Abstract
Although both a loss of spinal inhibitory neurotransmission and the involvement of oxidative stress have been regarded as important mechanisms in the pathogenesis of pain, the relationship between these two mechanisms has not been studied. To determine whether reactive oxygen species (ROS) involvement in pain mechanisms is related to the diminished inhibitory transmission in the substantia gelatinosa (SG) of the spinal dorsal horn, behavioral studies and whole cell recordings were performed in FVB/NJ mice. Neuropathic pain was induced by a tight ligation of the L5 spinal nerve (SNL). Pain behaviors in the affected foot were assessed by behavioral testing for mechanical hyperalgesia. Pain behaviors developed by three days and lasted over eight weeks. Both systemic and intrathecal administration of an ROS scavenger, phenyl-N-tert-butylnitrone (PBN), temporarily reversed mechanical hyperalgesia up to two hours one week after SNL. In non-ligated mice, an intrathecal injection of an ROS donor, tert-butyl hydroperoxide (t-BOOH), dose-dependently induced mechanical hyperalgesia for 1.5 hours. In whole cell voltage-clamp recordings of SG neurons, perfusion with t-BOOH significantly decreased the frequency of mIPSCs, and this effect was reversed by PBN. Furthermore, t-BOOH decreased the frequency of GABAA receptor-mediated mIPSCs without altering their amplitudes but did not affect glycine receptor-mediated mIPSCs. In SNL mice, mIPSC frequency in SG neurons was significantly reduced as compared to that of normal mice, which was restored by PBN. The anti-hyperalgesic effect of PBN on mechanical hyperalgesia was attenuated by intrathecal bicuculline, a GABAA receptor blocker. Our results indicate that the increased ROS in spinal cord may induce pain by reducing GABA inhibitory influence on SG neurons that are involved in pain transmission.
Keywords: free radicals, persistent pain, GABA dysfunction, spinal cord, inhibitory transmission
1. Introduction
Inhibitory synaptic transmission in the superficial dorsal horn mediated by GABA and glycine plays an important role in modulating the primary afferent signals [38]. One of the known mechanisms behind the development of neuropathic pain is the disinhibition of dorsal horn neurons, particularly through the disruption of the spinal GABAergic system. For instance, a loss of GABAA receptor-mediated postsynaptic inhibition has been observed in the superficial dorsal horn after peripheral nerve injury. Studies have also shown that intrathecal application of the GABAA receptor antagonist bicuculline produces behavioral signs of tactile hyperalgesia in the naïve rat [18, 37]. Furthermore, down-regulation of GABA synthesis related to neuropathic pain has been suggested with reduced GABA immunoreactivity [5, 9] without specific loss of GABAergic neurons in the superficial dorsal horn [2, 19, 24, 25]. While spinal GABAergic dysfunction has been demonstrated to contribute to pain, the mechanisms behind this loss of inhibition are still not understood clearly.
Reactive oxygen species (ROS) have been implicated in the development of persistent pain states that result from nerve injury or inflammatory insult [6, 7, 11, 13, 21, 22, 26, 27, 29, 33]. In fact, studies have shown that various antioxidants [e.g., phenyl N-tert-Butyl-α-phenylnitrone (PBN), 5,5-dimethyl-pyrroline-N-oxide (DMPO), 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL), and vitamin E] are very effective in alleviating mechanical hyperalgesia in spinal nerve ligated neuropathic rats [12, 13] and capsaicin-induced secondary mechanical hyperalgesia in rats and mice. Therefore, much evidence suggests ROS are important for the development and maintenance of persistent pain.
Several studies have proposed a vulnerability of GABA neurons to oxidative stress. For instance, resveratrol, an antioxidant found in red wine, imparted neuroprotective effects in vivo against kainate-induced excitotoxicity since it selectively attenuated the decrease in GABA synthesizing enzyme levels in the rat striatum [31]. Recently, superoxide, a type of ROS, was found to contribute to presynaptic inhibition of GABA release in the hypothalamic paraventricular nucleus by angiotensin II [3], indicating the susceptibility of GABA neurons to oxidative stress. Therefore, we hypothesize that presynaptic inhibition of GABAergic neurons by ROS in the spinal dorsal horn could be a crucial mechanism underlying neuropathic pain.
To investigate this hypothesis, the behavioral effects of a ROS scavenger on spinal nerve ligated mice and an ROS donor on non-ligated mice were investigated. In whole cell recordings of spinal substantia gelatinosa (SG) neurons, ROS donor and scavenger effects on miniature EPSCs (mEPSCs) and IPSCs (mIPSCs) were analyzed. The data from this study demonstrate that the increase of ROS in the spinal cord markedly reduced inhibitory transmission, mainly through decreased synaptic release of GABA, which probably contribute to persistent pain.
Parts of these data have previously been presented in abstract form [39].
2. Material and Methods
2.1. Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and are in accordance with NIH guidelines. Young FVB/NJ mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Four to 5 mice were housed in a plastic cage with standard bedding and were provided with free access to food and water under a 12/12 hour light-dark cycle (light cycle: 7:00 A.M. – 7:00 P.M.). All animals were acclimated at least 7 days before any experimental procedures.
2.2. Neuropathic pain model
Peripheral nerve injury was induced by tightly ligating the L5 spinal nerve (SNL) in 5 - 7 week old male mice under isoflurane anesthesia (2% induction and 1.5% maintenance). After a dorsal midline incision, the paraspinal muscles over the L5/6 vertebrae were removed. Forceps were used to remove the L6 transverse process and to expose the left L5 spinal nerve. The L5 spinal nerve was gently freed from adjacent structures and tightly ligated with 7-0 silk thread. The surgical site was closed, and the anesthesia was discontinued. Mice were returned to their cages to recover. Sham surgery was performed using the same procedure described above, but the L5 nerve was not touched or ligated.
2.3. Drug treatment
Seven days after SNL, mice were randomly divided into treatment and control groups. Mice in one treatment group were injected by one of two different routes with a ROS scavenger, phenyl-N-tert-butylnitrone, PBN (Sigma, St. Louis, MO). Doses of PBN were 150 mg/kg (i.p., 0.1 ml) or 100 μg (i.t., 5 μl), which were dissolved in saline at a concentration of 20 mg/ml. Mice in the control groups were treated with the same volume of saline. Non-ligated mice were divided into four groups, three being injected intrathecally with 0.05, 0.10, or 0.25 μg of an ROS donor, an organic hydroperoxide, tert-butyl hydroperoxide (t-BOOH) (Sigma) dissolved in 5 μl saline and the other group being injected intrathecally with 5 μl saline alone to serve as controls. For the behavioral experiment examining the effects of bicuculline, seven days after SNL, mice were randomly divided into two treatment groups and one control group. Mice in the two treatment groups received one of two doses (0.05 μg or 0.10 μg dissolved in 5 μl saline) of intrathecal bicuculline (1(S), 9(R)-(–)-Bicuculline methiodide), the GABAA receptor antagonist (Sigma). Mice in the control group received 5 μl saline intrathecally. Immediately after the intrathecal injection, all groups received an intraperitoneal injection of 150 mg/kg PBN dissolved in 0.1 ml saline.
