Abstract
Objective
To develop a new bioactive gas delivery method using echogenic liposomes (ELIP) as the gas carrier.
Background
Nitric oxide (NO) is a bioactive gas with potent therapeutic effects. Bioavailability of NO by systemic delivery is low with potential systemic effects.
Methods
Liposomes containing phospholipids and cholesterol were prepared using a new freezing under pressure method. The encapsulation and release profile of NO from NO containing-ELIP (NO-ELIP) or a mixture of NO/Argon (NO/Ar-ELIP was studied. Uptake of NO from NO-ELIP by cultured vascular smooth muscle cells (VSMC) both in the absence and presence of hemoglobin was determined. The effect of NO-ELIP delivery to attenuate intimal hyperplasia in a balloon-injured artery was determined.
Results
Coencapsulation of NO with argon (Ar) enabled the adjustment the amount of encapsulated NO. A total of 10 µl of gas can be encapsulated into 1 mg liposomes. The release profile of NO from NO-ELIP demonstrated an initial rapid release followed by a slower release over 8 hours. Sixty-eight percent of cells remained viable when incubated with 80 µg/ml of NO/Ar-ELIP for 4 hours. NO delivery to VSMC using NO/Ar-ELIP was 7-fold higher than unencapsulated NO. NO/Ar-ELIP remained effective NO delivery to VSMC even in the presence of hemoglobin. Local NO-ELIP administration to balloon-injured carotid arteries attenuated the development of intimal hyperplasia and reduced arterial wall thickening by 41±9%.
Conclusions
Liposomes can protect and deliver a bioactive gas to target tissues with the potential for both visualization of gas delivery and controlled therapeutic gas release.
Keywords: Intimal hyperplasia, liposomes, nitric oxide, contrast agent
1. Introduction
Nitric oxide (NO) is a potent bioactive gas with vasodilatatory, anti-inflammatory, anti-thrombotic, antiproliferative and possibly anti-atherogenic properties (1,2). The desirable characteristics of NO in modulating the development of various vascular diseases have prompted the development of various NO precursors, synthetic NO promoters such as L-arginine, endothelial NO synthase (eNOS) gene, NO donors and NO gas. One of the most popular methodologies for NO gas delivery clinically is by inhalation (3–5). In 1999, the US Food and Drug Administration approved an inhaled formulation of NO gas for the treatment of patients with persistent pulmonary arterial hypertension (6). Another potential use of NO would be to modulate neointimal hyperplasia (7). While NO delivery to the arterial wall has a number of potential benefits, successful NO delivery to targeted tissues is challenging due to the presence of endogenous NO scavengers such as hemoglobin (5).
Our laboratory has developed a number of liposomal formulations for molecular imaging and drug and gene delivery (8–12). The encapsulation of air into these liposomal formulations results in a contrast agent that is suitable for ultrasound image enhancement and is stable in serum at 37°C for prolonged duration (9). In this study, we demonstrated that gases, such as NO can also be encapsulated into our ELIP, resulting in a “theramatic” agent with theranostic both echogenic and bioactive gas-delivery properties.
The specific aims of this study were to (a) encapsulate NO into ELIP, (b) adjust the encapsulated gaseous composition by inclusion of argon (Ar) to modulate the rate of NO release, (c) determine the protective effects of NO encapsulation into ELIP for delivery to vascular smooth muscle cells (VSMC) in vitro in the presence of hemoglobin – an endogenous NO scanenger, and (d) demonstrate the potential therapeutic effects of NO delivery from NO/Ar-ELIP in attenuating neointimal hyperplasia in injured common carotid arteries of cholesterol-fed rabbits.
2. Methods
2.1. Preparation of NO-containing Liposomes (NO-ELIP) and NO/Ar-containing Liposomes (NO/Ar-ELIP)
Liposomes composed of 1,2-dipalmitoyl-sn-glycero-3-ethylphosphocholine (EDPPC, Genzyme Corporation, Cambridge, MA): 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Avanti Polar Lipids, Alabaster, AL): cholesterol (CH, Avanti Polar Lipids, Alabaster, AL) at a mole ratio of 60:30:10. liposomes were made by previous developed pressured-freeze method with modification(13). Briefly, after drying and hydrating lipid film, the liposomes were transferred to a 2 ml glass vial with a cap that was sealed with a Teflon-rubber septum. NO (specialty gases of America, Inc., Toledo, OH) was washed and deoxygenated with a saturated NaOH solution. Ten milliliters of NO alone or a gaseous mixture of NO and Ar was injected into the glass vial through the Teflon-rubber septum using a 12 ml syringe attached to a 27G × ½” needle. The pressurized liposomal dispersion was frozen at −70°C with dry ice for at least half an hour. The pressure in the vial was released by the removal of the cap and the liposomal dispersion was allowed to thaw. Gas encapsulation, release profiles and delivery characteristics of NO-ELIP or NO/Ar-ELIP were studied after thawing. The volume of encapsulated gas in liposomes was measured using a syringe method as previously described (12).
2.2. NO Release Study
The release of NO from pure NO-ELIP and NO/Ar-ELIP was determined by serial dialysis under sink conditions. A dispersion of 20 µl of NO-ELIP and 180 µl of phosphate-buffered saline (PBS) (donor buffer) was placed inside a dialysis tube (MWCO:12–14,000, Spectrum Laboratories, Inc. Rancho Dominguez, CA) and dialyzed against 1 ml of PBS (receiving buffer). The dialysis tube was transferred to a fresh PBS solution and the release of NO was measured at 5, 10, 20, 30, 60, 120, 240 and 480 minute. The measurement of NO release from NO-ELIP into the receiving buffer was based on the reaction between NO and oxygen to yield nitrite (4NO+O2+2H2O→4NO2−+4H+)(14). The nitrite concentration in the receiving solution was measured with a colorimetric NO assay kit (BioVision Inc, Mountain View, CA). As NO can exist in two different compartments in the dialysis system, namely encapsulated NO inside NO-ELIP or dissolved NO in the surrounding solution, NO saturated mannitol solution was used as a control to correct for NO release from NO-ELIP. Thus, the nitrite concentration in the receiving buffer with the NO-ELIP or NO/Ar-ELIP subtracted by the nitrite concentration in the receiving solution with the mannitol was taken to be equivalent to the NO released from NO-ELIP or NO/Ar-ELIP at each time point.
