated with mortality in men with RA. In contrast to other studies reliant solely on the use of billing or claims data for case identification, often lacking in disease specificity, the VARA includes patients with RA cases identified using gold-standard diagnostic criteria. Moreover, VARA combines longitudinal patient- and provider-reported outcomes with elements of administrative claims data and standardized serological assays, allowing for one of the most comprehensive assessments of mortality risk factors in RA to date.
Although men constitute up to one-fourth to one-third of all RA cases, there has been a paucity of data with regard to survival outcomes and predictors of all-cause mortality in men with RA. We have shown that men with RA receiving health care in the VA experience more than twice the mortality of men in the general population and, in the process, have identified potential modifiers of mortality risk that include RA disease activity and severity in addition to select RA-related treatments.

Acknowledgements
Funding: The VARA Registry has received research support from the Health Services Research & Development (HSR&D) Program of the Veterans Health Administration (VHA) in addition to unrestricted research funds from Abbott Laboratories and Bristol-Myers Squibb. T.R.M. receives research support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23 AR050004, R03 AR054539) and the VHA (VA Merit). L.C. is supported by a VA HSR&D Career Development Award.
Disclosure statement: L.C. is supported by VA HSR&D Career Development Award (CDP 09-388). All other authors have declared no conflicts of interest.
References
- 1.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(5 Suppl. 51):S35–61. [PubMed] [Google Scholar]
- 2.Ward MM. Recent improvements in survival in patients with rheumatoid arthritis: better outcomes or different study designs? Arthritis Rheum. 2001;44:1467–9. doi: 10.1002/1529-0131(200106)44:6<1467::AID-ART243>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Radovits BJ, Fransen J, Al Shamma S, Eijsbouts AM, van Riel PLCM, Laan RFJM. Excess mortality emerges after 10 years in an inception cohort of early rheumatoid arthritis. Arthritis Care Res. 2010;62:362–70. doi: 10.1002/acr.20105. [DOI] [PubMed] [Google Scholar]
- 4.Longo WE, Cheadle W, Fink A, et al. The role of the Veterans Affairs Medical Centers in patient care, surgical education, research and faculty development. Am J Surg. 2005;190:662–75. doi: 10.1016/j.amjsurg.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Veterans Affairs. 2001 National Survey of Veterans. [(11 September 2006, date last accessed)]. http://www.virec.research.va.gov/DataSources/NationalSurveyVeterans/2001NationalSurveyofVeterans.html.
- 6.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 7.Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One. 2009;4:e6242. doi: 10.1371/journal.pone.0006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikuls T, Saag K, Criswell L, et al. Mortality risk associated with rheumatoid arthritis in a prospective cohort of older women: results from the Iowa Women’s Health Study. Ann Rheum Dis. 2002;61:994–9. doi: 10.1136/ard.61.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naz SM, Farragher TM, Bunn DK, Symmons DP, Bruce IN. The influence of age at symptom onset and length of followup on mortality in patients with recent-onset inflammatory polyarthritis. Arthritis Rheum. 2008;58:985–9. doi: 10.1002/art.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus T, Callahan L. Taking mortality in rheumatoid arthritis seriously - predictive markers, socioeconomic status and comorbidity. J Rheumatol. 1986;13:841–5. [PubMed] [Google Scholar]
- 11.Troelsen LN, Garred P, Jacobsen S. Mortality and predictors of mortality in rheumatoid arthritis–a role for mannose-binding lectin? J Rheumatol. 2010;37:536–43. doi: 10.3899/jrheum.090812. [DOI] [PubMed] [Google Scholar]
- 12.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1530–42. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe F, Mitchell D, Sibley J, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Rasker JJ, Boers M, Wells GA, Michaud K. Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57:935–42. doi: 10.1002/art.22895. [DOI] [PubMed] [Google Scholar]
- 16.Mikuls TR, Kazi S, Cipher D, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34:1480–4. [PubMed] [Google Scholar]
- 17.Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease burden in U.S. veterans with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1292–7. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards JS, Peng J, Amdur RL, et al. Dual-energy X-ray absorptiometry and evaluation of the osteoporosis self-assessment tool in men with rheumatoid arthritis. J Clin Densitom. 2009;12:434–40. doi: 10.1016/j.jocd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Arnett F, Edworthy S, Bloch D, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 21.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol. 2008;35:2136–47. doi: 10.3899/jrheum.080182. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 24.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 25.Krause D, Schleusser B, Herborn G, Rau R. Response to methotrexate treatment is associated with reduced mortality in patients with severe rheumatoid arthritis. Arthritis Rheum. 2000;43:14–21. doi: 10.1002/1529-0131(200001)43:1<14::AID-ANR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 27.Caplan L, Wolfe F, Russell AS, Michaud K. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J Rheumatol. 2007;34:696–705. [PubMed] [Google Scholar]
- 28.Sihvonen S, Korpela M, Mustonen J, Huhtala H, Karstila K, Pasternack A. Mortality in patients with rheumatoid arthritis treated with low-dose oral glucocorticoids. A population-based cohort study. J Rheumatol. 2006;33:1740–6. [PubMed] [Google Scholar]
- 29.Wallberg-Jonsson S, Johansson H, Ohman ML, Rantapaa-Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–71. [PubMed] [Google Scholar]
- 30.Roubenoff R. Rheumatoid cachexia: a complication of rheumatoid arthritis moves into the 21st century. Arthritis Res Ther. 2009;11:108. doi: 10.1186/ar2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 32.Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450–7. doi: 10.1002/art.20612. [DOI] [PubMed] [Google Scholar]
- 33.Boscarino JA. Post-traumatic stress and associated disorders among Vietnam veterans: the significance of combat exposure and social support. J Trauma Stress. 1995;8:317–36. doi: 10.1007/BF02109567. [DOI] [PubMed] [Google Scholar]
- 34.Piette JD, Heisler M. Problems due to medication costs among VA and non-VA patients with chronic illnesses. Am J Manag Care. 2004;10:861–8. [PubMed] [Google Scholar]
- 35.Savas LS, del Junco DJ, Bastian LA, Vernon SW. Mortality ascertainment of women veterans: a comparison of sources of vital status information, 1979–2002. Med Care. 2009;47:125–8. doi: 10.1097/MLR.0b013e3181809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koivuniemi R, Paimela L, Leirisalo-Repo M. Causes of death in patients with rheumatoid arthritis from 1971 to 1991 with special reference to autopsy. Clin Rheumatol. 2009;28:1443–7. doi: 10.1007/s10067-009-1278-9. [DOI] [PubMed] [Google Scholar]
- 38.Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2009;48:1309–13. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 39.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. 1997;24:445–51. [PubMed] [Google Scholar]
- 40.Boyle CA, Decoufle P, Delaney RJ. Postservice mortality among Vietnam veterans. JAMA. 1987;257:790–5. [PubMed] [Google Scholar]
- 41.Rogot E, Murray JL. Smoking and causes of death among U.S. veterans: 16 years of observation. Public Health Rep. 1980;95:213–22. [PMC free article] [PubMed] [Google Scholar]