2.4. Behavioral testing
Testing for pain behavior consisted of assessing the mechanical hyperalgesia of the affected hind paw. All experiments were conducted by a person blinded to the treatment groups. Mechanical hyperalgesia of the hind paw was measured by determining the frequency of foot withdrawals to 10 stimuli applied with a von Frey (vF) filament (Stoelting, Wood Dale, IL). Mice were placed in a plastic box (4 × 4 × 12 cm) on a metal grid floor and were acclimated to the box for 8-10 minutes prior to testing. The vF filament was applied from underneath to the skin on the left hind-paw between the 3rd and 4th digits, which was found to be the most sensitive area after SNL. The hind-paw was stimulated with the filament vF# 3.0, equivalent to 0.1 grams of bending force. The vF filament was applied perpendicularly for 2-3 seconds with enough force to bend it slightly. A positive response consisted of an abrupt withdrawal of the foot during or immediately after stimulation. Response rates were calculated as a percentage of the number of positive responses/10 stimuli.
2.5. Intrathecal administration of drugs
For intrathecal injection, mice were anesthetized with isoflurane (2% induction and 1.5% maintenance) and were placed in the prone position. Hair of the caudal back was clipped, and the injection was performed with a modified method [15, 28] of the originally developed transcutaneous intrathecal injection [8]. The experimenter's thumb and middle finger held the lumbar vertebrae just cranial to both iliac crests, and the index finger palpated the highest spinous process (L6) to guide a 30 gauge hypodermic needle connected to a 10 μl Hamilton syringe. The needle was inserted caudally to the sixth lumbar spinous process at a 45° angle with respect to the vertebral column, facing the cranial direction. Penetration of the needle tip into the intervertebral space between the fifth and sixth lumbar vertebrae was signified by a sudden lateral movement of the tail. The agent (or saline, 5 μl in volume) was injected slowly for 5 seconds. Then, the syringe was held in place for 5 more seconds before removal in order to avoid drug spillage into the epidural space.
2.6. Spinal cord slice preparation and electrophysiological recording
Male mice (4-5 weeks old) were used. Under urethane (1.5 g/kg, i.p.) anesthesia, the lumbar spinal cord was removed from the mouse and placed in pre-oxygenated, cold (< 4°C) artificial cerebrospinal fluid (ACSF) (composition in mM: NaCl, 117; KCl, 3.6; CaCl2, 2.5; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 25; glucose 11), saturated with 95% O2 and 5% CO2 mixed gas. After the spinal cord was trimmed and embedded in a 3% agar block, the spinal cord was then sliced transversely at a thickness of 350 μm for whole-cell recordings, using a vibratome (Leica, VT1000S). The spinal cord slices were incubated for 1 hour in ACSF at 33°C. Then, the slice was placed in a recording chamber and fixed with a grid of parallel nylon threads supported by a U-shaped stainless steel weight for electrophysiological recordings. The ACSF saturated with 95% O2 − 5% CO2 was perfused into the chamber at 2 ml/min. Signals were recorded with the Multiclamp 700B amplifier (Axon Instruments; Union City,CA), digitized at 10 kHz with a DigiDATA (Axon Instruments; Union City,CA), and filtered at 1-2 kHz. The data files were saved in a PC-based computer using the pCLAMP9 acquisition software (Molecular Devices; Union City, CA). For whole-cell recordings, conventional whole-cell patch recording was made at room temperature, with patch pipettes (4-6 MΩ) filled with a potassium gluconate internal solution (composition in mM: K-gluconate, 120; KCl, 10; Mg-ATP, 2; Na-GTP, 0.5; EGTA, 0.5; HEPES, 20; phosphocreatine, 10). The position of the neurons in lamina II was identified using differential-interference contrast optics (DIC) at 40× magnification. In every voltage clamp recording, an equilibration period of 10 to 15 min was allowed after whole cell access was established, and the recording reached a steady state. The recording was discarded if the monitored input resistance changed >15%. The mEPSCs were recorded in the presence of 1 μM TTX and 10 μM bicuculline at a holding potential of -70 mV. The mIPSCs were recorded in the presence of 1 μM TTX, 20 μM CNQX, and 50 μM D-AP5 (D-2-amino-5-phosphonopentanoic acid, Sigma) at a holding potential of 0 mV. The mIPSCs were isolated into GABAA-receptor mediated and glycine-receptor mediated mIPSCs by 0.5 μM strychnine and 10 μM bicuculline, respectively, at a holding potential of 0 mV.
2.7. Drug preparation for electrophysiological recording
Each tested drug was dissolved in ACSF solution and perfused into the recording chamber by using a valve control system (4 channels, ALA-VM4, Scientific instruments, NY). The perfusate was sequentially switched to the perfusate containing the appropriate test drug. A ROS scavenger, PBN, an ROS donor, t-BOOH, a glycine receptor antagonist, strychnine, and a GABAA receptor antagonist, bicuculline (1(S), 9(R)-(–)-Bicuculline methiodide), were purchased from Sigma (St. Louis, MO). Tetrodotoxin (TTX) and 6-Cyano-7-nitroquinoxalin-2,3-dione (CNQX) were purchased from Tocris (Ellisville, MO). TTX, CNQX, bicuculline, and strychnine were prepared as a 1000× stock solution, stored at -80°C, and then diluted in the ACSF before use.
2.8. Statistical Analysis
All data are expressed as the mean ± standard error of the mean (SEM). For the behavioral tests for mechanical hyperalgesia, changes from the sham or vehicle controls were compared using the two-way repeated-measures ANOVA followed by Duncan's post hoc tests. The mEPSCs and mIPSCs of the substantia gelatinosa (SG) neurons were analyzed off-line with a peak detection program (MiniAnalysis, Synaptosoft, Leonia, NJ). Detection of events was accomplished by setting a threshold above the noise level. The distribution of cumulative probability of the inter-event interval and amplitude of mEPSCs and mIPSCs was estimated using the Komogorov-Smirov test. Electrophysiological experimental data were analyzed with one-way ANOVA. Tukey's post hoc analyses were utilized to determine differences between groups. In all tests, p < 0.05 was considered significant. The SigmaStat program (Version 3.1, Systat Software, San Jose, CA) was used to analyze all the data.