2.3. Cell Viability in the Presence of NO/Ar-ELIP
Rat VSMC were grown in 75 cm2 flasks containing a medium of Dulbecco’s Modified Eagle Medium (DMEM) (GIBCO-BRL, USA) and 10% FBS, 100 units/ml penicillin and 100 mg/ml streptomycin at 37°C under 5% CO2 and 95% air. Cells obtained between passages 5 and 8 were used.
Twenty four hours before experiment, rat VSMC were seeded in 48-well plates in DMEM and 10% fetal bovine serum (FBS). The medium was removed and replaced with a solution containing DMEM and 0.3% FBS. The concentration of FBS was decreased to minimize the interference with the NO assay. Differing concentrations of NO/Ar-ELIP were added to VSMC and incubated at 37°C for 4 hours. After incubation, the medium was removed and the cells were washed twice with PBS. Cell viability was measured with a commercially available calcein AM kit (Calbiochem, Gibbstown, NJ).
2.4. NO Delivery into Vascular Smooth Muscle Cells
Thirty microliters of NO/Ar(1:9)-ELIP or NO/Ar saturated mannitol solution were incubated with cultured VSMC in a 48-well plate for 5 minutes. The solutions were aspirated and VSMC were washed 3 times with 500 µl of PBS solution. The uptake of NO by VSMC was determined by measuring the nitrite concentration in cultured cells using the NO assay kit. Briefly, in this reaction, nitrate reductase in the VSMC converts nitrate to nitrite. Nitrite then reacts with the chromogenic substrate, sulfanilamide and N-(1-naphthyl) ethylenediamine, to produce a color reaction that was determined according to the optical density at 540 nm (OD540) using a Tecan microplate reader (Tecan US Inc, Raleigh, NC).
2.5. NO Delivery in the Presence of Hemoglobin
The uptake of NO by VSMC in the presence and absence of hemoglobin was measured by the NO fluorescent probes diaminofluorescein-2 diacetate (DAF-2DA, Calbiochem, Gibbstown, NJ) to localize NO production in cells by fluorescence microscopy. DAF-2DA is a non-fluorescent agent that can readily diffuse into cells and be converted into diaminofluorescein-2 (DAF-2) by cytosolic esterase inside living cells. When NO is delivered into VSMC, NO reacts with DAF-2, a non-fluorescent dye, to generate diaminofluorescein-2 triazole (DAF-2T), a fluorescent product. VSMC in the presence or absence of 1 mg/ml of hemoglobin were incubated with 30 µl of NO/Ar-ELIP or NO saturated mannitol solution for 5 minutes. The incubating solution was removed and VSMC washed three times with PBS. Ten microliters of DAF-2DA working solution (1:500 dilutions in PBS) were added to VSMC and incubated for 30 minutes in the dark. DAF-2DA was removed and the VSMC washed three times with PBS. The uptake of NO by VSMC under different conditions was qualitatively assessed by fluorescence microscopy at wavelength of 495nm.
2.6. Balloon Injury and Local Delivery of NO/Ar-ELIP in Vivo
All animal experiments were approved by the Animal Welfare Committee at the University of Texas Health Science Center at Houston. Twenty-one male New Zealand White rabbits weighing 3.0–4.5 kg were used. The animals were fed an atherogenic diet, containing 0.2% cholesterol and 4% coconut oil for two weeks before balloon denudation. On the day of surgery, the rabbit was anesthetized with ketamine (35 mg/kg), xylazine (5 mg/kg) and 1–3% isoflurane. Balloon injury to the common carotid artery was performed using a 2F Fogarty catheter (Edwards Lifesciences, Irvine, CA). A sheath was inserted into the right external carotid artery and the catheter was introduced through the sheath into the common carotid artery. The balloon was inflated with 200 µl of saline solution and passed back and forth for 3 times in the common carotid artery over a distance of 3 cm. Immediately following balloon injury, liposomes containing Ar alone or a gaseous mixture of 10% NO and 90% Ar was injected locally into the distally occluded common carotid artery through the sheath and allowed to dwell for 2 minutes. The occluding suture was then released, the sheath removed, the external carotid artery ligated, the wound closed and the rabbit allowed to recover. Carotid arteries subjected to a sham procedure, balloon injured carotid arteries without treatment and contralateral common carotid arteries without injury or treatment were used as controls.
The animals continued on a high cholesterol diet and were sacrificed 2 weeks after surgery. The carotid arteries were removed, cut into 2.5 mm segments and fixed with PBS containing 4% formalin for 24 hours. The fixed tissues were embedded in paraffin and sections were stained with H&E for histologic examination. All sections from the injured segment of each artery were examined in a blind fashion using Image-Pro Plus (Media Cybernetics, Bethesda, MD). Measurements were made of the cross-sectional area of the lumen and of the areas enclosed by the internal and external elastic laminae. The intimal cross-sectional area of carotid artery segments was determined by subtracting the area of the lumen from the area enclosed by the internal elastic lamina. The medial area was determined by subtracting the area enclosed by the internal elastic lamina from the area enclosed by the external elastic lamina.
2.7. Statistical Analysis
Statistical analysis between groups was performed by a t-test or one-way analysis of variance (ANOVA) using SigmaStat (Version 3.5, Systat Software Inc, Point Richmond, CA) and Statistica (Version 8.0, StatSoft, Inc., Tulsa, OK) software. Differences between multiple groups were assessed with the post-hoc Tukey HSD test for unequal N. A value of P < 0.05 was considered significant. All data are presented as mean ± SEM.