3. Results
3.1. Spinal nerve ligation produces a long lasting mechanical hyperalgesia in mice
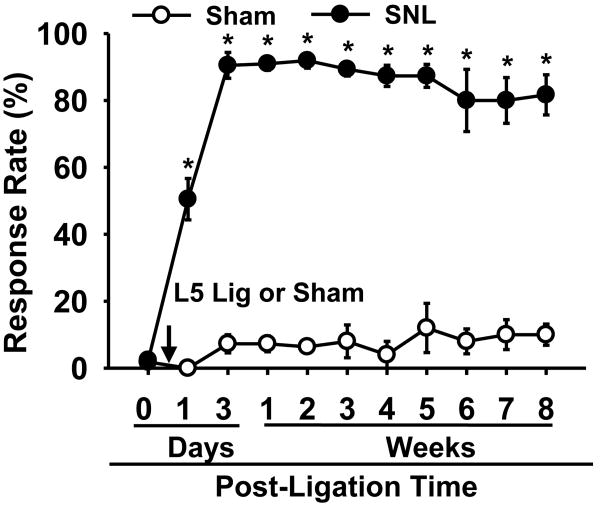
To determine the time course of pain behavior initiated by neuropathic injury, the response rates of mice to the von Frey stimuli (vF filament # 3.0) were examined before and after either sham or L5 spinal nerve ligation (Fig. 1). Before SNL, the response rates of the left hind paw were 2 ± 1% (mean ± SEM). Following SNL, the response rates increased significantly one day after surgery, reached 90 ± 4% by the third day, and remained at high levels for eight weeks. In contrast, the sham-operated mice did not develop pain behaviors after surgery.
Figure 1. The time course of mechanical hyperalgesia in SNL and sham mice.
Response rates to von Frey filament 3.0, corresponding to 0.1 g, were measured at 0 day for pre-surgical levels and were gradually increased following SNL. The mice exhibited signs of mechanical hyperalgesia at 1 day which peaked by 3 day and were stably maintained, lasting longer than 8 weeks in all operated mice (n = 6). Sham operation (n = 6) did not result in a significant change in response rates to the stimuli throughout the testing period. Data are presented as means ± SEM. Arrow with L5 Lig or Sham, time of L5 SNL or sham surgery; *, the value is significantly (p < 0.05) different from that of the sham control by two-way repeated-measures ANOVA.
3.2. Reactive oxygen species scavenger, PBN, reduces pain behaviors in neuropathic mice
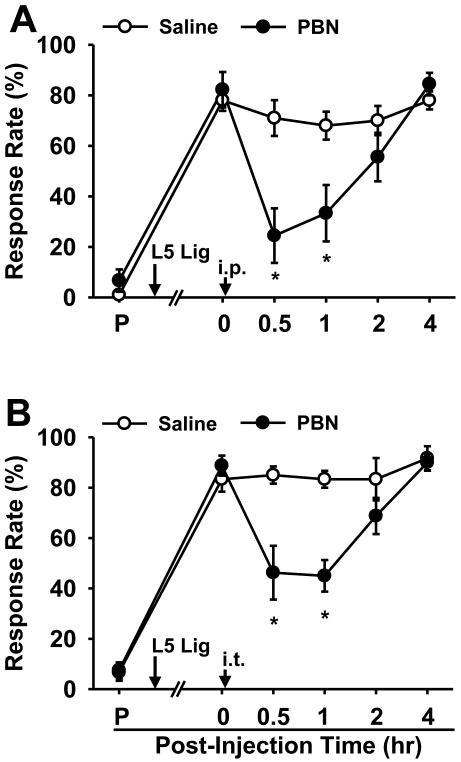
To determine whether ROS play a role in mechanical hyperalgesia in the SNL mouse, the effect of a ROS scavenger on pain behavior was examined. The effect of a single, systemic PBN injection on pain behaviors is shown in Fig. 2A. Seven days after surgery, the response rates were 82 ± 7% in the SNL group (n = 8). PBN (150 mg/kg, i.p., 0.1 ml) significantly decreased the response rates up to 1 hr after injection. On the other hand, the same volume of saline (i.p., 0.1 ml) had little effect on the response rates (n = 10). In addition, intrathecal injection of PBN (100 μg in 5 μl, n = 8) at seven days after SNL significantly reduced foot withdrawal frequencies to von Frey stimuli up to 1 hr after injection (Fig. 2B). The 200 μg of intrathecal PBN (data not shown) did not result in any further reduction in response rates (n = 6) compared to that with 100 μg PBN; therefore, the maximal effective dose of PBN was estimated to be 100 μg. The maximum effect of intrathecal PBN was smaller than that of systemic PBN, suggesting that PBN has additional sites of action besides the spinal cord. Nevertheless, the fact that intrathecal PBN produces a powerful analgesic effect suggests that the spinal cord is an important site of action of PBN. In summary, scavenging ROS in the spinal cord can temporarily but significantly relieve pain behaviors in neuropathic mice, suggesting that spinal ROS is an important component for the maintenance of neuropathic pain.
Figure 2. The effects of a ROS scavenger on mechanical hyperalgesia in SNL mice.
(A) The effect of i.p. PBN. SNL resulted in significantly increased response rates to von Frey filament 3.0 from pre-surgical levels (P) in all operated mice (n = 18). One week after surgery, a single systemic injection of 150 mg/kg PBN (n = 8) significantly alleviated mechanical hyperalgesia up to 1 hr after injection. Vehicle treatment (n = 10) resulted in little change in response rates. (B) The effect of i.t. PBN. SNL resulted in significantly increased response rates from pre-surgical levels (P) in all operated mice (n = 14). One week after surgery, a single intrathecal injection of 100 μg PBN in 5 μl saline (n = 8) significantly alleviated mechanical hyperalgesia up to 1 hr. Vehicle treatment did not affect response rates (n = 6). Data are presented as means ± SEM. Arrow with L5 Lig, time of L5 SNL, arrow with ip, intraperitoneal injection; arrow with it, intrathecal injection; *, the value is significantly (p < 0.05) different from that of the vehicle control by two-way repeated-measures ANOVA followed by Duncan's post hoc tests.