3. Results
3.1. NO Encapsulation and Release
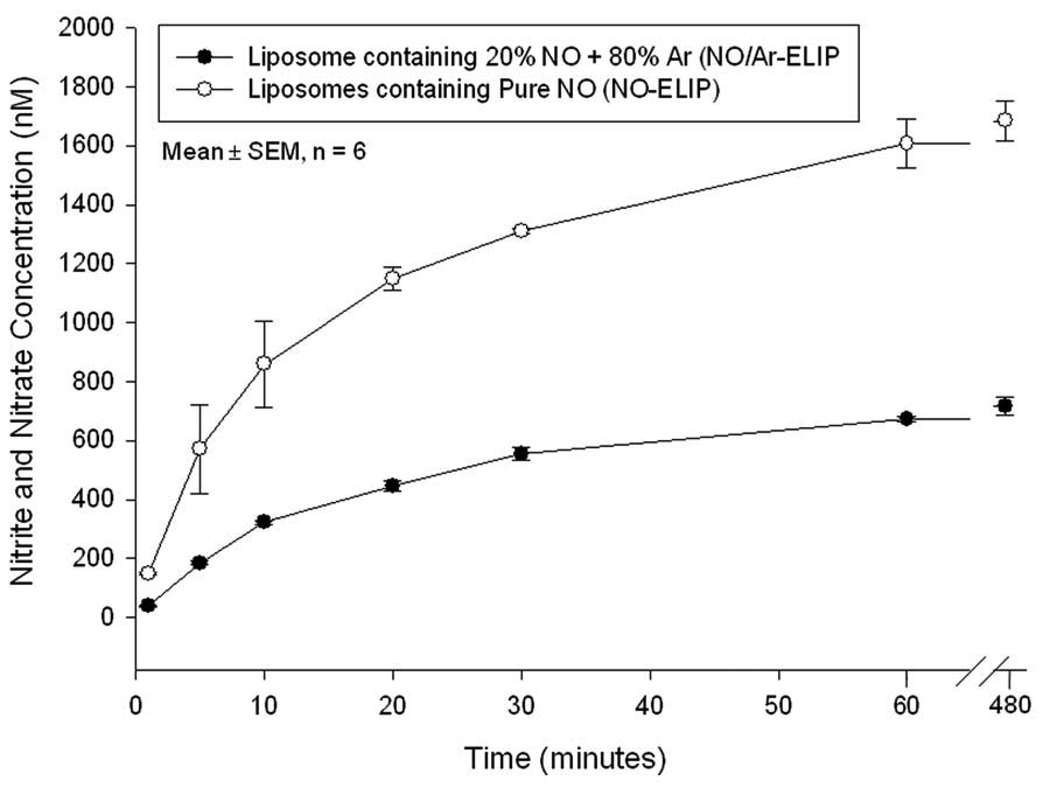
Ten microliters of NO gas were encapsulated into 1 mg of liposomes using the freezing under pressure method. The release of NO from NO-ELIP was rapid, with 50% of the encapsulated gas released within 10 min, followed by slower release over 8 hours (Figure 1). In contrast, NO release was slower in the presence of Ar (liposomes containing 20% NO and 80% Ar). The total volume of releasable NO was 2 µl from 1 mg 20% NO/ 80% Ar-containing liposomes,1 µl from 1 mg 10% NO/ 90% Ar-containing liposomes, and 10 µl from 1 mg NO-ELIP. For the rest of our experiments, we used a NO/Ar ratio of 1 to 9, with a NO concentration of 0.045 µmol/mg lipids.
Figure 1. NO release profile.
This figure illustrates the amount of released NO when pure NO (circles) or a mixture of 20% NO with 80% Ar (dots) were encapsulated into the liposomes.
3.2. Cell Viability in the Presence of NO-ELIP
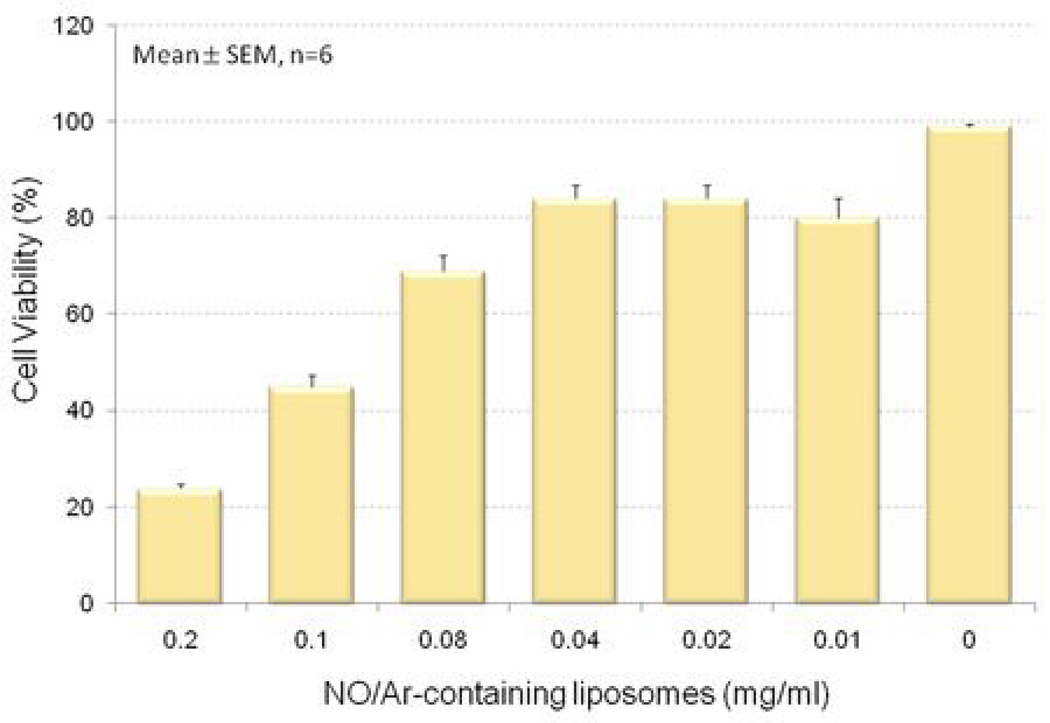
The effect of the lipid and gaseous components of 10% NO and 90% Ar on cell viability was evaluated by measuring the extent of calcein AM uptake in cultured VSMC. As shown in Figure 2, 68% of cells remained viable after incubating with 0.08 mg/ml NO/Ar-ELIP (2 µmol/L NO) for 4 hours. Eighty-two percent of the cells were viable in a culture media containing 0.01–0.04 mg/ml NO-ELIP. We arbitrarily selected concentrations in the range from 0.01 to 0.04 mg/ml (containing 0.26 to 1.0 µM/L of NO) of NO/Ar-ELIP in subsequent in-vitro cell culture experiments because of the relatively high cell viability and the substantial amount of NO that was deliverable by NO/Ar-ELIP.
Figure 2. Cell viability with NO-ELIP.
Vascular smooth muscle cell viability at 4 hours following differing amounts of NO/Ar(1:9)-containing liposomes. Viability was measured utilizing calcein AM.