3.3. A reactive oxygen species donor, t-BOOH, induces pain behaviors in non-injured mice
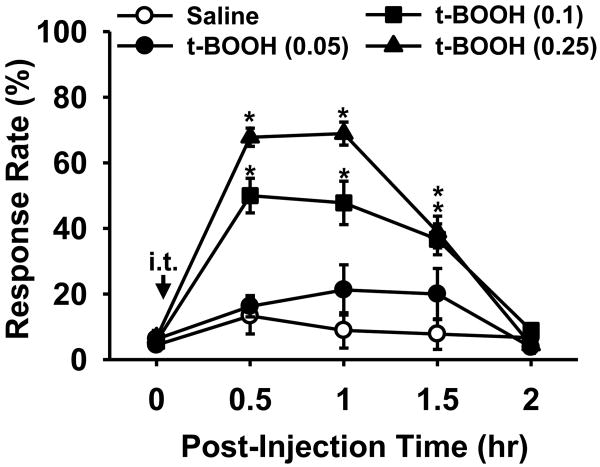
To determine whether an increase in spinal ROS would be sufficient to induce pain behaviors in non-ligated mice, the effects of intrathecal administration of an ROS donor were investigated. An ROS donor, t-BOOH, was injected into the intrathecal space and the effects on paw-withdrawal response rates in normal mice are shown in Fig. 3. Baseline responses to vF filament #3.0 were recorded in four groups of mice prior to receiving a single intrathecal injection of either 0.05, 0.10, or 0.25 μg t-BOOH (n = 8, 9, 9, respectively) dissolved in 5 μl saline or an injection of 5 μl saline (n = 9, vehicle control). t-BOOH dose-dependently increased response rates compared to vehicle injection. These changes lasted up to 1.5 hr and then returned to normal levels by 2 hr. A single injection of 0.25 μg t-BOOH significantly increased response rates from 6 ± 2% to 68 ± 4% at 0.5 hr after injection. These data show that increased levels of spinal ROS can induce mechanical hyperalgesia in mice, which resemble the pain behaviors seen after peripheral nerve injury.
Figure 3. The effects of an ROS donor on paw withdrawal response in non-ligated mice.
Response rates to von Frey filament 3.0 were measured prior to and after injection of an ROS donor. A single intrathecal injection of 0.05, 0.10, or 0.25 μg t-BOOH dissolved in 5 μl saline (n = 8, 9, 9, respectively) dose-dependently increased response rates, which lasted up to 1.5 h. Vehicle injection did not affect response rates (n = 9). Data are presented as means ± SEM. Arrow with it, intrathecal injection; *, the value is significantly (p < 0.05) different from that of the vehicle control by two-way repeated-measures ANOVA followed by Duncan's post hoc tests.
3.4. ROS do not affect mEPSCs in the spinal dorsal horn
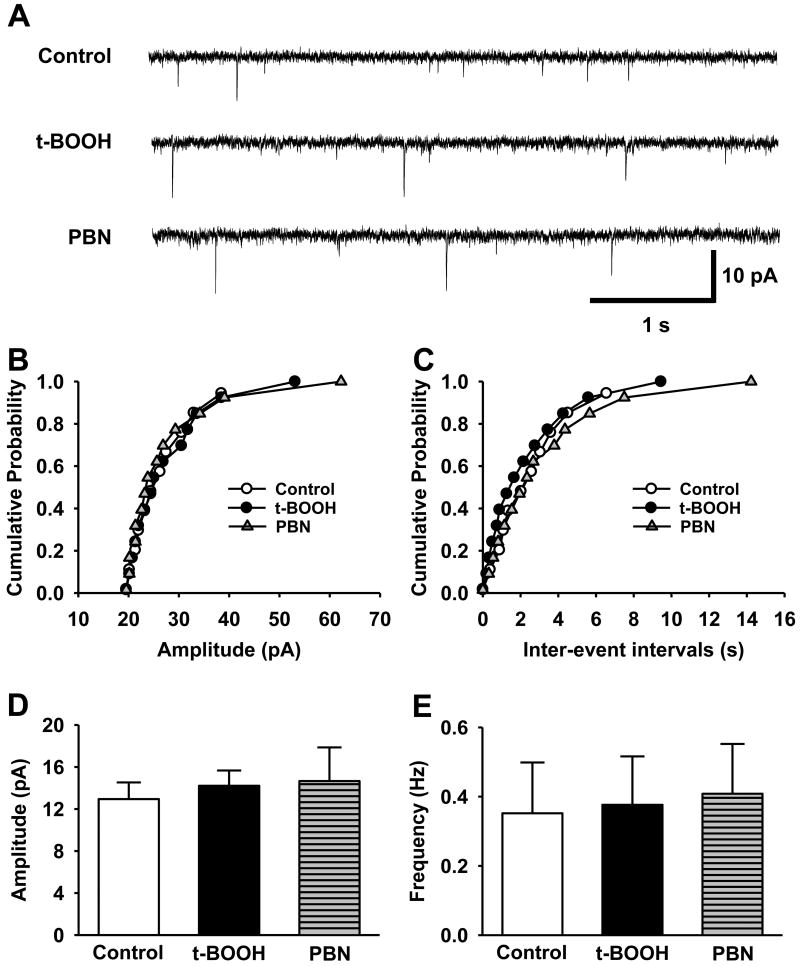
The results from the above behavioral studies indicate that elevated levels of spinal ROS, whether originating from an endogenous or exogenous source, induce pain behaviors in mice. These pain behaviors may result from a phenomenon called central sensitization, where spinal neurons involved in pain transmission become more responsive to peripheral inputs after neuropathic injury [4, 35, 36]. The enhanced responsiveness can be achieved by one of two different mechanisms - either through the enhancement of excitatory transmission or the reduction in inhibitory transmission. In order to determine whether ROS affect the excitatory or inhibitory transmission, whole cell recordings were performed on substantia gelatinosa (SG) neurons in the spinal dorsal horn in non-ligated mice. For miniature excitatory post-synaptic currents (mEPSCs) of SG neurons, we routinely waited for 10 -15 min until the baseline current recording was stabilized after the whole cell recording configuration. The baseline was recorded for 5 min under a control solution (1 μM TTX+10 μM bicuculline in ACSF), superfused with an ROS donor, t-BOOH (2 mM in the control solution) for 10 min, and then washed out with a ROS scavenger, PBN (1 mM in the control solution) for 15 min. Neither t-BOOH nor PBN significantly affected the amplitude and the frequency of the mEPSCs in the studied neurons (Fig. 4, n = 10). These data suggest that excessive levels of ROS did not change either frequency or amplitude of mEPSCs, thus suggesting that ROS do not affect either presynaptic or postsynaptic mechanisms of excitatory transmission in the SG neurons in normal mice.
Figure 4. The effects of an ROS donor and a ROS scavenger on mEPSCs of SG neurons.
This experiment was performed in ten neurons in the substantia gelatinosa (SG). The SG neurons were sequentially perfused with control solution (Control), the same solution containing 2 mM t-BOOH (t-BOOH) or 1 mM PBN (PBN). (A) Representative tracings of mEPSCs were observed before and during t-BOOH followed by PBN infusion. Cumulative probability analysis for the current amplitude (B) and inter-event interval (C) of spontaneous mEPSCs were recorded from the same SG neuron. (D, E) The summary graph of the averaged amplitudes and the frequency of the mEPSCs at various conditions: control baseline, during t-BOOH superfusion (t-BOOH), and during PBN superfusion (PBN). Overall, there were no significant differences between the control condition and the test conditions. Statistical analyses were carried out by one-way analysis of variance (ANOVA).
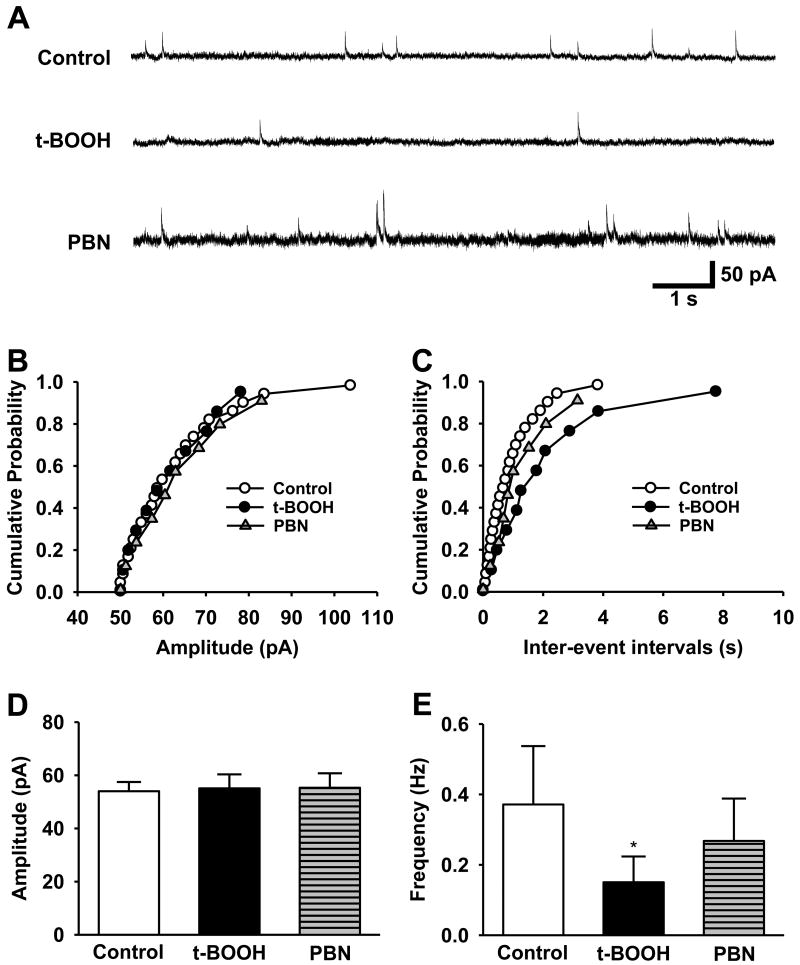
3.5. ROS reduce the mIPSCs in the spinal dorsal horn through presynaptic mechanisms
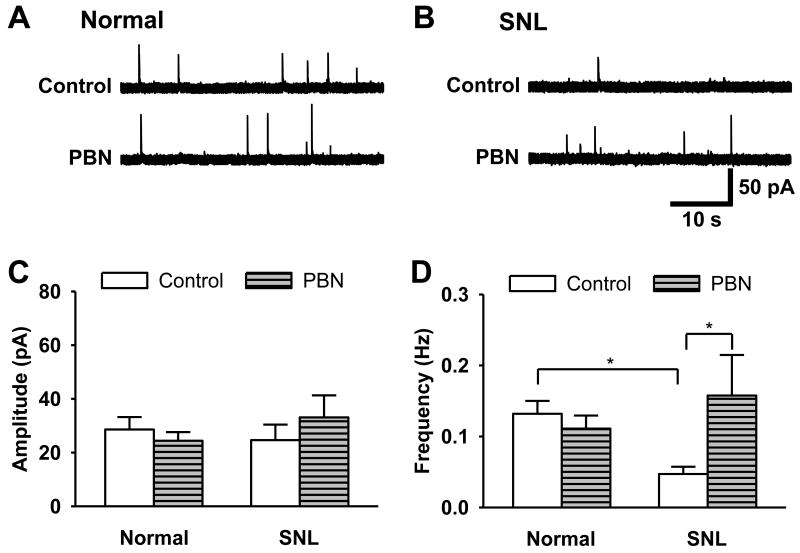
The effects of ROS on inhibitory transmission were investigated next. To determine the effects of ROS on inhibitory transmission, 2 mM t-BOOH was applied into the recording chamber, and miniature inhibitory post-synaptic currents (mIPSCs) were then examined in the SG neurons of non-ligated mice (n = 6). t-BOOH alone significantly decreased the frequency of mIPSCs from 0.37 ± 0.13 Hz to 0.15 ± 0.03 Hz (p < 0.05) without affecting the amplitude (Fig. 5). Therefore, ROS mainly reduced inhibitory neurotransmission through presynaptic mechanisms. This reduction was reversed with the perfusion of 1 mM PBN, demonstrating the reversible nature of these pre-synaptic changes. To verify that peripheral nerve injury reduces the inhibitory transmission through presynaptic action of ROS, the effect of PBN on mIPSCs was compared in spinal cord slices taken from normal and SNL mice. Seven days after left SNL surgery (about 4-5 weeks old mice), mIPSC recordings were made from the left L5 segment of spinal cord slices. After a 5 min baseline recording with control perfusion solution (1 μM TTX, 20 μM CNQX, and 50 μM D-AP5 in ACSF), an ROS scavenger, PBN (1 mM in the control solution) was perfused for 10 min. Baseline and PBN data [taken for 4 min (4 - 8 min after PBN application)] were averaged in normal (n=6) and SNL (n=6) groups. After SNL, the frequencies of mIPSCs, but not amplitude, were significantly decreased as compared to the normal group, which was restored to normal group level by PBN perfusion. However, PBN did not affect either amplitude or frequency of mIPSCs in the normal group (Fig. 6). Overall, these data suggest that ROS are critically involved in diminishing presynaptic inhibitory neurotransmitter release in neuropathic mice.
Figure 5. The effects of an ROS donor and ROS scavenger on mIPSCs of SG neurons.