3.3. NO Delivery into Cultured Vascular Smooth Muscle Cells
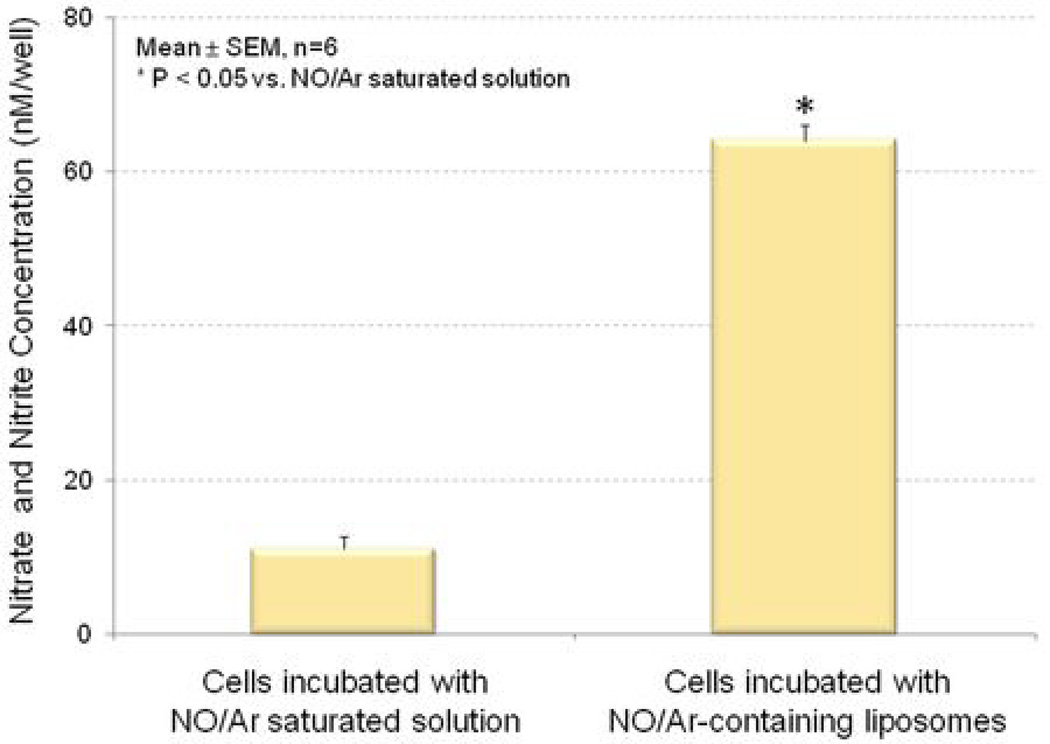
NO uptake by cells was measured on the basis of the corresponding nitrate and nitrite concentration using a colorimetric NO assay kit. VSMC were incubated with a solution containing 30 µg/ml NO/Ar-saturated mannitol or 30 µg/ml NO/Ar-ELIP. The amount of NO that can be delivered to cells was 7-fold higher with NO/Ar-ELIP than with NO/Ar-saturated mannitol solution (Figure 3).
Figure 3. NO delivery into cultured VSMC.
Cells were cultured in 48-well plates and incubated with NO/Ar(1:9) saturated mannitol (left) or 30 µg of NO/Ar(1:9)-containing liposomes (right) for 10 minutes. The uptake of NO was expressed as nitrate and nitrite concentrations.
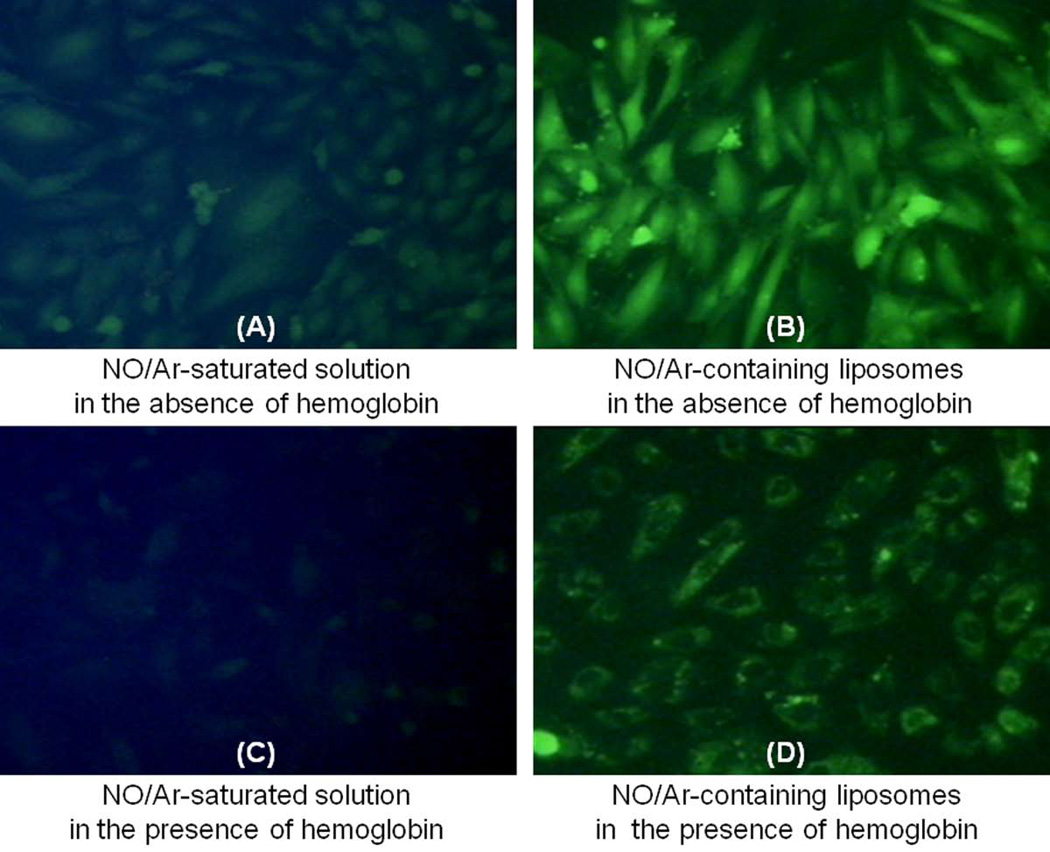
As hemoglobin is the primary scavenger of NO in the circulation, which drastically reduces the bioavailability of NO to target tissues, we evaluated the delivery of NO/Ar-ELIP in the presence of hemoglobin (Figure 4C and 4D). Cellular uptake of NO was visualized by DAF-2DA fluorometric imaging. In the absence of hemoglobin, both the NO/Ar-saturated mannitol solution and the NO/Ar-ELIP efficiently delivered NO to VSMC (Figures 4A & 4B). The fluorescence signal was stronger inside cells incubated with NO/Ar-ELIP than with the NO/Ar-saturated mannitol solution. In the presence of hemoglobin, NO delivery to cells from NO/Ar-saturated mannitol solution was poor (Figure 4C), whereas, that from NO/Ar-ELIP was effective, as indicated by the bright cellular fluorescence (Figure 4D).
Figure 4. Cellular uptake of NO.
Representative DAF-2DA fluorescence images of VSMC treated with (A) NO/Ar-saturated mannitol solution in the absence of hemoglobin; (B) NO/Ar(1:9)-containing liposomes in the absence of hemoglobin; (C) NO/Ar(1:9)-saturated mannitol solution in the presence of hemoglobin; and (D) NO/Ar(1:9)-containing liposomes in the presence of hemoglobin.
3.4. Effects of Local Delivery of NO/Ar-ELIP on the Development of Neointimal Hyperplasia in Balloon Injured Arteries
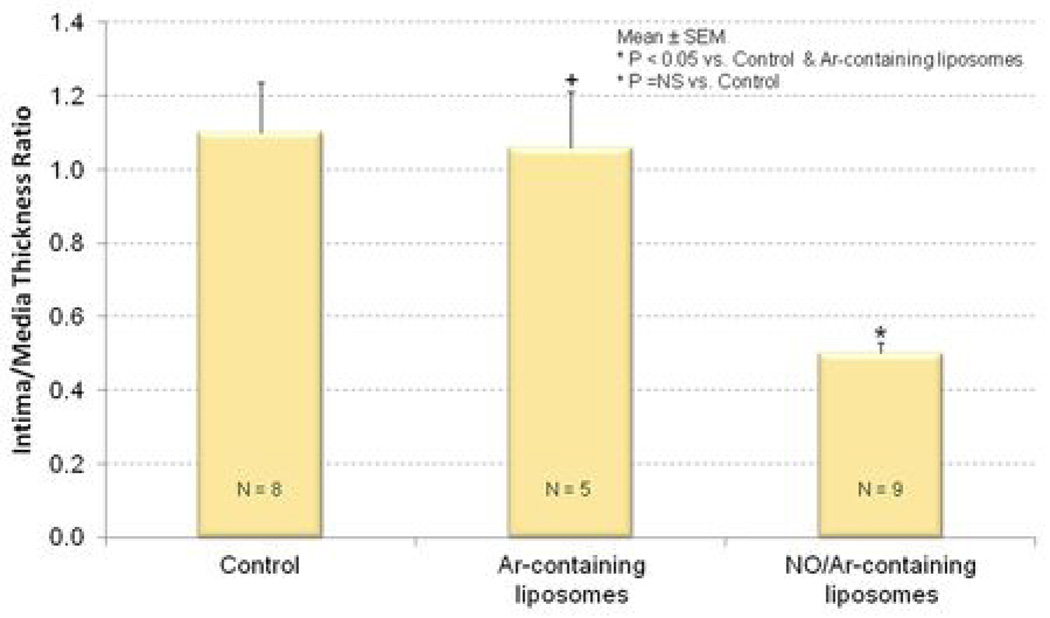
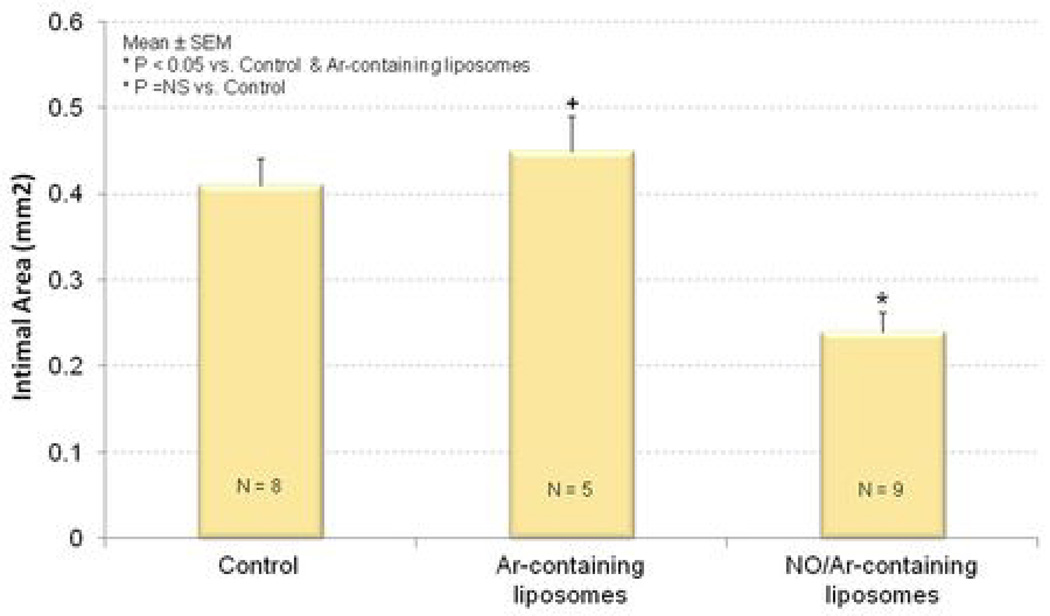
The combination of balloon denudation of the carotid arteries and cholesterol feeding induced extensive neointimal hyperplasia and luminal narrowing relative to the uninjured arteries (Figure 5A and 5B). Ar-ELIP did not attenuate the hyperplastic reaction in balloon-injured arteries (Figure 5C). On the other hand, NO/Ar-ELIP were able to inhibit the hyperplastic response in balloon-injured arteries (Figure 5D and 6). Intima/media area ratios decreased from 1.1±0.39 in injured control animals without any specific treatment to 0.5±0.09 in animals treated with NO/Ar-ELIP (Figure 6A). Similarly, intimal area was reduced from 0.4±0.09 mm2 in the injured controls to 0.2±0.07 mm2 in the NO/Ar-ELIP treated animals (n=9, p<0.05) (Figure 6B, and 5B vs. 5D). Ar-ELIP had no effect on the intima/ media area ratio or intimal area. The decrease in intimal area induced by NO/Ar-ELIP accounted for the decrease in the arterial wall thickness (Table 1).