The SG neurons were sequentially perfused with control solution (Control), the same solution containing 2 mM t-BOOH (t-BOOH) or 1 mM PBN (PBN). (A) Representative tracings of mIPSCs observed before and during t-BOOH and then PBN infusion in ten neurons in the substantia gelatinosa (SG). Cumulative probability analysis for the current amplitude (B) of spontaneous mIPSCs and inter-event interval (C, p < 0.001, control vs t-BOOH; p = 0.23, t-BOOH vs PBN) were recorded from the same neuron. (D, E) Averaged data show that t-BOOH caused a significant decrease in the frequency of the mIPSCs while their amplitudes remained unaffected. Perfusion with 1 mM PBN partially recovered the frequency of mIPSCs (*p < 0.05, t-BOOH vs. control and PBN). Statistical analyses were carried out by one-way analysis of variance (ANOVA).
Figure 6. The effect of an ROS scavenger on mIPSCs of SG neurons in SNL mice.
(A, B) Representative tracings of mIPSCs before (Control) and during 1 mM PBN perfusion (PBN) in the substantia gelatinosa (SG) neuron in normal (A) and SNL (B) mice. (C, D) The summary graphs of the averaged amplitudes and the frequency of the mEPSCs in normal and SNL mice (6 neurons/group). *, the value is significantly (p < 0.05) different from that of normal control or SNL control by one-way repeated-measures ANOVA followed by the Tukey's post hoc tests.
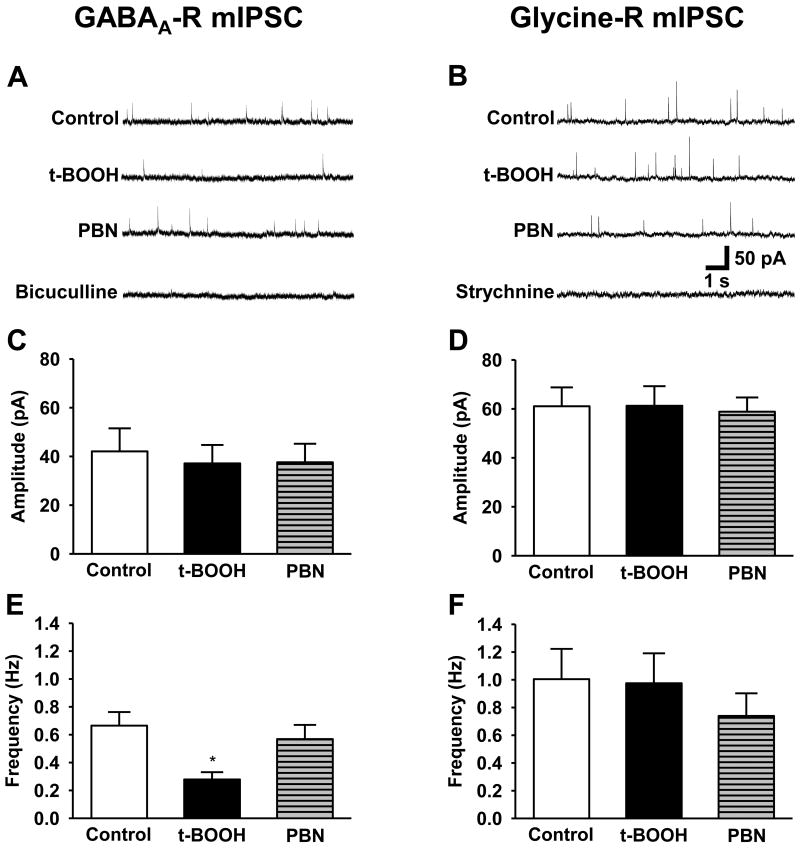
3.6. ROS reduce GABA transmission through presynaptic mechanisms without affecting glycinergic transmission
Since ROS affected inhibitory synaptic transmission in particular, the next step was to determine which inhibitory neurotransmitter system in the spinal cord was altered. Although GABA is known as a major inhibitory neurotransmitter which can modulate spinal nociceptive transmission [35, 38], glycine is another important inhibitory neurotransmitter in the spinal dorsal horn [30, 32, 38]. Moreover, a large portion of IPSCs recorded from SG neurons have both GABAA- and glycine-mediated components. To identify which neurotransmitter system is affected by ROS, GABAA- and glycine-mediated mIPSCs were isolated in SG neurons. Specifically, whole cell recordings of SG neurons were performed using either 10 μM bicuculline with 1 μM TTX to isolate only glycine-mediated mIPSCs (n = 9) or 0.5 μM strychnine with 1 μM TTX to isolate only GABAA-mediated mIPSCs (n = 7). Confirmation of the inhibitory system that produced the mIPSCs was performed with the application of either bicuculline or strychnine to the recording chamber (Fig. 7A, B). Superfusion with 2 mM t-BOOH resulted in a significant reduction in GABAA-receptor mediated mIPSC frequency, from 0.66 ± 1.10 Hz to 0.28 ± 0.05 Hz without an effect on the mIPSC amplitude (Fig. 7C). This response is consistent with a decrease in presynaptic GABA release. When 1 mM PBN was applied to the recording chamber, the mIPSC frequency significantly recovered to 0.57 ± 0.10 Hz, compared to the frequency during t-BOOH perfusion (Fig. 7E, p < 0.05). On the other hand, both t-BOOH and PBN did not significantly affect either the amplitude or the frequency of the glycine-receptor mediated mIPSCs (Fig. 7D, F). Therefore, the data show that the increase of ROS inhibits the release of GABA through presynaptic mechanisms in the spinal dorsal horn, and this effect is reversible when the ROS are scavenged.
Figure 7. The effects of an ROS donor and a ROS scavenger on GABAA- vs. glycine-mediated mIPSCs of SG neurons.
0.5 μM strychnine with 1 μM TTX were used to isolate only GABAA-receptor mediated mIPSCs (A, C, E, n = 9) and 10 μM bicuculline with 1 μM TTX were used to isolate glycine-receptor mediated mIPSCs (B, D, F, n = 7). The SG neurons were sequentially perfused with control solution (Control), the same solution containing 2 mM t-BOOH (t-BOOH), 1 mM PBN (PBN), 10 μM bicuculline (Bicuculline) or 0.5 μM strychnine (Strychnine).
(A) Representative tracings of GABAA-receptor mediated mIPSCs (GABAA-R mIPSC) during control baseline (Control), t-BOOH superfusion (t-BOOH), PBN superfusion (PBN) and bicuculline superfusion (Bicuculline). (C, E) The summary graph of the averaged amplitudes (C) and frequencies (E) of the GABAA-R mIPSCs at various conditions. The ROS donor caused a significant reduction in the GABA-R mIPSC frequency compared with control (**p < 0.001), with a near complete recovery by PBN (**p < 0.001, t-BOOH vs PBN).