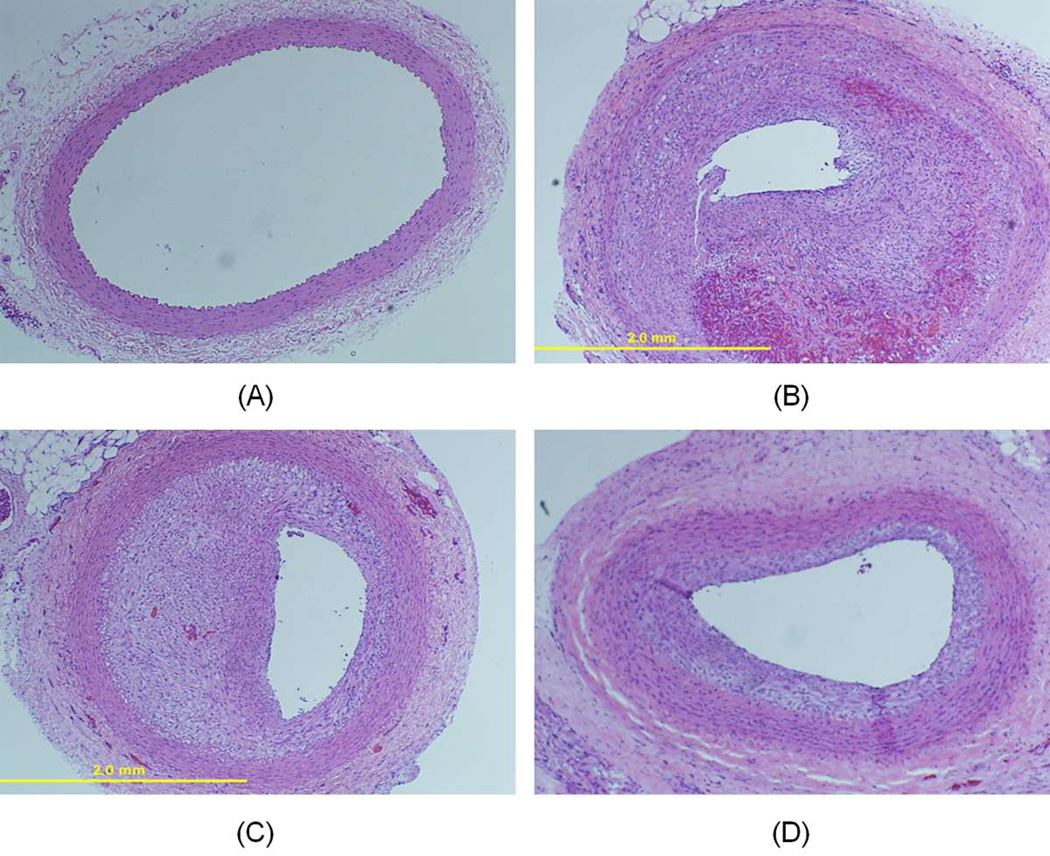
Figure 5. NO/Ar-ELIP effect on inhibition of neointimal hyperplasia.
Representative histological sections of (A) normal common carotid artery (no balloon injury); (B) common carotid artery 14 days after balloon injury; (C) common carotid artery 14 days after balloon injury treated with Ar-ELIP; and (D) common carotid artery 14 days after balloon injury treated with NO/Ar(1:9)-containing liposomes (H&E ×400).
Figure 6. Quantitation of neointimal hyperplasia.
(A) intima/media thickness ratio and (B) intimal area of injured common carotid arteries; control (left); treated with Ar-ELIP (middle); and treated with NO/Ar(1:9)-containing liposomes (right).
Table 1.
Morphometric analysis of the carotid arteries 14 days after balloon injury
| Treatment group | Intimal area (mm2) |
Intimal depth (mm) |
Medial area (mm2) |
Medial depth (mm) |
I/M ratio | I/(I+M) |
|---|---|---|---|---|---|---|
| Injury alone (Mean ± SEM) (n=8) | 0.41± 0.03 | 0.18± 0.02 | 0.42 ± 0.03 | 0.12 ± 0.01 | 1.1 ± 0.14 | 0.5 ± 0.04 |
| Ar-liposomes (Mean ± SEM) (n=5) | 0.45 ± 0.04 | 0.15 ± 0.01 | 0.44± 0.04 | 0.13 ± 0.004 | 1.1 ± 0.15 | 0.5 ± 0.08 |
| NO/Ar-liposomes (Mean ± SEM) (n=9) | 0.24 ± 0.02** | 0.09 ± 0.01* | 0.45 ± 0.03 | 0.12 ± 0.01 | 0.5 ± 0.03** | 0.3 ± 0.03** |
P < 0.05 versus Injury alone and Ar-containing liposomes.
P < 0.005 versus Injury alone and Ar-containing liposomes.
4. Discussion
Impaired endothelial nitric oxide production is implicated in many cardiovascular diseases, such as essential hypertension, stroke, coronary artery disease, atherosclerosis, platelet aggregation and restenosis after percutaneous transluminal coronary angioplasty, and ischemia/reperfusion injury (15,16). Administration of exogenous NO can potentially to supplement NO to the arterial wall and has been explored over the past decade as a therapeutic agent (16). This study has demonstrated that encapsulation of a bioactive gas, NO, into ELIP can be achieved with a freezing under pressure technique. The resultant NO-ELIP have a biphasic release profile. The amount of releasable NO from ELIP can be modulated by mixing with Ar, thus prolonging NO delivery for in vivo applications. We clearly demonstrate from in vitro, ex vivo and animal models that liposomal encapsulation of NO protect the payload against NO scavenging by hemoglobin and effective NO delivery by NO-ELIP inhibits intimal hyperplasia in injured arterial segments. This is the first study to describe liposomal encapsulation of a bioactive gas for controlled release with in vivo biologic effects. NO was used as a model gas in the current study and this new methodology of gas encapsulation into liposomes can be applied to other therapeutic gases.
4.1. Liposome for Bioactive Gas Entrapment and Delivery
Pathological changes to vascular wall have been observed in cardiovascular diseases such as atherosclerosis (17). Numerous pharmaceutics have been investigated to treat or prevent atherosclerosis. Gases can be thought of as small molecules that readily cross the endothelial barrier (18). The obstacles of using bioactive gases for the treatment of other vascular conditions have been related to the lack of suitable administration methods and potential adverse effects due to systemic delivery. With our new methodology, we have demonstrated that local delivery of NO-ELIP into carotid arteries can inhibit intimal hyperplasia in a balloon-injured arterial model.
The freezing under pressure technique is effective in encapsulating precise amounts of NO in liposomes. In the current freezing under pressure technique, we postulate that the increased concentrations of NO dissolved in solution provides a means for the entrapment of NO in the hydrophobic bilayer of NO-ELIP during the freezing and thawing process (13). NO is a colorless and odorless gas that readily reacts with oxygen to form NO2 gas, which is toxic to cells. In our formulations, we took special precautions to prevent NO from reacting with oxygen. This would have resulted in the formation of toxic NO2. This included mixing NO with an inert gas, Ar, and deoxygenating all gases by passing them through a saturated solution of sodium hydroxide.
The release of NO from NO-ELIP is rapid and half of the payload is released within the first 10 minutes followed by an extended period of slower release over at least 8 hours (Figure 1). Such a release profile is highly desirable when treating acute arterial injury. In contrast, the other ELIP formulation that contains a mixture of NO and Ar has slower NO release kinetics that may be suitable for clinical settings when chronic low level delivery is desirable.
Sixty percent of the lipid shell of NO-ELIP is comprised of positively charged phospholipids, which are known to facilitate attachment of liposomes and other lipid particles to negatively charged endothelial surfaces and exposed extracellular matrix in damaged arteries. We have previously demonstrated that our ELIP formulations are suitable for carrying air and/or a variety of drugs such as papaverine hydrochloride (19), azithromycin (20), tissue plasminogen activator (21,22) and rosiglitazone (23). In the current study, we demonstrated that 6 µl of NO can be encapsulated into 1 mg of lipids in NO-ELIP using a relatively low pressure. We have also previously demonstrated that surface modifications of ELIP with antibodies can target ELIP to inflamed arterial walls and localize the therapeutics (10). NO-ELIP are echogenic and provide a means for monitoring in the circulation and delivery of NO-ELIP with contrast-enhanced ultrasound imaging. As needed, a rapid, but controlled payload release from ELIP can be achieved using Doppler ultrasound at a specific site(24). All the listed techniques have the potential to maximize the effects of drugs and/or bioactive gases in a localized manner.
We have demonstrated that local administration of NO-ELIP is effective in reducing neointimal hyperplasia in injured carotid arteries in hyperlipidemic rabbits. Such a model resembles the clinical situation of balloon angioplasty in coronary and other vascular beds. The in vivo effect of inhaled NO on neointimal hyperplasia has been inconsistent. Lee et al. have described a beneficial effect of inhaled NO in reducing neointimal formation in balloon injured carotid arteries in rats (25). Others, however, have been unable to demonstrate NO effects in the pulmonary vasculature (26). Hemoglobin binding to inhaled NO is likely responsible for the inability of NO to reach the intended vascular beds. The affinity of NO for hemoglobin is 1500 times greater than that of carbon monoxide. It has been demonstrated that red blood cells, due to their high hemoglobin content, can reversibly bind, transport, and release NO within the cardiovascular system (27). In Figure 4, the NO/Ar-saturated mannitol solution was less efficient in delivering NO to VSMC than the NO/Ar-ELIP; both methodologies were rendered less effective for NO delivery to VSMC in the presence of hemoglobin. However, Figure 4A and 4D appear similar and there is consistency between the fluorescent enhancement of Figure 4B and 4D. This clearly demonstrates the ability of the liposomes to protect NO from hemoglobin scavenging. This mixture of NO/Ar further modulates the rate of NO delivery into cells. This mimics local NO delivery by NO/Ar-ELIP in vivo and animal experiments also substantiate the biologic effects of NO/Ar-ELIP when delivered locally in balloon-injured arteries (28)
4.2. Limitations
To demonstrate NO release, we delivered NO-ELIP (without targeting) to an occluded artery locally. This may mimic certain interventions such as angioplasty of an occluded coronary or carotid artery but may not simulate physiologic flow conditions when arterial flow is present. Although we have demonstrated the protective effects of NO encapsulation into liposomes for preventing NO scavenging by hemoglobin, we assume that continuous blood flow will probably increase the scavenging effect of NO by hemoglobin. This may be overcome by local delivery of NO-ELIP, targeting of the NO-ELIP and ultrasound-enhanced NO release. Local delivery of antiproliferative or immunosuppressive agents immediately post angioplasty has been successful in clinical trials and has been proven more efficient compared with systemic drug delivery (29). Recently, Cyrus et al. have developed a "contact facilitated drug delivery" methodology(30) to incorporate αvβ3-targeted nanoparticles containing rapamycin for targeting and content release through fusion of the lipid membrane with the targeted cell membrane. This methodology could also be useful in targeting and release of our NO_ELIP. In another study, we have also found that local ultrasound application enhances NO delivery to the arterial wall under physiological flow conditions demonstrating another potiential methodology to increase NO delivery. The spontaneous release pattern, in which release is initially rapid and then later much slower, may limit the amount of gas deliverable to targeted sites; with the consequence of reducing localized delivery and increasing systemic exposure. Further modifications of the lipid shell composition as to make it less permeable and reduce spontaneous gas release may overcome this problem. Surface modifications of NO-ELIP such as the addition of polyethylene glycol and antibodies to the lipid shell to prolong circulation duration and provide specific targeting mechanisms were not evaluated in this study.
In spite of a lack of sophistication of our initial approaches, marked in vivo effects were realized which induced inhibition of intima/media ratio by 40% by NO-ELIP in injured arteries compared to Ar-ELIP or no treatment. Therapeutic effects should be improved by co-encapsulating NO with other NO donors or synthetic genes involved in NO synthesis, although the benefit of such procedures must be assessed by future research.
4.3. Summary
A new technique has been described for encapsulating NO into liposomes for bioactive gas delivery. Such formulations have the desirable properties of high payload concentration, modifiable gas release profiles, sufficient protection of the NO payload against exogenous scavengers, and excellent payload delivery to cells for biologic effects. With future formulation modifications, this methodology may expand the repertoire of NO donors in the payload, providing greater stability, as well as site-specific triggered delivery with ultrasound, to maximize bioactive effect at the local tissue level.
Acknowledgments
This work was in part supported by NIH grants (R01 HL-74002 and R01 HL-059586, McPherson) and American Heart Association Award (0535512Z, Huang) We thank Dianna B. Roberts, Ph.D, Manager of Clinical Data Management Systems in the Dept. of Head and Neck Surgery at the University of Texas M. D. Anderson Cancer Center for assisting with the statistical analysis of the data. We thank Ryan Murphy, Susan Laing, Melvin Klegerman, Beverly Smulevitz for their assistance.