(B) Representative tracings of glycine-receptor mediated mIPSCs (glycine-R mIPSC) during control baseline (Control), t-BOOH superfusion (t-BOOH), PBN superfusion (PBN) and strychnine superfusion (Strychnine). (D, F) The summary graph of the averaged amplitudes (D) and frequencies (F) of the glycine-R mIPSCs at various conditions. The glycine-mediated mIPSC frequency was unchanged by either the ROS donor or the ROS scavenger. *, the value is significantly (p < 0.05) different from that of normal control or SNL control by one-way repeated-measures ANOVA followed by Tukey's post hoc tests.
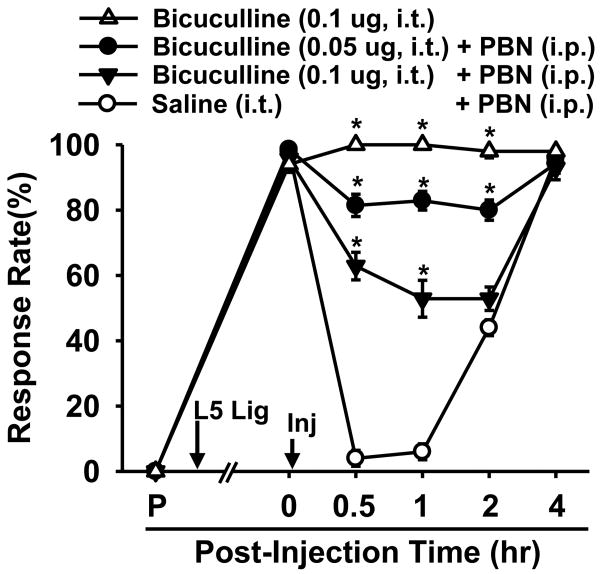
3.7. The anti-hyperalgesic effect of PBN is abolished by bicuculline in SNL mice
The above electrophysiological data suggest that the ROS scavenger, PBN, restores GABAA-receptor mediated inhibitory transmission in the SG. PBN's ability to restore spinal GABAergic function is thus hypothesized to be linked to PBN's analgesic effect. Therefore, the antagonism of the GABAA receptors in the SG is predicted to interfere with PBN's analgesic effect. In order to test this hypothesis, the effect of the GABAA receptor antagonist, bicuculline, on PBN-treated SNL mice was investigated using the behavioral test for mechanical hyperalgesia. Seven days after SNL, mice were given an intrathecal injection of either 0.05 μg or 0.1 μg bicuculline (i.t., 5 μl, n = 7 per group) or vehicle (i.t., 5 μl, n = 5). Immediately afterwards, mice received a systemic injection of 150 mg/kg PBN (i.p., 0.1 ml). The analgesic effect of PBN was greatly reduced by 0.05 μg bicuculline, while 0.1 μg of bicuculline almost completely abolished PBN's effect (Fig. 8). In contrast, 0.1 μg bicuculline alone did not affect mechanical hyperalgesia in SNL mice (Fig. 8) nor changed mechanical sensitivity in normal mice (n=3; data not shown). Thus, these data suggest that ROS are involved in the pathogenesis of pain by interfering with the inhibitory function of the spinal GABAergic system.
Figure 8. The effects of a GABAA antagonist on the PBN induced analgesic effect in SNL mice.
SNL resulted in significantly increased response rates to von Frey filament 3.0 from pre-surgical levels (P) in all operated mice (n = 19). One week after surgery, a single injection of 150 mg/kg PBN i.p. given immediately after 5 μl saline i.t. (n = 5) alleviated mechanical hyperalgesia up to 2 hr. A single intrathecal injection of 0.05 or 0.10 μg bicuculline dissolved in 5 μl saline (n = 7, 7 respectively) given immediately before PBN (150 mg/kg, i.p.) dose-dependently antagonized PBN's analgesic effect. Single injection of bicuculline (0.10 μg, i.t., n=6) itself did not affect mechanical hyperalgesia in SNL mice (n=6). Data are presented as means ± SEM. Arrow with L5 Lig, time of L5 SNL, arrow with Inj, time of intrathecal followed by intraperitoneal injection. *, the value is significantly (p < 0.05) different from that of the vehicle control by two-way repeated-measures ANOVA followed by Duncan's post hoc tests.
4. Discussion
This study examined the modulation of pain by ROS in mice. Spinal nerve ligation produced mechanical hyperalgesia lasting longer than eight weeks, and removal of ROS with a single, systemic injection of PBN transiently reduced pain behaviors. An intrathecal injection of PBN also produced a significant anti-hyperalgesic effect, suggesting that ROS in the spinal cord were an important factor for the maintenance of pain. The intrathecal injections of an ROS donor, t-BOOH, were also sufficient to induce pain behaviors without nerve injury. In addition, the present study showed that ROS donor or spinal nerve injury reduced inhibitory synaptic transmission on spinal SG neurons, specifically modulating GABAA- but not glycine-receptor mediated neurotransmission, which was recovered by scavenging ROS with PBN. Finally, the anti-hyperalgesic effect of a ROS scavenger on pain behaviors was attenuated with a GABAA receptor antagonist. Combined together, results from this study suggest that ROS are involved in neuropathic pain and one possible mechanism involved is by way of reducing spinal GABA neurotransmission.
The behavioral data in this study showed that an exogenous ROS donor, t-BOOH, increased pain behaviors dose-dependently in non-ligated mice. This pain behavior was a transient response that lasted for approximately 90 minutes after intrathecal injection. As a donor of ROS, t-BOOH has been used widely in ‘oxidative stress’ experiments because it easily penetrates the cell membranes, breaks down to produce ROS as a simple chemical reaction, and oxidizes the endogenous antioxidant system to further increase levels of ROS [1, 17, 23]. In the present study, intrathecal injection of t-BOOH produced rapid onset of pain-like behaviors in normal animals at a dose dependent fashion. Furthermore, it has been shown that t-BOOH induces spinal LTP in the superficial dorsal horn at the comparable dose that is used in the present study (1-2 mM) and this LTP is maintained for 20-30 min after t-BOOH is washed out [16]. Thus, it is speculated that the immediate reduction of tBOOH-induced mIPSCs by a sequential PBN infusion is due to PBN's antioxidant effects. Lowering the level of ROS in the spinal cord after treatment with PBN was also observed by others [34]. These results suggest that t-BOOH can exert its effect on the spinal cord by producing ROS and the effect can be reversed by PBN, an ROS scavenger. On the other hand, a contrasting situation may be found in the SNL model where the neurons may continuously overproduce ROS due to injury and overload the cellular machinery that disposes ROS.
It has been proposed that a loss of inhibitory transmission in the spinal cord induced by peripheral nerve injury contributes to neuropathic pain [2, 4]. Consistent with this proposal, the electrophysiological data show that peripheral nerve injury or an ROS donor reduces inhibitory transmission while excitatory transmission remains intact. The present data further suggest that the increase of spinal ROS may cause a reduction of inhibitory transmission in the spinal dorsal horn. Supporting data include that the frequency of GABAA-receptor mediated mIPSCs is reduced by perfusion with an ROS donor, suggesting that there is a reduction in presynaptic GABA release. While a study suggested there was an enhanced endogenous GABAergic tone in the spinal cord after peripheral nerve injury [14], the contrary has also been demonstrated where decreased GABAA-receptor mediated currents in SG neurons were seen in various neuropathic models [20]. Therefore, it is reasonable to suggest that excessive ROS cause heightened neuronal excitability of the spinal dorsal horn by reducing inhibitory transmission.
Another important point brought up by this study is that ROS selectively attenuate GABAergic transmission but not glycinergic transmission. Glycine, in addition to its role in activating postsynaptic glycine receptors, is a co-agonist for the glycine binding site of NMDA receptors [10]. In this regard, increased glycine levels may further enhance nociceptive transmission by enhancing NMDA receptor activity regardless of ROS levels. With respect to the modulation of GABA and glycine transmissions in the spinal cord, it has been shown that the action sites and synaptic release of GABA and glycine are regulated differentially [30, 38]. For example, activation of muscarinic acetylcholine receptors differentially regulates synaptic GABA and glycine release to SG neurons. The M3 receptor subtype is found on glycinergic neurons while the M2 and M4 receptor subtypes are important for GABAergic neurons [32, 40]. Furthermore, it has been shown that nociceptive input with capsaicin injection suppresses GABAergic sIPSCS [42] but augments glycinergic sIPSCs in SG neurons [41]. The present study provides further evidence that GABAergic but not glycinergic transmission is reduced by an increase of ROS in spinal cord; thus, the data suggest that a more complex mechanism is responsible for the differential regulation of synaptic release of these two inhibitory neurotransmitters in the spinal dorsal horn.
In conclusion, this study demonstrated that a ROS scavenger effectively produced anti-hyperalgesia in the mouse model of SNL-induced peripheral neuropathy, and this anti-hyperalgesia is achieved in part by restoring GABAA-mediated inhibitory transmission. An ROS donor temporarily induced pain behaviors in the mouse and reduced GABA-mediated inhibitory transmission without affecting excitatory transmission or glycine-mediated inhibitory transmission. Overall, these data demonstrate the importance of GABA dysfunction in the spinal cord for the development of pain and the possible role of oxidative stress in this dysfunction. Excessive levels of ROS may induce pain by interfering with inhibitory transmission in the form of reducing synaptic GABA release.
Acknowledgments
This work was supported by NIH Grants R01 NS31680 and P01 NS 11255.
Footnotes
The authors report no conflicts of interest through financial or other relationships.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alia M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Mol Toxicol. 2005;19:119–28. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- 2.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Pan HL. Signaling mechanisms of angiotensin II-induced attenuation of GABAergic input to hypothalamic presympathetic neurons. J Neurophysiol. 2007;97:3279–87. doi: 10.1152/jn.01329.2006. [DOI] [PubMed] [Google Scholar]
- 4.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 5.Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–71. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL., Jr Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006;173:211–6. doi: 10.1016/j.bbr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 9.Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–58. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 11.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548:167–73. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–24. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Kontinen VK, Stanfa LC, Basu A, Dickenson AH. Electrophysiologic evidence for increased endogenous gabaergic but not glycinergic inhibitory tone in the rat spinal nerve ligation model of neuropathy. Anesthesiology. 2001;94:333–9. doi: 10.1097/00000542-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KY, Chung K, Chung JM. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol. 2010;103:382–91. doi: 10.1152/jn.90906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemasters JJ, Nieminen AL. Mitochondrial oxygen radical formation during reductive and oxidative stress to intact hepatocytes. Biosci Rep. 1997;17:281–91. doi: 10.1023/a:1027332611839. [DOI] [PubMed] [Google Scholar]
- 18.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–7. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–22. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 20.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–31. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–11. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 23.Park JE, Yang JH, Yoon SJ, Lee JH, Yang ES, Park JW. Lipid peroxidation-mediated cytotoxicity and DNA damage in U937 cells. Biochimie. 2002;84:1199–205. doi: 10.1016/s0300-9084(02)00039-1. [DOI] [PubMed] [Google Scholar]
- 24.Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–66. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–39. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 26.Salvemini D, Ischiropoulos H, Cuzzocrea S. Roles of nitric oxide and superoxide in inflammation. Methods Mol Biol. 2003;225:291–303. doi: 10.1385/1-59259-374-7:291. [DOI] [PubMed] [Google Scholar]
- 27.Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect. 1998;11:204–14. [PubMed] [Google Scholar]
- 28.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29:159–68. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996;7:1382–4. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- 30.Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J Neurosci. 1996;16:974–82. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett. 2000;281:123–6. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang XL, Zhang HM, Li DP, Chen SR, Pan HL. Dynamic regulation of glycinergic input to spinal dorsal horn neurones by muscarinic receptor subtypes in rats. J Physiol. 2006;571:403–13. doi: 10.1113/jphysiol.2005.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–78. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 34.Westlund KN, Kochukov MY, Lu Y, McNearney TA. Impact of central and peripheral TRPV1 and ROS levels on proinflammatory mediators and nociceptive behavior. Mol Pain. 2010;6:46–59. doi: 10.1186/1744-8069-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis WD. Long-term potentiation in spinothalamic neurons. Brain Res Brain Res Rev. 2002;40:202–14. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- 36.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 37.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–23. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. J Physiol. 1995;482(Pt 1):29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yowtak J, Kim HK, Chung K, Chung JM. Reactive oxygen species in a mouse model of neuropathic pain. Society for neuoroscience. 2006 [Google Scholar]
- 40.Zhang HM, Li DP, Chen SR, Pan HL. M2, M3, and M4 receptor subtypes contribute to muscarinic potentiation of GABAergic inputs to spinal dorsal horn neurons. J Pharmacol Exp Ther. 2005;313:697–704. doi: 10.1124/jpet.104.079939. [DOI] [PubMed] [Google Scholar]
- 41.Zhou HY, Zhang HM, Chen SR, Pan HL. Increased C-fiber nociceptive input potentiates inhibitory glycinergic transmission in the spinal dorsal horn. J Pharmacol Exp Ther. 2008;324:1000–10. doi: 10.1124/jpet.107.133470. [DOI] [PubMed] [Google Scholar]
- 42.Zhou HY, Zhang HM, Chen SR, Pan HL. Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J Neurophysiol. 2007;97:871–82. doi: 10.1152/jn.00964.2006. [DOI] [PubMed] [Google Scholar]