Abbreviations
- NO
Nitric oxide
- Ar
Argon
- ELIP
Echogenic liposomes
- NO-ELIP
Nitric oxide-containing Liposomes
- NO/Ar-ELIP
Nitric oxide/argon-containing Liposomes
- EDPPC
1,2-dipalmitoyl-sn-glycero-3-ethylphosphocholine
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- CH
Cholesterol
- VSMC
Vascular smooth muscle cells
- DAF-2DA
diaminofluorescein-2 diacetate
- DAF-2
diaminofluorescein-2
- DAF-2T
diaminofluorescein-2 triazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no conflicts to disclose.
References
- 1.Fischer JW, Hawkins S, Clowes AW. Pharmacologic inhibition of nitric oxide synthases and cyclooxygenases enhances intimal hyperplasia in balloon-injured rat carotid arteries. J Vasc Surg. 2004;40:115–122. doi: 10.1016/j.jvs.2004.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 5.Tsao PS, McEvoy LM, Drexler H, Butcher EC, Cooke JP. Enhanced endothelial adhesiveness in hypercholesterolemia is attenuated by L-arginine. Circulation. 1994;89:2176–2182. doi: 10.1161/01.cir.89.5.2176. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 7.Jagadeesha DK, Miller FJ, Jr, Bhalla RC. Inhibition of Apoptotic Signaling and Neointimal Hyperplasia by Tempol and Nitric Oxide Synthase following Vascular Injury. J Vasc Res. 2008;46:109–118. doi: 10.1159/000151444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkan-Onyuksel H, Demos SM, Lanza GM, et al. Development of inherently echogenic liposomes as an ultrasonic contrast agent. J Pharm Sci. 1996;85:486–490. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan KD, Huang S, Kim H, Macdonald RC, McPherson DD. Echogenic liposome compositions for increased retention of ultrasound reflectivity at physiologic temperature. J Pharm Sci. 2008;97:2242–2249. doi: 10.1002/jps.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton A, Huang SL, Warnick D, et al. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002;105:2772–2778. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton A, Rabbat M, Jain P, et al. A physiologic flow chamber model to define intravascular ultrasound enhancement of fibrin using echogenic liposomes. Invest Radiol. 2002;37:215–221. doi: 10.1097/00004424-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Hamilton AJ, Tiukinhoy SD, et al. Liposomes as ultrasound imaging contrast agents and as ultrasound-sensitive drug delivery agents. Cell Mol Biol Lett. 2002;7:233–235. [PubMed] [Google Scholar]
- 13.Huang SL, McPherson DD, Macdonald RC. A method to co-encapsulate gas and drugs in liposomes for ultrasound-controlled drug delivery. Ultrasound Med Biol. 2008;34:1272–1280. doi: 10.1016/j.ultrasmedbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007;45 Suppl A:A64–A73. doi: 10.1016/j.jvs.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Wimalawansa SJ. Nitric oxide: new evidence for novel therapeutic indications. Expert Opin Pharmacother. 2008;9:1935–1954. doi: 10.1517/14656566.9.11.1935. [DOI] [PubMed] [Google Scholar]
- 17.Mannarino E, Pirro M. Endothelial injury and repair: a novel theory for atherosclerosis. Angiology. 2008;59:69S–72S. doi: 10.1177/0003319708320761. [DOI] [PubMed] [Google Scholar]
- 18.Sibley CP, Bauman KF, Firth JA. Permeability of the foetal capillary endothelium of the guinea-pig placenta to haem proteins of various molecular sizes. Cell Tissue Res. 1982;223:165–178. doi: 10.1007/BF00221507. [DOI] [PubMed] [Google Scholar]
- 19.Kee PH, Abruzzo TA, Smith DA, et al. Synthesis, Acoustic Stability, and Pharmacologic Activities of Papaverine-Loaded Echogenic Liposomes for Ultrasound Controlled Drug Delivery. J Liposome Res. 2008:1–15. doi: 10.1080/08982100802354558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiukinhoy SD, Khan AA, Huang S, Klegerman ME, MacDonald RC, McPherson DD. Novel echogenic drug-immunoliposomes for drug delivery. Invest Radiol. 2004;39:104–110. doi: 10.1097/01.rli.0000111207.92580.44. [DOI] [PubMed] [Google Scholar]
- 21.Tiukinhoy-Laing SD, Buchanan K, Parikh D, et al. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J Drug Target. 2007;15:109–114. doi: 10.1080/10611860601140673. [DOI] [PubMed] [Google Scholar]
- 22.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Kee P, McPherson DD, MacDonald RC. Multi-functional echogenic liposomes for image-guided and ultrasound-controlled PPAR agonist delivery. J Am Coll Card. 2007;49:365A. [Google Scholar]
- 24.Smith DA, Porter TM, Martinez J, et al. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Adrie C, Jacob HJ, Roberts JD, Jr, Zapol WM, Bloch KD. Chronic inhalation of nitric oxide inhibits neointimal formation after balloon-induced arterial injury. Circ Res. 1996;78:337–342. doi: 10.1161/01.res.78.2.337. [DOI] [PubMed] [Google Scholar]
- 26.Morris GN, Rich GF. Inhaled nitric oxide as a selective pulmonary vasodilator in clinical anesthesia. AANA J. 1997;65:59–67. [PubMed] [Google Scholar]
- 27.Gladwin MT. Role of the red blood cell in nitric oxide homeostasis and hypoxic vasodilation. Adv Exp Med Biol. 2006;588:189–205. doi: 10.1007/978-0-387-34817-9_17. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Kee PH, M.R M, Zou Y, McPherson DD, MacDonald RC. Nitric Oxide loaded echogenic liposomes inhibit intimal hyperplasia in an acute arterial injury model. Circulation. 2007;116 II_294. [Google Scholar]
- 29.Sheiban I, Carrieri L, Catuzzo B, et al. Drug-eluting stent: the emerging technique for the prevention of restenosis. Minerva Cardioangiol. 2002;50:443–453. [PubMed] [Google Scholar]
- 30.Cyrus T, Zhang H, Allen JS, et al. Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arterioscler Thromb Vasc Biol. 2008;28:820–826. doi: 10.1161/ATVBAHA.107.156281. [DOI] [PMC free article] [PubMed] [Google Scholar]